Effects of Growth Hormone (GH) Supplementation on Dermatoscopic Evolution of Pigmentary Lesions in Children with Growth Hormone Deficiency (GHD)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Walker, G.J.; Hayward, N.K. Pathways to Melanoma Development: Lessons from the Mouse. J. Investig. Dermatol. 2002, 119, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Situm, M.; Buljan, M.; Kolić, M.; Vučić, M. Melanoma—Clinical, dermatoscopical, and histopathological morphological characteristics. Acta Dermatovenerol. Croat. ADC 2014, 22, 2. [Google Scholar]
- Tsao, H.; Chin, L.; Garraway, L.A.; Fisher, D.E. Melanoma: From mutations to medicine. Genes Dev. 2012, 26, 1131–1155. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.R.; Eliades, P.; Gupta, S.; Tsao, H. Genetics of melanocytic nevi. Pigment Cell Melanoma Res. 2015, 28, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Magnoni, C.; Tenedini, E.; Ferrari, F.; Benassi, L.; Bernardi, C.; Gualdi, G.; Bertazzoni, G.; Roncaglia, E.; Fantoni, L.; Manfredini, R.; et al. Transcriptional profiles in melanocytes from clinically unaffected skin distinguish the neoplastic growth pattern in patients with melanoma. Br. J. Dermatol. 2007, 156, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Gualdi, G.; Panarese, F.; Meogrossi, G.; Marchioni, M.; DE Tursi, M.; Di Martino, P.; Angelucci, D.; Amatetti, M.; Proietto, G.; Di Nicola, M.; et al. Speed rate (SR) as a new dynamic index of melanoma behavior. Pigment. Cell Melanoma Res. 2020, 33, 709–718. [Google Scholar] [CrossRef]
- Orthaber, K.; Pristovnik, M.; Skok, K.; Perić, B.; Maver, U. Skin Cancer and Its Treatment: Novel Treatment Approaches with Emphasis on Nanotechnology. J. Nanomater. 2017, 2017, 2606271. [Google Scholar] [CrossRef]
- Bourneuf, E. The MeLiM Minipig: An Original Spontaneous Model to Explore Cutaneous Melanoma Genetic Basis. Front. Genet. 2017, 8, 146. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Farhat, A.M.; Arce, A.; Ziogas, A.; Taylor, T.; Wang, Z.; Yourk, V.; Liu, J.; Wu, J.; McEligot, A.J.; et al. Sex differences in the association of cutaneous melanoma incidence rates and geographic ultraviolet light exposure. J. Am. Acad. Dermatol. 2016, 76, 499–505.e3. [Google Scholar] [CrossRef]
- Roh, M.R.; Eliades, P.; Gupta, S.; Grant-Kels, J.M.; Tsao, H. Cutaneous melanoma in women. Int. J. Women’s Dermatol. 2017, 3, S11–S15. [Google Scholar] [CrossRef]
