Behavioural Interventions in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analysis of Randomised Clinical Trials
Abstract
:1. Introduction
2. Methods
2.1. Information Sources
2.2. Search Strategies
2.3. Inclusion and Exclusion Criteria
2.4. Systematic Review
2.5. Meta-Analysis
3. Results
3.1. Study Selection
3.2. Description of Studies
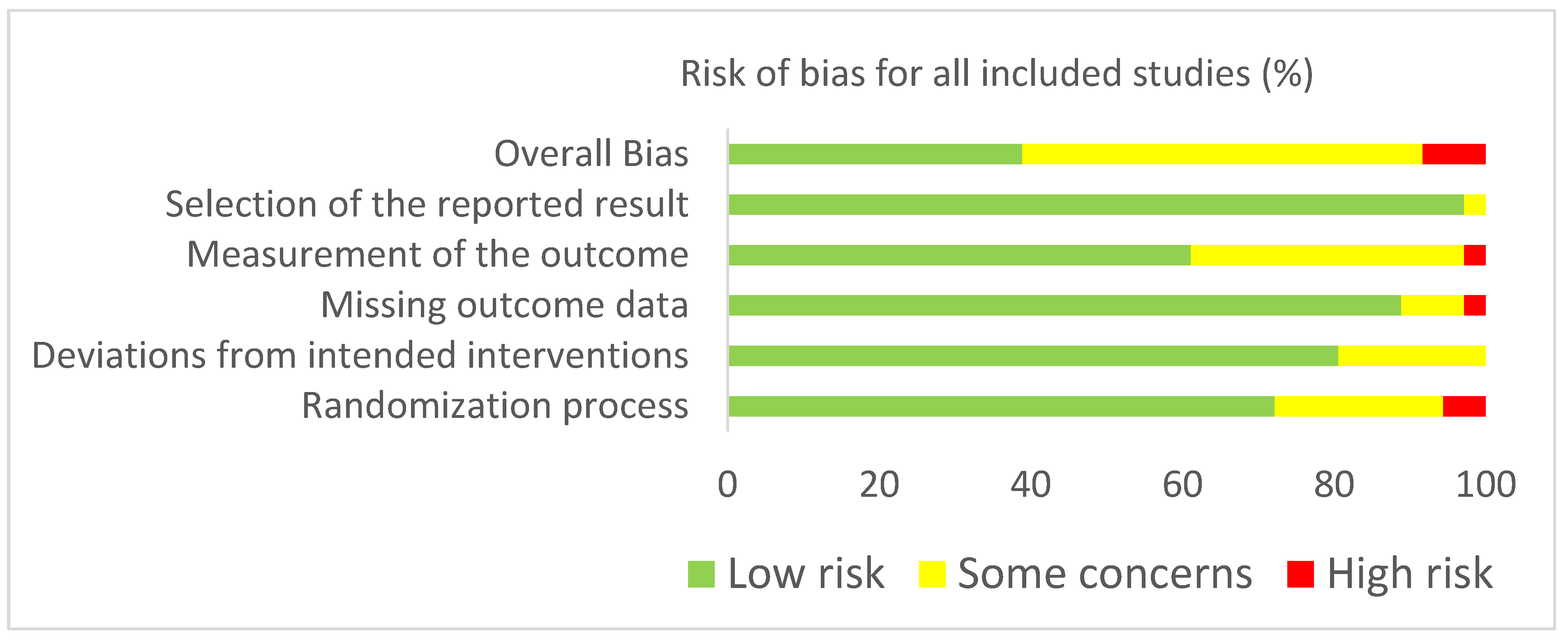
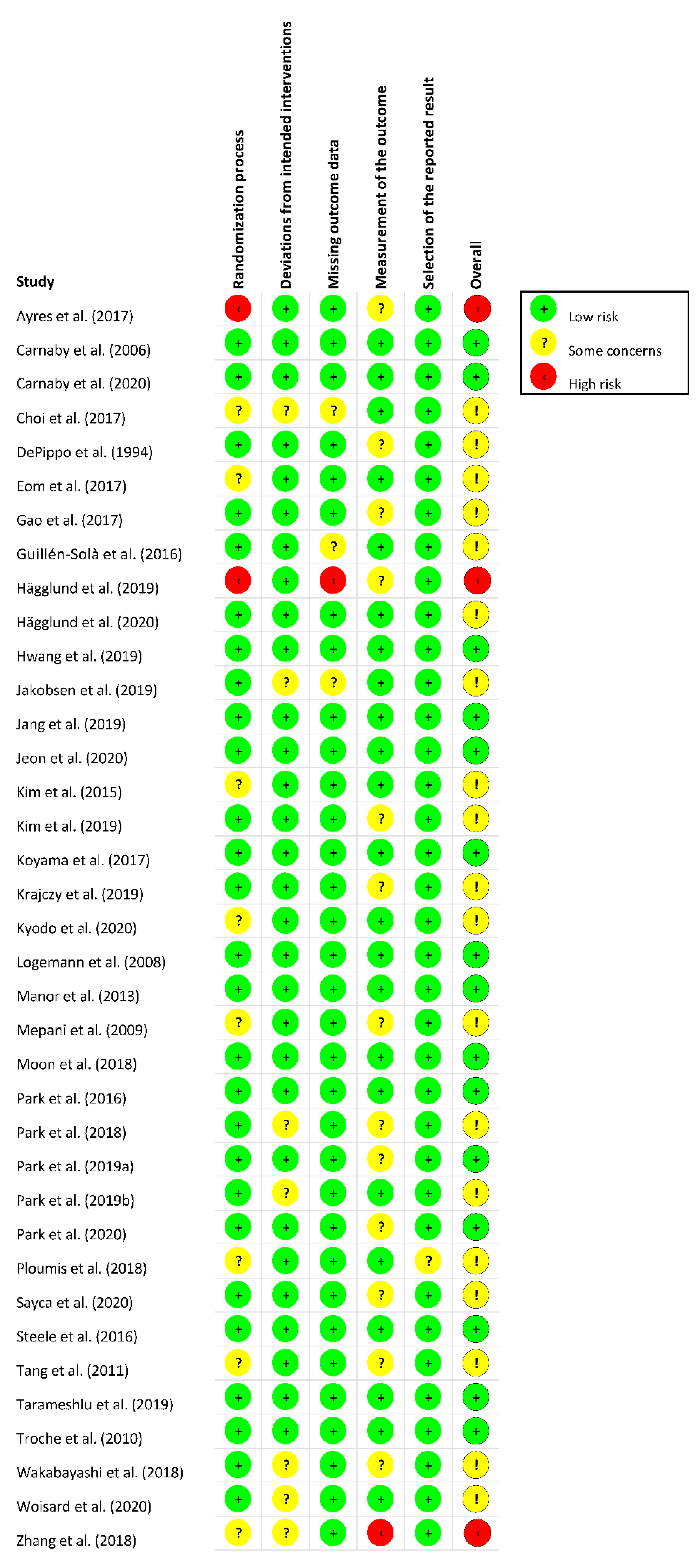
3.3. Risk of Bias Assessment
3.4. Methodological Quality
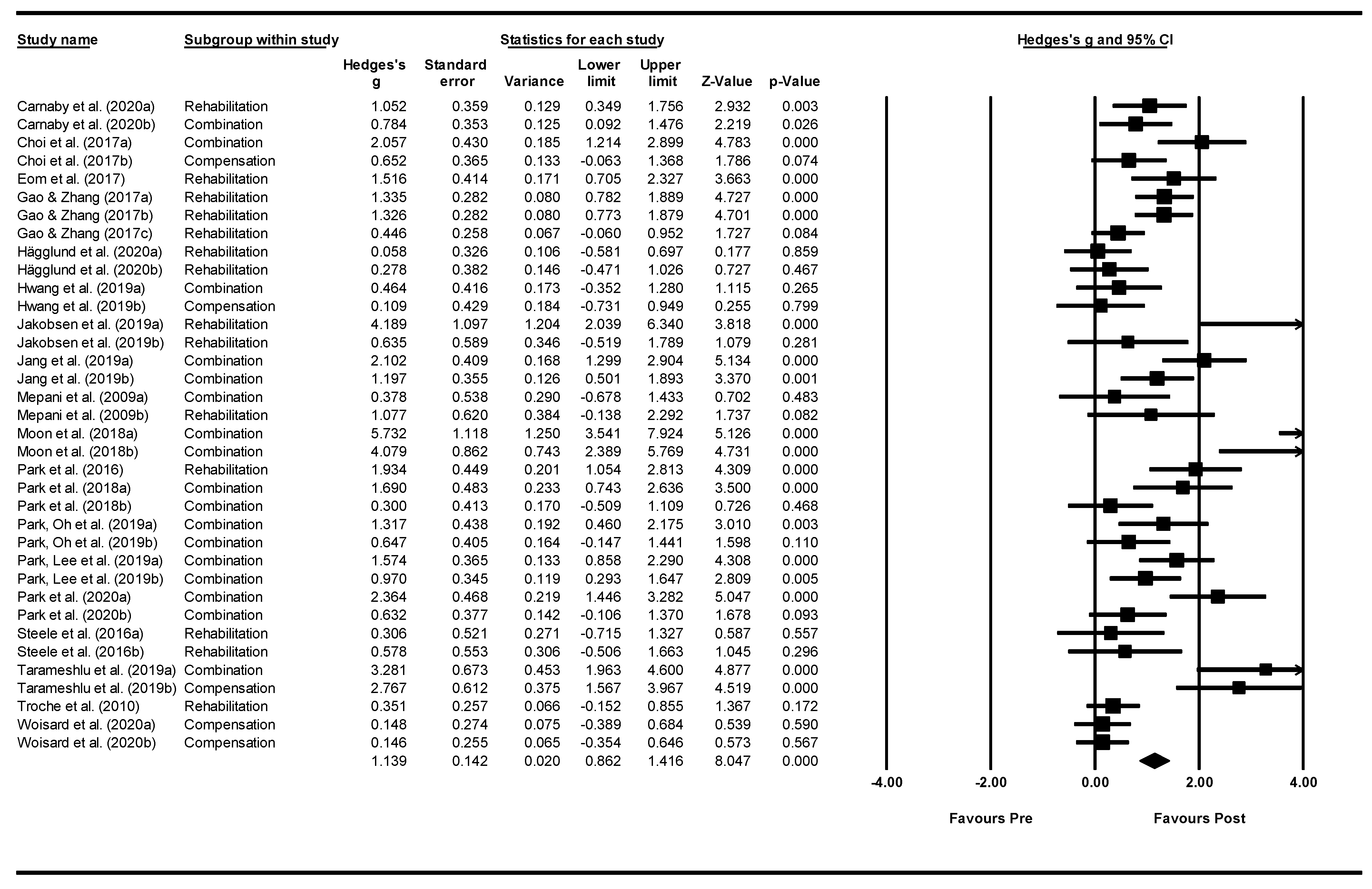
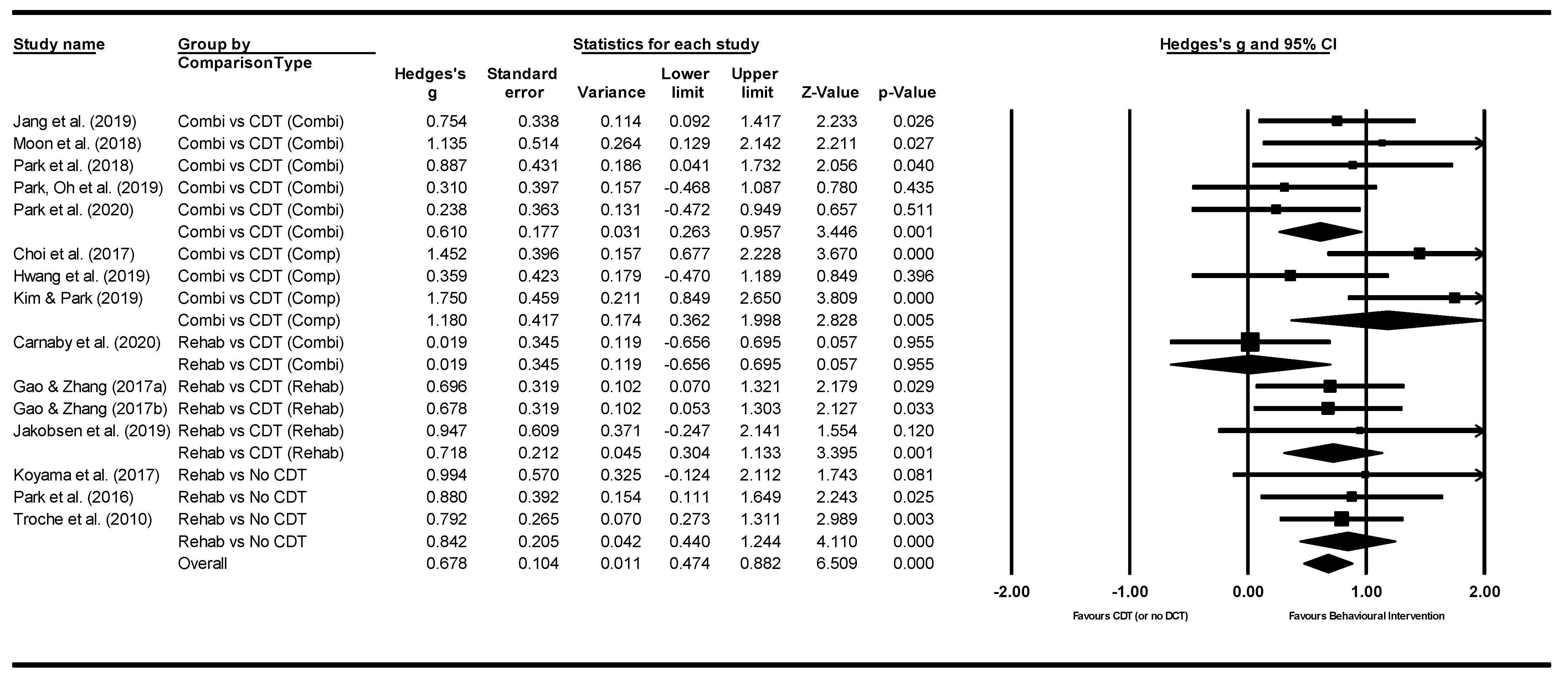
3.5. Meta—Analysis: Effect of Interventions
4. Discussion
4.1. Systematic Review Findings
4.2. Meta-Analysis Findings
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kertscher, B.; Speyer, R.; Fong, E.; Georgiou, A.; Smith, M. Prevalence of oropharyngeal dysphagia in the Netherlands: A telephone survey. Dysphagia 2015, 30, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Speyer, R.; Cordier, R.; Farneti, D.; Nascimento, W.; Pilz, W.; Verin, E.; Walshe, M.; Woisard, V. White paper by the European society for Swallowing Disorders: Screening and non-instrumental assessment for dysphagia in adults. Dysphagia 2021. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, C.; Gemmell, E.; Kenworthy, J.; Speyer, R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson’s disease, Alzheimer’s disease, head injury, and pneumonia. Dysphagia 2016, 31, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Speyer, R.; Cordier, R.; Kim, J.-H.; Cock, N.; Michou, E.; Wilkes-Gillan, S. Prevalence of drooling, feeding and swallowing problems in cerebral palsy across the lifespan: Systematic review and meta-analysis. Dev. Med. Child Neurol. 2019, 61, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, E.; Speyer, R.; Kertscher, B.; Swan, K.; Wagg, B.; Cordier, R. Health-related quality of life in oropharyngeal dysphagia. Dysphagia 2018, 33, 141–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speyer, R. (Ed.) Behavioural Treatment of Oropharyngeal Dysphagia; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Speyer, R.; Baijens, L.; Heijnen, M.; Zwijnenberg, I. The effects of therapy in oropharyngeal dysphagia by speech therapists: A systematic review. Dysphagia 2010, 25, 40–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, N.; Teasell, R.; Salter, K.; Kruger, E.; Martino, R. Dysphagia treatment post stroke: A systematic review of randomised controlled trials. Age Ageing 2008, 37, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Cheng, I.; Sasegbon, A.; Hamdy, S. Effects of neurostimulation on poststroke dysphagia: A synthesis of current evidence from randomised controlled trials. Neuromodulation Technol. Neural Interface 2021, 24, 1388–1401. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Guidelines for the Development and Implementation of Clinical Guidelines, 1st ed.; Australian Government Publishing Service: Canberra, Australia, 1995.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Aki, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Brittish Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. Brittish Med. J. 2021, 372, n160. [Google Scholar] [CrossRef]
- Sterne, J.; Savović, J.; Page, M.; Elbers, R.; Blencowe, N.; Boutron, I.; Cates, C.; Cheng, H.-Y.; Corbett, M.; Eldridge, S.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. Brittish Med. J. 2019, 366, 14898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. Comprehensive Meta-Analysis; Biostat: Englewood, NJ, USA, 2014; Volume 3. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Brittish Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R. The file drawer problem and tolerance for null results. Psychol. Bull. 1979, 86, 638–664. [Google Scholar] [CrossRef]
- Ayres, A.; Jotz, G.P.; Rieder, C.R.M.; Olchik, M.R. Benefit from the Chin-Down Maneuver in the swallowing performance and self-perception of Parkinson’s Disease patients. Parkinson’s Dis. 2017, 8. [Google Scholar] [CrossRef]
- Carnaby, G.; Hankey, G.J.; Pizzi, J. Behavioural intervention for dysphagia in acute stroke: A randomised controlled trial. Lancet Neurol. 2006, 5, 31–37. [Google Scholar] [CrossRef]
- Carnaby, G.; LaGorio, L.; Silliman, S.; Crary, M. Exercise-based swallowing intervention (McNeill Dysphagia Therapy) with adjunctive NMES to treat dysphagia post stroke: A double blind placebo-controlled trial. J. Oral Rehabil. 2020, 47, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-B.; Shim, S.-H.; Yang, J.-E.; Kim, H.-D.; Lee, D.-H.; Park, J.-S. Effects of Shaker exercise in stroke survivors with orophagyngeal dysphagia. Neurorehabilitation 2017, 41, 753–757. [Google Scholar] [CrossRef]
- DePippo, K.L.; Holas, M.A.; Reding, M.J.; Mandel, F.S.; Lesser, M.L. Dysphagia therapy following stroke: A controlled trial. Neurology 1994, 44, 1655–1660. [Google Scholar] [CrossRef]
- Eom, M.-J.; Chang, M.-Y.; Oh, D.-H.; Kim, H.-D.; Han, N.-M.; Park, J.-S. Effects of resistance expiratory muscle strength training in elderly patients with dysphagic stroke. Neurorehabilitation 2017, 41, 747–752. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.-J. Effects of chin tuck against resistance exercise versus Shaker exercise on dysphagia and psychological state after cerebral infarction. Eur. J. Phys. Rehabil. Med. 2017, 53, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Solà, A.; Sartor, M.M.; Soler, N.B.; Duarte, E.; Barrera, M.C.; Marco, E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 31, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, P.; Hägg, M.; Wester, P.; Jäghagen, E.L. Effects of oral neuromuscular training on swallowing dysfunction among older people in intermediate care—A cluster randomised, controlled trial. Age Aging 2019, 48, 533–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hägglund, P.; Hägg, M.; Jäghagen, E.L.; Larsson, B.; Wester, P. Oral neuromuscular training in patients with dysphagia after stroke: A prospective, randomized, open-label study with blinded evaluators. BMC Neurol. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.-K.; Kim, H.-H.; Shim, J.-M.; Park, J.-S. Tongue stretching exercises improve tongue motility and oromotor function in patients with dysphagia after stroke: A preliminary randomized controlled trial. Arch. Oral Biol. 2019, 108, 104521. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, D.; Poulson, I.; Schultheiss, C.; Riberholt, C.G.; Curtis, D.J.; Peterson, T.H.; Seidl, R.O. The effect of intensified nonverbal facilitation of swallowing on dysphagia after severe acquired brain injury: A randomised controlled pilot study. Neurorehabilitation 2019, 45, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, K.W.; Lee, S.J.; Kim, S.B.; Lee, K.W.; Lee, J.H.; Park, J.G. Effects of mechanical inspiration and expiration exercise on velopharyngeal incompetence in subacute stroke patients. J. Rehabil. Med. 2019, 51, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, Y.H.; Cho, K.H.; Park, S.J. Effects of Neuromuscular Electrical Stimulation (NMES) plus upper cervical spine mobilization on forward head posture and swallowing function in stroke patients with dysphagia. Brain Sci. 2020, 10, 478. [Google Scholar] [CrossRef]
- Kim, K.D.; Lee, H.J.; Lee, M.H.; Ruy, H.J. Effects of neck exercises on swallowing function of patients with stroke. J. Phys. Ther. Sci. 2015, 27, 1005–1008. [Google Scholar]
- Kim, H.-H.; Park, J.-S. Efficacy of modified chin tuck against resistance exercise using hand-free device for dysphagia in stroke survivors: A randomised controlled trial. J. Oral Rehabil. 2019, 46, 1042–1046. [Google Scholar] [CrossRef]
- Koyama, Y.; Sugimoto, A.; Hamano, T.; Kasahara, T.; Toyojura, M.; Masakado, Y. Proposal for a modified jaw opening exercise for dysphagia: A randomized, controlled Trial. Tokai J. Exp. Clin. Med. 2017, 42, 71–78. [Google Scholar] [PubMed]
- Krajczy, E.; Krajczy, M.; Luniewski, J.; Bogacz, K.; Szczegielniak, J. Assessment of the effects of dysphagia therapy in patients in the early post-stroke period: A randomised controlled trial. Neurol. I Neurochir. Pol. 2019, 53, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Kyodo, R.; Kudo, T.; Horiuchi, A.; Sakamoto, T.; Shimizu, T. Pureed diets containing a gelling agent to reduce the risk of aspiration in elderly patients with moderate to severe dysphagia. Medicine 2020, 99, e21165. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A.; Gensler, G.; Robbins, J.; Lindbland, A.S.; Brandt, D.; Hind, J.A.; Kosek, S.; Dikeman, K.; Kazandjian, M.; Gramigna, G.D.; et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s Disease. J. Speech Lang. Hear. Res. 2008, 51, 173–183. [Google Scholar] [CrossRef]
- Manor, Y.; Mootanah, R.; Freud, D.; Giladi, M.; Cohen, J.T. Video-assisted swallowing therapy for patients with Parkinson’s disease. Parkinisonism Relat. Disord. 2013, 19, 207–211. [Google Scholar] [CrossRef]
- Mepani, R.; Antonik, S.; Massey, B.; Kern, M.; Logemann, J.A.; Pauloski, B.R.; Rademaker, A.; Easterling, C.; Shaker, R. Augmentation of deglutitive thyrohyoid muscle shortening by the Shaker Exercise. Dysphagia 2009, 24, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Moon, J.-H.; Hahm, S.-C.; Won, Y.S.; Cho, H.-Y. The effects of tongue pressure strength and accuracy training on tongue pressure strength, swallowing function, and quality of life in subacute stroke patients with dysphagia: A preliminary randomized clinical trial. Int. J. Rehabil. Res. 2018, 41, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Oh, D.-H.; Chang, M.-Y.; Kim, K.M. Effects of expiratory muscle strength training on oropharyngeal dysphagia in subacute stroke patients: A randomised controlled trial. J. Oral Rehabil. 2016, 43, 364–372. [Google Scholar] [CrossRef]
- Park, J.-S.; An, D.-H.; Oh, D.-H.; Chang, M.-Y. Effect of chin tuck against resistance exercise on patients with dysphagia following stroke: A randomized pilot study. Neurorehabilitation 2018, 42, 191–197. [Google Scholar] [CrossRef]