- Manganoni, A.M.; Pizzatti, L.; Pavoni, L.; Consoli, F.; Damiani, E.; Manca, G.; Gualdi, G.; Calzavara-Pinton, P. Melanoma in the elderly. G. Ital. di Dermatol. Venereol. 2017, 152, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, M.; Di Nicola, M.; Varrati, S.; Carbone, A.; Pamio, A.; Capo, A.; Tracanna, M.; Castigliego, A.; Tiboni, G.; Amerio, P. Mole modifications following controlled ovarian stimulation for assisted reproduction technologies. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.K.; Emerald, B.S.; Mertani, H.C.; Lobie, P.E. The oncogenic potential of growth hormone. Growth Horm. IGF Res. 2006, 16, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Brunet-Dunand, S.E.; Vouyovitch, C.; Araneda, S.; Pandey, V.; Vidal, L.J.-P.; Print, C.; Mertani, H.C.; Lobie, P.E.; Perry, J. Autocrine Human Growth Hormone Promotes Tumor Angiogenesis in Mammary Carcinoma. Endocrinology 2008, 150, 1341–1352. [Google Scholar] [CrossRef]
- Pandey, V.; Perry, J.K.; Mohankumar, K.M.; Kong, X.-J.; Liu, S.-M.; Wu, Z.-S.; Mitchell, M.D.; Zhu, T.; Lobie, P.E. Autocrine Human Growth Hormone Stimulates Oncogenicity of Endometrial Carcinoma Cells. Endocrinology 2008, 149, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Kopchick, J.J.; List, E.O.; Kelder, B.; Gosney, E.S.; Berryman, D.E. Evaluation of growth hormone (GH) action in mice: Discovery of GH receptor antagonists and clinical indications. Mol. Cell. Endocrinol. 2013, 386, 34–45. [Google Scholar] [CrossRef]
- Tavakkol, A.; Elder, J.T.; Griffiths, C.E.M.; Cooper, K.D.; Talwar, H.; Fisher, G.J.; Keane, K.M.; Foltin, S.K.; Voorhees, J.J. Expression of Growth Hormone Receptor, Insulin-Like Growth Factor 1 (IGF-1) and IGF-1 Receptor mRNA and Proteins in Human Skin. J. Investig. Dermatol. 1992, 99, 343–349. [Google Scholar] [CrossRef]
- Slominski, A.; Malarkey, W.B.; Wortsman, J.; Asa, S.L.; Carlson, A.; Carlson, J.A. Human skin expresses growth hormone but not the prolactin gene. J. Lab. Clin. Med. 2000, 136, 476–481. [Google Scholar] [CrossRef]
- Lincoln, D.T.; Sinowatz, F.; Kölle, S.; Takahashi, H.; Parsons, P.; Waters, M. Up-regulation of growth hormone receptor immunoreactivity in human melanoma. Anticancer. Res. 1999, 19, 1919–1931. [Google Scholar]