- Park, H.-S.; Oh, D.-H.; Yoon, T.; Park, J.-S. Effect of effortful swallowing training on tongue strength and oropharyngeal swallowing function in stroke patients with dysphagia: A double-blind, randomized controlled trial. Int. J. Lang. Commun. Disord. 2019, 54, 479–484. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, G.; Jung, Y.-J. Effects of game-based chin tuck against resistance exercise vs head-lift exercise in patients with dysphagia after stroke: An assessor-blind, randomized controlled. J. Rehabil. Med. 2019, 51, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-S.; An, D.-H.; Kam, K.-Y.; Yoon, T.; Kim, T.; Chang, M.-Y. Effects of resistive jaw opening exercise in stroke patients with dysphagia: A doubleblind, randomized controlled study. J. Back Musculoskelet. Rehabil. 2020, 33, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Ploumis, A.; Papadopoulou, S.L.; Theodorou, S.J.; Exarchakos, G.; Givissis, P.; Beris, A. Cervical isometric exercises improve dysphagia and cervical spine malalignment following stroke with hemiparesis: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Sayaca, C.; Serel-Arslan, S.; Sayaca, N.; Demir, N.; Somay, G.; Kaya, D.; Karaduman, A. Is the proprioceptive neuromuscular facilitation technique superior to Shaker exercises in swallowing rehabilitation? Eur. Arch. Oto-Rhino-Larynology 2020, 277, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Bayley, M.T.; Peladeau-Pigeon, M.; Nagy, A.; Namasivayam, A.M.; Stokely, S.L.; Wolkin, T. A randomized trial comparing two tongue-pressure resistance training protocols for post-stroke dysphagia. Dysphagia 2016, 31, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Shen, Q.; Wang, Y.; Lu, K.; Wang, Y.; Peng, Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther. Onkol. 2011, 187, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Tarameshlu, M.; Ghelichi, L.; Azimi, A.R.; Ansari, N.N.; Khatoonabadi, A.R. The effect of traditional dysphagia therapy on the swallowing function in patients with Multiple Sclerosis: A pilot double-blinded randomized controlled trial. J. Bodyw. Mov. Ther. 2019, 23, 171–176. [Google Scholar] [CrossRef] [Green Version]
- Troche, M.S.; Okun, M.S.; Rosenbek, J.C.; Musson, N.; Fernandez, H.H.; Rodriguez, R.; Romrell, J.; Pitts, T.; Wheeler-Hegland, K.M.; Sapienza, C.M. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST: A randomized trial. Neurology 2010, 75, 1912–1919. [Google Scholar] [CrossRef] [Green Version]
- Wakabayashi, H.; Matsushima, M.; Momosaki, R.; Yoshida, S.; Mutai, R.; Yodoshi, T.; Murayama, S.; Hayashi, T.; Horiguchi, R.; Ichikawa, H. The effects of resistance training of swallowing muscles on dysphagia in older people: A cluster, randomized, controlled trial. Nutrition 2018, 48, 111–116. [Google Scholar] [CrossRef]
- Woisard, V.; Costes, M.; Colineaux, H.; Lepage, B. How a personalised transportable folding device for seating impacts dysphagia. Eur. Arch. Oto-Rhino-Larynology 2020, 277, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Ju, X.-M. Clinical improvement of nursing intervention in swallowing dysfunction of elderly stroke patients. Biomed. Res. 2018, 29, 1099–1102. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.H.; Sohn, H.-J.; Park, J.-S.; Ahn, T.G.; Shin, Y.B.; Park, M.; Ko, S.-H.; Shin, Y.-I. Effect of bihemispheric anodal transcranial direct current stimulation for dysphagia in chronic stroke patients: A randomized control trial. J. Rehabil. Med. 2017, 49, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltringham, S.A.; Kilner, K.; Gee, M.; Sage, K.; Bray, B.D.; Smith, C.J.; Pownall, S. Factors associated with risk of stroke-associated pneumonia in patients with dysphagia: A systematic review. Dysphagia 2020, 35, 735–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninfa, A.; Crispiatico, V.; Pizzorni, N.; Bassi, M.; Casazza, G.; Schindler, A.; Delle Fave, A. The care needs of persons with oropharyngeal dysphagia and their informal caregivers: A scoping review. PLoS ONE 2021, 9, e0257683. [Google Scholar] [CrossRef]
- Attrill, S.; White, S.; Murray, J.; Hammond, S.; Doeltgen, S. Impact of oropharyngeal dysphagia on healthcare cost and length of stay in hospital: A systematic review. BMC Health Serv. Res. 2018, 18, 594. [Google Scholar] [CrossRef] [Green Version]
- Speich, B.; von Niederhäusern, B.; Schur, N.; Hemkens, L.G.; Fürst, T.; Bhatnagar, N.; Alturki, R.; Agarwal, A.; Kasenda, B.; Pauli-Magnus, C.; et al. Systematic review on costs and resource use of randomized clinical trials shows a lack of transparent and comprehensive data. J. Clin. Epidemiol. 2018, 96, 1–11. [Google Scholar] [CrossRef]
- McHutchion, L.D.; Pringle, J.M.; Tran, M.-H.N.; Ostevik, A.V.; Constantinescu, G. A survey of public awareness of dysphagia. Int. J. Speech-Lang. Pathol. 2021, 23, 614–621. [Google Scholar] [CrossRef]
- Hróbjartsson, A.; Forfang, E.; Haahr, M.T.; Als-Nielsen, B.; Brorson, S. Blinded trials taken to the test: An analysis of randomised clinical trials that report tests for the success of blinding. Int. J. Epidemiol. 2007, 36, 654–663. [Google Scholar] [CrossRef]
- Kahan, B.C.; Rehal, S.; Cro, S. Risk of selection bias in randomised trials. Trials 2015, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Baijens, L.W.; Clave, P.; Cras, P.; Ekberg, O.; Forster, A.; Kolb, G.F.; Leners, J.C.; Masiero, S.; Mateos-Nozal, J.; Ortego, O.; et al. European Society for Swallowing Disorders—European Union Geriatric Medicine Society white paper: Oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 2016, 11, 1403–1428. [Google Scholar] [CrossRef] [Green Version]
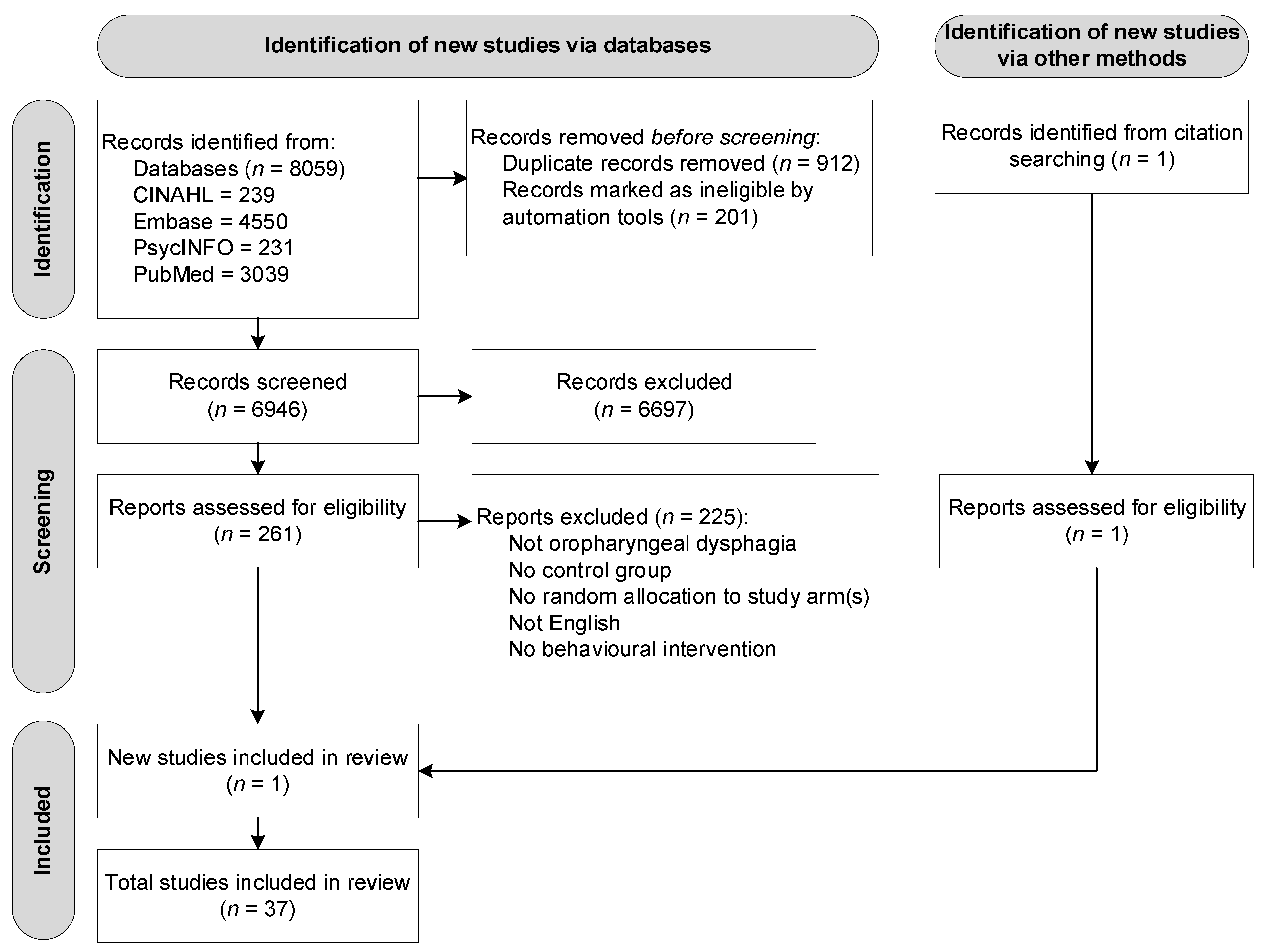
| Database and Search Terms | Number of Records |
|---|---|
| Cinahl: ((MH “Deglutition”) OR (MH “Deglutition Disorders”)) AND (MH “Randomized Controlled Trials”) | 239 |