- Wyatt, D. Melanocytic nevi in children treated with growth hormone. Pediatrics 1999, 104, 1045–1050. [Google Scholar] [CrossRef]
- Caldarola, G.; Battista, C.; Pellicano, R. Melanoma onset after estrogen, thyroid, and growth hormone replacement therapy. Clin. Ther. 2010, 32, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Handler, M.Z.; Ross, A.L.; Shiman, M.I.; Elgart, G.W.; Grichnik, J.M. Potential Role of Human Growth Hormone in Melanoma Growth Promotion. Arch. Dermatol. 2012, 148, 1179–1182. [Google Scholar] [CrossRef][Green Version]
- Sustarsic, E.G.; Junnila, R.K.; Kopchick, J.J. Human metastatic melanoma cell lines express high levels of growth hormone receptor and respond to GH treatment. Biochem. Biophys. Res. Commun. 2013, 441, 144–150. [Google Scholar] [CrossRef]
- Iyengar, B. Hormone Expression in Melanomas Related to Tumor Angiogenesis. J. Solid Tumors 2012, 2, p36. [Google Scholar] [CrossRef][Green Version]
- Chatzistamou, I.; Volakaki, A.-A.; Schally, A.V.; Kiaris, H.; Kittas, C. Expression of growth hormone–releasing hormone receptor splice variant 1 in primary human melanomas. Regul. Pept. 2008, 147, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Szalontay, L.; Schally, A.V.; Popovics, P.; Vidaurre, I.; Krishan, A.; Zarandi, M.; Cai, R.-Z.; Klukovits, A.; Block, N.L.; Rick, F.G. Novel GHRH antagonists suppress the growth of human malignant melanoma by restoring nuclear p27 function. Cell Cycle 2014, 13, 2790–2797. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Bourguignon, J.; Ernould, C.; Arrese, J.; Franchimont, C.; Piérard, G.; Heinrichs, C.; Craen, M.; Rochiccioli, P. Effects of human growth hormone therapy on melanocytic naevi. Lancet 1993, 341, 1505–1506. [Google Scholar] [CrossRef]
- Piérard, G.E.; Piérard-Franchimont, C.; Nikkels, A.; Nikkels-Tassoudji, N.; Arrese, J.E.; Bourguignon, J.P. Naevocyte triggering by recombinant human growth hormone. J. Pathol. 1996, 180, 74–79. [Google Scholar] [CrossRef]
- Bozzola, E.; Giacchero, R.; Barberi, S.; Borroni, G. Sutton’s nevus and growth hormone therapy. Minerva Pediatr. 2004, 56, 349–351. [Google Scholar]