| Embase: (swallowing/OR dysphagia/) AND (randomization/or randomized controlled trial/OR “randomized controlled trial (topic)”/OR controlled clinical trial/) | 4550 |
| PsycINFO: (swallowing/OR dysphagia/) AND (RCT OR (Randomised AND Controlled AND Trial) OR (Randomized AND Clinical AND Trial) OR (Randomised AND Clinical AND Trial) OR (Controlled AND Clinical AND Trial)).af. | 231 |
| PubMed: (“Deglutition” [Mesh] OR “Deglutition Disorders” [Mesh]) AND (“Randomized Controlled Trial” [Publication Type] OR “Randomized Controlled Trials as Topic” [Mesh] OR “Controlled Clinical Trial” [Publication Type] OR “Pragmatic Clinical Trials as Topic” [Mesh]) | 3039 |
Study
|
| Sample (N)
| Group Descriptive (Mean ± SD) (Age, Gender, Relevant Medical Diagnoses) |
|---|---|---|---|
Ayres, et al. [19]
| OD: Oropharyngeal dysphagia determined by FEES Diagnosis: PD Inclusion: PD and oro-pharyngeal dysphagia. Exclusion: Presenting language and/or hearing disorders that could complicate the understanding of intervention; diagnosis of dementia, or other neurological illnesses. | n = 32:
| Experimental group/Orientation group/control group Age years: 62 (11.5)/64.5 (5.6)/62.8 (6.2) Male: 80%/66.7%/75% Schooling: 5.9 (4.1)/12 (9.1)/10.3 (8.4) Time of disease: 10.7 (4.7)/11.8 (8)/8.8 (6) H & Y disability score: 2.8 (0.8)/2.5 (0.7)/2.5 (0.8) MOCA: 21.9 (4.9)/20.5 (7.7)/21.2 (8.4) PDQ-39: 41.4 (13.8)/38.7 (16.7)/36.5 (17.1) BDI: 13.8 (7.7)/17.1 (9.2)/14.7 (9.3) FOIS: 5.9 (1.3)/6.8 (0.5)/6.8 (0.4) |
Carnaby, et al. [20]
| OD: Diagnosis of swallowing difficulty by speech pathologist, <85 on Hospital’s dysphagia assessment Diagnosis: Clinician diagnosed Stroke, WHO definition Inclusion: Stroke < 7 days Exclusion: NR | n = 306:
| High intensity/low intensity/UC: mean (SD) Age yr: 69.8 (12.5)/72 (12.4)/71.4 (12.7) Male: 59%/58%/58% Severity Barthel index <15: 78%/78%/79% Rankin score >3: 85%/79%/83% Length hospital stay, days: 19.1/19.2/21.4 |
Carnaby, et al. [21]
| OD: Dysphagia on admission- score < 178 on MASA, no history of swallowing disability, head/neck surgery. Diagnosis: Sub-acute stroke confirmed by attending neurologist according to the WHO definition Inclusion: Able to adhere to behavioural treatment regimens Exclusion: NR | n = 53: | NMES/MDTP/UC: mean (SD) Age yr: 62.7 (12.2)/70.6 (11.8)/64.3 (14.7) Male: 55%/44%/41% Modified Rankin: 4.5 (0.6)/4.46 (0.5)/4.56 (0.5) Modified Barthel: 5.3 (3.4)/5.5 (2.8)/5.6 (2.6) Days post stroke: 7.83 (3.9)/8.47 (7.17)/6.7 (5.1) MASA score: 157.8 (16.5)/154.62 (18.87)/158.4 FOIS score: 3.72 (1.44)/3.25 (1.61)/4.35 (1.8) |
Choi, et al. [22]
| OD: Dysphagia after stroke confirmed by VFSS Diagnosis: Stroke (method NR) Inclusion: No major cognitive deficit (MMSE >20), >fair grade on neck muscle testing, symmetric neck posture Exclusion: neck pain or neck surgery, poor general condition, severe communication problem, unstable medical condition, presence of a tracheostomy tube | n = 32: | Experimental SE + CDT/control (CDT): mean (SD) Age yr: 60.8 (10.9)/60.4 (10.5) Gender (male/female): 10/6/9/6 Time since stroke onset months: 3.4 (1.6)/4.1 (1.0) PAS: 4.6 (0.8)/4.9 (0.1) FOIS: 3.1 (1.0)/3.2 (0.68) |
DePippo, et al. [23]
| OD: MBS, BDST, VFSS, speech pathologists determined dysphagia Diagnosis: Stroke by clinical history, neurologic examination CT/MRI Inclusion: 20–90 yrs, no history of oral or pharyngeal anomaly Exclusion: aspirated >50% of all consistencies, | n = 115, allocated to graded therapist treatment levels:
| Group A/Group B/Group C Age yr: 76/74.5/73 Male/Female: 22/16/19/19/27/12 Mini-Mental State score: 16 (12)/17 (10)/18 (10) Barthel-ADL Mobility: 37 (23)/48 (20)/46 (38) Weeks post stroke: 4.6/4.5/4.9 |
Eom, et al. [24]
| OD: Dysphagia caused by a stroke, confirmed by VFSS Diagnosis: Stroke Inclusion: Age > 65, onset duration < 3 months, score ≥ 24 on MMSE. Exclusion: Presence of severe orofacial pain, significant malocclusion or facial asymmetry, unstable breathing or pulse, tracheostomy, aphasia or apraxia, inadequate lip closure | n = 30:
| Experimental/Placebo Age yr: 69.2 (4.1)/70.2 (3.6) Male/Female: 5/8/6/7 PAS baseline: 5.1 (0.8)/4.9 (0.6) |
Gao and Zhang [25]
| OD: VFSS evaluation Diagnosis: Chinese diagnosis guidelines for acute ischemic stroke, CT or MRI Inclusion: >60 yrs, positive Neill screening test, first-time cerebral infaction. Exclusion: unstable conditions, previous abnormality in mouth, throat or neck, multiple organ dysfunction syndromes, uncooperative patients, severe mental illness, complete or sensory aphasia | n = 90:
| Control/Shaker/CTAR Age yr: 71.1 (6.4)/71.1 (7.1)/70.9 (6.6) Male: 14/15/13 Therapeutic course (day): 12.2 (1.4)/13.0 (1.4)/ 13.0 (1.6) |
Guillén-Solà, et al. [26]
| OD: Dysphagia confirmed by VFSS score ⩾3 in 8-point PAS Diagnosis: Subacute ischemic stroke Inclusion: Stroke within 1–3 wks. Exclusion: Cognitive impairment and/or history of previous neurological diseases associated with dysphagia | n = 62:
| Control/IEMT/NMES Age yr: 68.9 (7.0)/67.9 (10.6)/70.3 (8.4) Male: 12 (57.1%)/16 (76.2%)/10 (47.6%) Modified Rankin: 3.7 (0.8)/3.9 (0.5)/3.6 (0.8) Barthel Index: 44.0 (18.5)/42.7 (14.6)/41.8 (12.2) Stroke onset (days): 9.3 (5.1)/10.8 (8.7)/11.0 (5.5) FOIS: 4.3 (0.6)/4.5 (0.5)/4.4 (1.0) PAS: 5.4 (2.3)/5 (2.7)/5.5 (2.2) |
Hägglund, et al. [27]
| OD: Swallowing function assessed with timed water swallow test; diagnosed dysfunction when swallowing rate did not exceed 10 mL/s. Diagnosis: NR Inclusion: ≥65 yrs, No cognitive impairment, ≥3 days intermediate care. Exclusion: Patients receiving end of life care, moderate or severe cognitive impairment | n = 116:
| Control/Intervention Age yr: 85/83 Male: 29 (43.3)/27 (55.1) Dysphagia risk condition: 32 (47.8)/25 (52.1) Care moderate dependence: 27 (40.9)/18 (36.7) Swallowing rate (mL/s): 4.10/5.31 |
Hägglund, et al. [28]
| OD: Swallowing dysfunction (pathological TWST test 4-weeks post-stroke) Diagnosis: Stroke Inclusion: First-time stroke and a pathological timed water swallow test. Exclusion: Inability to cooperate; neurological diseases other than stroke, known history of dysphagia prior to the stroke, prominent horizontal overbite (contra-indication due to the oral device’s design), or hypersensitivity to the acrylate | n= 40:
| Control/Intervention Age years: 75 (56–90) yrs/75 (60–85) Male = 14/11; Female: 6/9. Stroke type: Ischemic (16 16); ICH= 3/4; ischemic and ICH = 1/0; left hemisphere = −6/7; right hemisphere = 10/10; supratentorial = 15/16; infratentorial = 3/4; supra-and infratentorial = 1/0. Lowered consciousness at hospital admission: 6/6 |
Hwang, et al. [29]
| OD: OD confirmed by VFSS Diagnosis: Stroke Inclusion: Dysphagia <3 months, swallow voluntarily. Exclusion: trigeminal neuropathy, tongue deviation, facial asymmetry, communication disorders. | n = 25: | Experimental/Control Age (yrs): 60.5 (12.5)/62.2 (10.3) Male: 6/5 Time since stroke, weeks: 8.2 (2.9)/9.1 (2.7) Type of stroke (n) Haemorrhage: 7/6 Type of stroke (n) Infarction: 4/4 |
Jakobsen, et al. [30]
| OD: Clinical signs of dysphagia; score ≥3 on PAS, FEES. Diagnosis: Severe ABI, non-sedated GCS <9, <24 hrs of injury Inclusion: 18–65 yrs Exclusion: formerly acquired or congenital brain damage, psychiatric diagnosis, history of treatment for head and neck cancer, need for a tracheostomy tube, agitated behaviour | n = 10: | Control/Intervention Age yrs: 45.6 (37.5–57.8)/53.8 (41.8–61.4) Male: 4/2 Days from injury: 70.4 (43.0)/76.4 (21.8) GCS at injury (3–15 points): 6.8 (4.4)/6.0 (5.2) |
Jang, et al. [31]
| OD: Swallowing difficulty VFSS-patients who showed velopharyngeal incompetence (VPI) on VFSS were enrolled Diagnosis: Subacute stroke Inclusion: Diagnosis of subacute stroke Exclusion: Previous stroke, pharyngeal structural abnormalities, unable to cooperate | n = 36: | Study/Control Age yrs: 67.3 (9.5)/71.15 (8.6) Male, n: 10/9 Stroke type, n Haemorrhage: 8/6 Days from stroke onset: 20.5 (13.6)/18.4 (12.5) |
Jeon, et al. [32]
| OD: Swallowing dysfunction/dysphagia as determined by VDS and PAS scores on VFSS Diagnosis: Stroke disease Inclusion: MMSE-K score ≥19 points; stroke disease duration ≥6 mths and <2 years Exclusion: Altered neck posture. VitalStim contraindications or cardiopulmonary disease. | n= 34:
| Experimental/Control Age yrs: 63.12 (13.5)/64.47 (8.43) Male: 11/6; 11/6 Side of stroke (left/right): 6/11; 7/10 Haemorrhage/infarction: 14/3/12/5 Weight: 69.11 (11.95); 65.55 (12.66) K-MMSE (point): 24.53 (2.62)/24.2 (2.91) K-NIHSS (point): 10.41 (3.06)/10.76 (3.75) |
Kim, et al. [33]
| OD: Dysphagia defined as a disorder that causes difficulty with chewing and swallowing food Diagnosis: Stroke Inclusion: Diagnosed with dysphagia between May and July 2014; Symptoms of dysphagia for 6 months prior to treatment; 24 points or higher on MMSE- K; fair grade of manual muscle testing of neck flexors. Exclusion: Heart/internal/musculoskeletal disease | n= 26:
| Experimental/Control Age yrs: 63.2 (10.2)/63.6 (8.1) Male: 8/5; 7/8 Side of stroke (right/left): 7/6/7/6 |
Kim and Park [34]
| OD: Dysphagia confirmed by VFSS Diagnosis: Diagnosed as having had stroke within 6 months post-onset Inclusion: Liquid aspiration or penetration on VFSS, nasogastric tube able to communicate, no cognitive deficit Exclusion: Secondary stroke, gastronomy tube, tracheostomy, neck or shoulder pain, cervical herniated nucleus, cervical spine orthosis or brainstem stroke | n = 30:
| Experimental/Control Age yrs: 63.5 (5.5)/65.2 (6.2) Male: 6/6 Type of stroke–haemorrhage: 5/7 Side of stroke (right/left): 5/7/4/9 Facial palsy: 1/1 Dysarthria: 1/0 |
Koyama, et al. [35]
| OD: Stroke related dysphagia, hypopharyngeal residue found by VFSS Diagnosis: Stroke Inclusion: able to perform real or sham exercise Exclusion: Level 1 to 4 on FOIS, pulmonary aspiration with 2 mL of barium water in VFSS, past or present temporomandibular joint disease and/or tumor in head or neck, past or present progressive disease | n = 12:
| Intervention/control Age yrs: 66.0 (9.3)/71.8 (7.6) Male: 5/5 Post-onset weeks, mean (SD): 6.7 (2.1)/9.2 (4.0) FOIS, n, Level 5/Level 6: 3/3/4/2 |
Krajczy, et al. [36]
| OD: Level 1–3 or 5–7 on SRS Diagnosis: Ischaemic stroke- using the National Institutes of Health Stroke Scale Inclusion: Early post-stroke (first stroke) period (<30 days) Exclusion: 2nd or 3rd stroke, level 1–3 dysphagia or level 5–7 dysphagia according to SRS, cognitive function disorders, total aphasia, anarthria, bilateral facial nerve paralysis, tracheostomy | n = 60:
| Study/Control Age yrs: 55–65 (3.3)/55–65 (1.5) Male: 12/14 Paresis, right side: 15/12 |