- Zvulunov, A.; Wyatt, D.; Laud, P.; Esterly, N. Lack of effect of growth hormone therapy on the count and density of melanocytic naevi in children. Br. J. Dermatol. 1997, 137, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Manganoni, A.M.; Rossi, M.T.; Sala, R.; Venturini, M.; Sereni, E.; Ungari, M.; Marocolo, D.; Lonardi, S.; Calzavara-Pinton, P. Dermoscopic, histological and immunohistochemical evaluation of cancerous features in acquired melanocytic nevi that have been repeatedly exposed to UVA or UVB. Exp. Dermatol. 2011, 21, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.G.; Howe, S.F.; Graf, L.H. Expression of Insulin-like Growth Factor I (IGF-I) in Nevi and Melanomas. Am. J. Dermatopathol. 1994, 16, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, P.; Naville, D.; Avallet, O.; Penhoat, A.; Jaillard, C.; Sanchez, P.; Saez, J. Paracrine and Autocrine Regulation of Insulin-Like Growth Factor I. Acta Paediatr. 1991, 80, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Satyamoorthy, K.; Li, G.; Vaidya, B.; Kalabis, J.; Herlyn, M. Insulin-like growth factor-I-induced migration of melanoma cells is mediated by interleukin-8 induction. Cell Growth Differ. Mol. Boil. J. Am. Assoc. Cancer Res. 2002, 13, 87–93. [Google Scholar]
- Kanter-Lewensohn, L.; Dricu, A.; Girnita, L.; Wejde, J.; Larsson, O. Expression of insulin-like growth factor-1 receptor (IGF-1R) and p27Kip1 in melanocytic tumors: A potential regulatory role of IGF-1 pathway in distribution of p27Kip1 between different cyclins. Growth Factors 2000, 17, 193–202. [Google Scholar] [CrossRef] [PubMed]


| Baseline | Follow-Up | Relative Variation | p-Value | |
|---|---|---|---|---|
| Weight (kg) | 27.10 (22.40–33.10) | 28.20 (23.70–32.30) | 0.41 (0.16–0.46) | 0.043 |
| Height (cm) | 124.60 (118.00–137.80) | 127.50 (121.30–141.50) | 0.23 (0.18–0.32) | 0.018 |
| SDS Height | −2.27 (−2.73–−1.76) | −2.19 (−2.25–−1.68) | −0.46 (−0.18–0.18) | 0.075 |
| BMI (kg/m2) | 16.40 (15.30–17.80) | 16.10 (15.10–17.50) | −0.13 (−0.18–0.00) | 0.246 |
| IGF1 (ng/mL) | 152.90 (121.50–167.00) | 266.50 (189.40–371.00) | 0.84 (0.28–1.51) | 0.018 |
| Baseline | Follow-Up | Relative Variation | p-Value | |
|---|---|---|---|---|
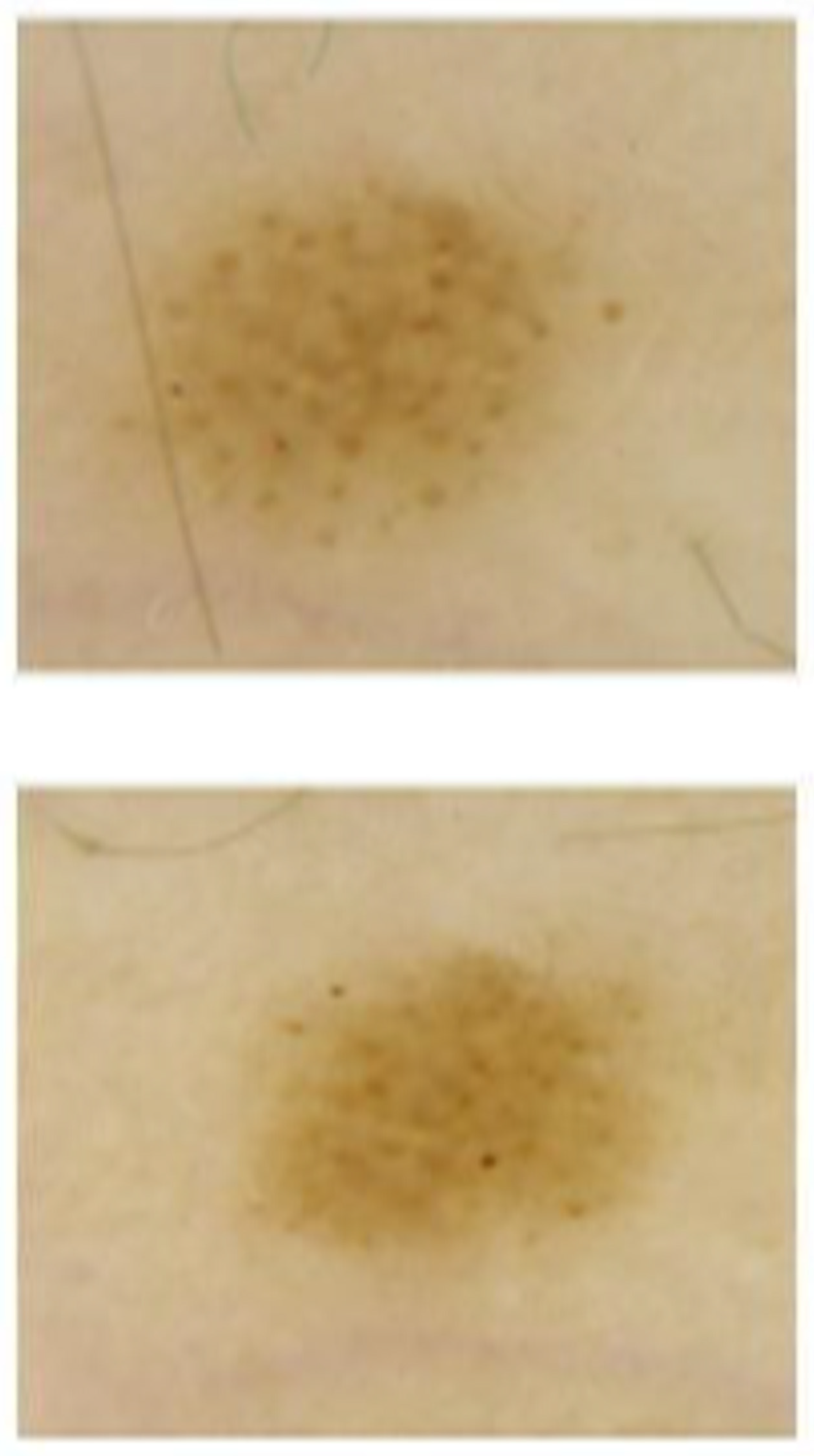
| Diameter Maximum S | 2.22 ± 1.07 | 2.35 ± 1.10 | 0.07 ± 0.12 | <0.001 |
| Diameter Maximum C | 2.30 ± 0.47 | 2.38 ± 0.81 | 0.02 ± 0.07 | 0.12 |
| Diameter Minimum S | 1.72 ± 0.85 | 1.85 ± 0.88 | 0.09 ± 0.13 | <0.001 |
| Diameter Minimum C | 1.79 ± 0.66 | 1.86 ± 0.75 | 0.03 ± 0.08 | 0.27 |
| Baseline | Follow-Up | Absolute Variation | p-Value | |