Kyodo, et al. [37]
| OD: Dysphagia determined by endoscopic swallowing evaluation Diagnosis: Elderly patients with moderate-to-severe dysphagia. Diagnosis: NR Inclusion: Patients hospitalized between May 2017 and Sept 2018 who underwent endoscopic swallowing evaluation Exclusion: Patients ≥65 years old; the presence of an acute infection; patients who developed cerebrovascular disease, myocardial infarction, aspiration pneumonia within 2 weeks | n= 62 (randomized crossover trial):
| Total sample Age years: 83 (9) Male/female: 36/26 Height (cm): 153.4 (6) Weight (kg): 51.8 (5) Concurrent medical conditions:
Mild 0–3: 8 (13%) Moderate 4–7: 35 (56%) Severe 8–9: 19 (31%) |
Logemann, et al. [38]
| OD: Speech pathologist referral after swallow screening, patient aspirating thin liquids. Diagnosis: Physician’s diagnosis of dementia or PD. Bedford Alzheimer Nursing Severity Scale; neurologist rated PD using Hoehn and Yahr scale. Inclusion: 50–95 yrs Exclusion: Inability to perform chin down intervention | n = 742
| Age range: 50–79, 41% Age range: 80–95, 59% Male: 70% PD–No dementia: 32% PD–Dementia: 19% Dementia–Other: 19% Dementia–Single or multistroke: 15% Dementia–Alzheimer’s: 15% |
Manor, et al. [39]
| OD: Referred to speech pathologist for evaluation of swallowing disturbances, confirmed via FEES. Diagnosis: PD had been diagnosed according to the UK Brain Bank criteria Inclusion: Diagnosis as above Exclusion: History of other uncontrolled neurological or medical disorders interfering with swallowing | n = 42:
| Vast/Conventional therapy Age yrs: 67.7 (8.3)/69.9 (9.7) Disease duration (years) 7.4 (4.7)/8.8 (5.7) Disease severity (H&Y-1–5) 2.2 (0.8)/2.2 (0.8) MMSE score (range 0–30) 28.1 (1.6)/27.8 (1.5) Swallowing disturbances questionnaire: 14.7 (5.8)/14.3 (7.2) Fiberoptic endoscopic evaluation of swallowing: 0.7 (0.4)/0.6 (0.4) |
Mepani, et al. [40]
| OD: Post deglutitive dysphagia, pharyngeal phase dysphagia, VFSS to confirm Diagnosis: Stroke or chemoradiation for head and neck cancer Inclusion: Pharyngeal phase dysphagia, incomplete UES opening and postdeglutitive aspiration, hypopharyngeal residue, able to comply with protocol, dysphagia with aspiration of at least 3 month duration Exclusion: History of pharyngeal surgical procedures excluded. | n = 11: | Traditional/Shaker Age years: 70.5 (9.5)/64 (22.8) Males: 5 (83%)/3 (60%) Etiology of dysphagia:
|
Moon, et al. [41]
| OD: Aspiration or penetration, oropharyngeal residue, confirmed VFSS. Diagnosis: Subacute stage 3–12 weeks after the onset of stroke Inclusion: Diagnosis as above, could follow instructions provided, score of > 21 on Mini Mental State Exam, decreased lingual pressures with either anterior or posterior tongue as 40 kPa Exclusion: Nonstroke patients with dysphagia. | n = 16: | TPSAT/Control Age years: 62.0 (4.2)/63.5 (6.1) Male: 3/4 Stroke type (ischemic/hemorrhagic): 6/2/6/2 Poststroke duration days: 56.0 (17.4)/59.9 (20.0) MMSE: 22.87 ± 2.47 23.50 ± 2.00 |
Park, et al. [42]
| OD: Dysphagia confirmed by VFSS Diagnosis: Stroke Inclusion: Onset within 6 months; score ≥24 on the MMSE Exclusion: Stroke prior to that resulting in dysphagia, severe orofacial pain, significant malocclusion or facial asymmetry, unstable breathing or pulse, tracheostomy, severe communication disorder, inadequate lip closure | n = 27:
| Experimental/Placebo Age years: 64.3 (10.7)/65,8 (11.3) Male n: 6/6 Time since onset weeks: 27. 4 (6.3)/26.6 (6.8) |
Park, et al. [43]
| OD: Dysphagia following stroke was confirmed by VFSS Diagnosis: Stroke Inclusion: Onset duration was <12 months, swallow voluntarily, MMSE score ≥20 Exclusion: Secondary stroke, severe communication disorder, pain in the neck region, unstable medical conditions, head and neck cancer | n = 22: | Experimental/Control Age years: 62.2 (17.3)/58.4 (12.5) Male: 6/4 Infarction: 7/6 Time after stroke (weeks): 37.2 (54 3)/14 (14.4) Oral feeding: 4/5 Tube feeding: 7/6 |
Park, et al. [44]
| OD: OD after stroke by VFSS Diagnosis: Stroke based on computed tomography or MRI Inclusion: Inpatient, no significant cognitive problems (MMSE score > 24) Exclusion: Secondary stroke, trigeminal neuropathy, significant malocclusion or facial symmetry, parafunctional oral habits, tongue strength could not be measured, severe communication disorders, neck pain or neck surgery, presence of tracheostomy tube | n = 24: | Experimental/Control Age years: 66.5 (9.5)/64.8 (11.2) Male: 6/5 Stroke lesion middle cerebral artery: 6/6 Time since stroke onset, wks: 24.4 (8.6)/25.7 (6.3) |
Park, et al. [45]
| OD: pharyngeal dysphagia confirmed through VFSS Diagnosis: Diagnosed as having stroke Inclusion: Within 6 months post-onset, nasogastric tube; absence of cognitive deficits. Exclusion: Secondary stroke, presence of other neurological, pain in the disc and cervical spine, cervical spine orthosis, presence of gastronomy tube, problems with the oesophageal phase of dysphagia | n = 37 patients: | Experimental/Control Age years: 60.9 (11.2)/59.5 (9.3) Male n: 13/10 Type of stroke, haemorrhage, n: 12/14 Paretic side, right, n: 11/13 Time since stroke, months: 3.60 (1.19)/3.85 (1.18) |
Park, et al. [46]
| OD: Dysphagia after stroke, by VFSS Diagnosis: Stroke due to hemorrhage or infarction Inclusion: <6 months of onset, liquid aspiration or penetration on VFSS; nasogastric tube; voluntary swallowing; coughing after water swallow test. Exclusion: Secondary stroke, difficulty in using both upper limbs, significant malocclusion or facial asymmetry, pain in the disc and cervical spine, limitations in opening jaw, use of cervical spine orthosis, tracheostomy, severe communication difficulties associated with dementia or aphasia, presence of gastronomy tube, problems with the oesophageal phase of dysphagia | n = 40: | Experimental/Placebo Age years: 62.1 (10.1)/61.8 (12.1) Male: 9/8 Infarction: 7/8 |
Ploumis, et al. [47]
| OD: Dysphagia screening-at least one severe symptom, validated in Greek Ohkuma questionnaire Diagnosis: Hemiparesis following stroke Inclusion: Hemiparesis following stroke, at least one severe symptom of the validated Greek Ohkuma questionnaire Exclusion: Exclusion-Barthel Index >20, Motor Function Hemispheric Stroke Scale <25, history of OD. | n = 70:
| Experimental/Control Age years (all participants): 52 (15) Barthel Index: 22.8 (2.4)/23.4 (2.7) Motor function, Stroke Scale: 22.8 (2.4)/23.4 (2.7) Sagittal C2-C7 Cobb angle: 16.9 (18.5)/14.0 (16.2) Coronal C2-C7 Cobb angle: 6.9 ± 5.3/6.2 ± 5.0 VFSS Score: 1.0 (0)/1.0 (1.0) |
Sayaca, et al. [48]
| OD: ‘Swallowing difficulties’ determined with Turkish version of the eating assessment tool (T-EAT-10) Diagnosis: No neurological problems after neurologist’s examination Inclusion: Over 65 yrs, adequate cognitive status. Exclusion: Head/neck conditions affecting swallowing | n = 50:
| Shaker/PNF Age years: 69 (4.9)/67 (2.1) Male: 10/10 T-EAT-10 scores: 3.5 (1.8)/3.6 (1.3) Peak amplitude (μV): 425.1 (170.7)/417.9 (143.0) Swallow speed (secs): 1.3 (0.3)/1.3 (0.3) Swallow capacity (mL/sec): 1.2 (0.1)/1.2 (0.1) Swallow volume (mL/sec): 1.3 (0.1)/1.3 (0.1) |
Steele, et al. [49]
| OD: Dysphagia post stroke (VFSS) Diagnosis: Recent stroke (4–20 wks) Inclusion: Recent stroke, one repetition maximum posterior maximum isometric tongue-palate pressure measure <40 kPa at intake, stage transition duration if < 350 ms on at least one liquid barium swallow at intake VFSS Exclusion: Severe dysphagia with no functional opening of upper esophageal sphincter; pre-existing dysphagia or diagnoses of head and neck. | n = 14: | TPPT/TPSAT Age years, range: 56–84/49–89 Male: 4/5 Days post onset, range: 28–126/33–150 |
Tang, et al. [50]
| OD: Radiation-induced dysphagia and trismus by non-instrumental clinical assessment Diagnosis: Nasopharyngeal carcinoma (NPC) patients after radiotherapy Inclusion: Diagnosed as above Exclusion: Dysphagia or trismus as initial symptoms of NPC excluded | n = 43:
| Rehabilitation group/Control group Age years (total sample): 49.3 (11) Male (total sample), n: 32 Postradiotherapy, years: 4.6 (1.8)/4.8 (1.6) Interincisor distance (IID), cm: 1.9 (0.7)/1.8 (0.6) |
Tarameshlu, et al. [51]
| OD: Dysphagia based on DYMUS questionnaire (patient self-report) Diagnosis: Established diagnosis of MS according to McDonald’s criteria Inclusion: 20–60 years, lack of acute relapse in past two months, no other conditions such as stroke Exclusion: severe reflux, dysphagia due to drug toxicity, pregnancy | n = 20: | TDT/UC Age years: 47.5 (12.9)/39.9 (9.7) Male: 2/5 Disease Duration (years): 6.8 (2.9)/6.1 (2.7) Expanded Disability Status Scale: 3.6(2.1)/3.2(2.5) MS Type-Relapse-Remitting: 4/7 MS Type-Primary Progressive: 4/1 MS Type-Secondary Progressive: 2/2 |
Troche, et al. [52]
| OD: Swallowing disturbance (screening followed by VFSS) Diagnosis: PD-diagnostic criteria of the UK Brain Bank Inclusion: 55–85 yrs, same PD medication, >24 MMSE. Exclusion: other neurologic disorders; head/neck cancer | n = 60:
| EMST/Sham Age years: 66.7 (8.9)/68.5 (10.3) Male: 25/22 Hoehn & Yahr stage 2.5: 8/13, stage 3: 14/8 Unified Parkinson’s Disease Rating Scale III motor total: 39.4 (9.2)/40.0 (8.5) |
Wakabayashi, et al. [53]
| OD: Dysphagia, Eating Assessment Tool (EAT-10) score ≥3 points Diagnosis: NR (Community-dwelling, ≥65 yrs) Inclusion: Receiving long-term care via day-service or day-care program, mild cognitive impairment/dementia Exclusion: Severe or moderate dementia, inability to perform training | n = 91:
| Intervention/Control Age years: 80 (7)/79 (7) Male: 19/28 Tongue pressure (kPa): 23.3 (8.3)/23.3 (10.0) EAT-10, median (IQR): 7 (5–13)/8 (4–11) Barthel Index: 81 (9)/81 (21) |
Woisard, et al. [54]
| OD: Dysphagia- by Deglutition Handicap Index (DHI) Diagnosis: NR. (Sitting abnormality- by seated postural control measure, SPCM). Inclusion >18 years; DHI score >11, score >0 on 1 item SPCM, chronic dysphagia. Exclusion: NR | n = 56: | D-/D+ Age years (total sample): 61.5 (11.8) Male, n (total sample): 35 Degenerative dysphagia, N (total sample): 24 NIHSS: 1.3 (1.4)/1.3 (1.6) PAS: 1.7 (1.3)/1.9 (1.9) FOIS: 6.0 (0.9)/5.8 (1.1) |
Zhang and Ju [55]
| OD: Swallowing dysfunction (water swallow test upon inclusion) Diagnosis: Stroke Inclusion: Swallowing dysfunction exclusion: NR (admitted patients with dysphagia) | n = 120:
| Control/intervention Age years: 70.6 (7.4)/70.3 (7.4) Males: 33/32 |
| Study | Intervention Goal | Intervention Agent, Delivery and Dosage a | Materials and Procedures a | Outcome Measures | Treatment Outcome a |