|---|---|---|---|---|
| Asymmetry | 0.26 ± 0.59 | 0.34 ± 0.57 | 0.07 ± 0.64 | 0.091 |
| Borders | 0.03 ± 0.10 | 0.05 ± 0.14 | 0.02 ± 0.14 | 0.032 |
| Colors | 0.62 ± 0.22 | 0.71 ± 0.26 | 0.09 ± 0.26 | <0.001 |
| Structures | 0.77 ± 0.28 | 0.86 ± 0.28 | 0.09 ± 0.30 | <0.001 |
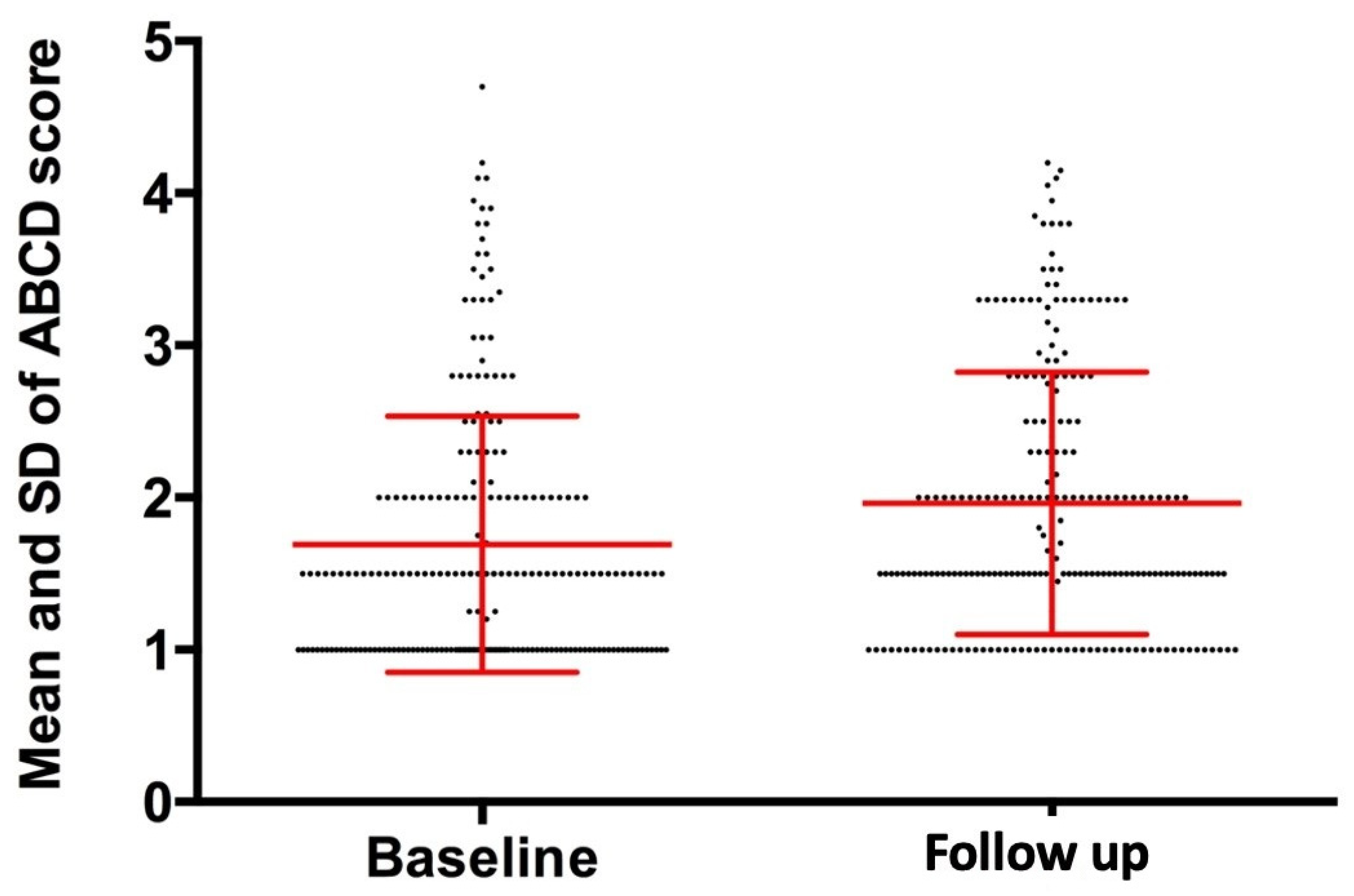
| ABCD Score | 1.69 ± 0.84 | 1.96 ± 0.86 | 0.27 ± 0.87 | <0.001 |
| Diameter Max | Diameter Min | Asymmetry | Borders | Colors | Structures | ABCD Score | |
|---|---|---|---|---|---|---|---|
| Weight (kg) | R = 0.188 p = 0.005 | R = 0.255 p < 0.001 | |||||
| Height (cm) | R = −0.212 p = 0.002 | ||||||
| SDS Height | R = −0.146 p = 0.030 | R = 0.162 p = 0.015 | R = 0.151 p = 0.025 | ||||
| BMI (kg/m2) | R = 0.266 p < 0.001 | R = 0.142 p = 0.034 | R = 0.200 p = 0.003 | R = 0.346 p < 0.001 | R = 0.157 p = 0.019 | R = 0.268 p < 0.001 | |
| IFG1 (ng/mL) | R = −0.267 p < 0.001 | R = −0.242 p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panarese, F.; Gualdi, G.; Di Nicola, M.; Giannini, C.; Polidori, N.; Giuliani, F.; Mohn, A.; Amerio, P. Effects of Growth Hormone (GH) Supplementation on Dermatoscopic Evolution of Pigmentary Lesions in Children with Growth Hormone Deficiency (GHD). J. Clin. Med. 2022, 11, 736. https://doi.org/10.3390/jcm11030736
Panarese F, Gualdi G, Di Nicola M, Giannini C, Polidori N, Giuliani F, Mohn A, Amerio P. Effects of Growth Hormone (GH) Supplementation on Dermatoscopic Evolution of Pigmentary Lesions in Children with Growth Hormone Deficiency (GHD). Journal of Clinical Medicine. 2022; 11(3):736. https://doi.org/10.3390/jcm11030736
Chicago/Turabian StylePanarese, Fabrizio, Giulio Gualdi, Marta Di Nicola, Cosimo Giannini, Nella Polidori, Federica Giuliani, Angelika Mohn, and Paolo Amerio. 2022. "Effects of Growth Hormone (GH) Supplementation on Dermatoscopic Evolution of Pigmentary Lesions in Children with Growth Hormone Deficiency (GHD)" Journal of Clinical Medicine 11, no. 3: 736. https://doi.org/10.3390/jcm11030736
APA StylePanarese, F., Gualdi, G., Di Nicola, M., Giannini, C., Polidori, N., Giuliani, F., Mohn, A., & Amerio, P. (2022). Effects of Growth Hormone (GH) Supplementation on Dermatoscopic Evolution of Pigmentary Lesions in Children with Growth Hormone Deficiency (GHD). Journal of Clinical Medicine, 11(3), 736. https://doi.org/10.3390/jcm11030736