|---|---|---|---|---|---|
| Ayres et al. [19] | To verify the effectiveness of a manoeuvre application in swallowing therapy in patients with PD. | Intervention agent: NR Dosage: Experimental group: chin-down manoeuvre and swallowing orientation: 4 sessions per week (30 min each). Orientation group: Swallowing orientation only: 4 sessions per week (30 min each). | Three groups: Experimental group: Chin-down posture manoeuvre (patient instructed to ‘swallow lowering the head until chin touches in the neck’). Patients performed manoeuvre twice a day, swallowing saliva, during meals, throughout the week, at home. Patients were given a form to record the number of times the manoeuvre was performed at home. Patients also given instructions for optimal feeding and swallowing related to ‘swallowing orientations’: (1) environment during feeding (2) posture (3) meal-time (4) oral hygiene. Written instructions given. Orientation group: Patients also given instructions for optimal feeding and swallowing related to ‘swallowing orientations’: (1) environment during feeding (2) posture (3) meal-time (4) oral hygiene. Written instructions given. Control group: No intervention received during 4-week period. Written instructions given. | Primary outcomes: FEES; Clinical evaluation (checking 21 signs and symptoms of oropharyngeal dysphagia and rating these as present or absent); FOIS; SWAL-QOL. | Experimental group showed significant improvement in clinical evaluation of dysphagia compared to two other groups regarding solid (p = < 0.001) and liquid (p = 0.022). Analysis of FEES did not show differences between groups. Experimental group presented with significant improvement in scores of domains frequency of symptoms (p = 0.029) and mental health (p = 0.004) on the SWAL-QOL when compared with the groups that did not receive intervention. |
| Carnaby et al. [20] | Compare standard low-intensity and high-intensity behavioural interventions with usual care (UC) for dysphagia | Intervention agent: Speech pathologist (Low/high intensity); physician and speech pathologist when referred (UC) Dosage (average): Swallowing sessions = 8.1, treatment days = 15.3, duration of session = 21.6 min | UC (control): Physician management. Patient referred to hospital speech pathology if needed. Treatment- feeding supervision, safe swallowing. If prescribed–VFSS. Standard low-intensity: Swallowing techniques, environmental modifications (upright for feeding); safe swallowing advice (eating rate); dietary modification (speech pathologist, 3 times per wk for 1 month. Strategies VFSS. Standard high-intensity: Direct swallowing exercises (effortful swallow, supraglottic swallow technique), dietary modification (from speech pathologist, daily for 1 month. Swallowing exercises established by examination and VFSS. | Primary outcomes: return to pre stroke diet < 6 months Secondary outcomes: time to return to normal diet, proportion recovered, functional swallowing, dysphagia-related complications, died, were institutionalised, or dependent in daily living 6 months post stroke. | Compared with usual care and low-intensity therapy, high-intensity therapy was associated with an increased proportion of patients who returned to a normal diet (p = 0.04) and recovered swallowing (p = 0.02) by 6 months. |
| Carnaby et al. [21] | Effectiveness and safety of exercise based swallowing therapy and neuromuscular electrical stimulation for dysphagia | Intervention agent: NMES & MDTP-Speech pathologists, >5 years dysphagia experience. UC-experienced therapist Dosage: 1 h/day × 3 wks (15 sessions) | McNeill Dysphagia Therapy Program (MDTP): Exercise-based swallowing-criteria for initial oral bolus materials for therapy and advancement on 11-step “food hierarchy”. Simple swallowing. Clinicians monitor each swallow. Neuromuscular Electrical stimulation (NMES): VitalStim®-Active NMES/sham, common single electrode placement-midline above hyoid bone to superior to cricoid cartilage)-ascending amplitude until amplitude reached. Usual care treatment control (UC): Behavioural swallowing treatment strategies common in dysphagia treatment. | Primary outcomes: Ability to swallow (MASA), oral intake (FOIS). Secondary outcomes: Barium swallow outcomes, self-perceived swallowing, weight, time to pre-stroke diet, complications. | Post treatment dysphagia severity significant between groups (p ≤ 0.01). MDTP greater change vs. NMES or UC for increased oral intake (p ≤ 0.02), functional outcomes at 3-mnths (RR = 1.7, 1.0–2.8), earlier time for “return to pre-stroke diet” (p < 03). |
| Choi et al. [22] | Effects of Shaker exercise on aspiration and oral diet | Intervention agent: Caregiver (SE), occupational therapist (CDT) Dosage: 30 min/day, 5 days/wk × 4 wks | Shaker Exercise (SE): Isometric and isokinetic movements. 3 head lifts held for 60 s in supine; 60 s rest. 30 reps head lifts observe toes without raising shoulders-without hold. Conventional Dysphagia Therapy (CDT): Orofacial muscle exercises, thermal tactile stimulation, therapeutic/compensatory manoeuvres. | Primary outcomes: PAS from VFSS. Oral diet level by FOIS. | Experimental group greater improvement on PAS (p < 0.05) and FOIS (p < 0.05) vs. control group. |
| DePippo et al. [23] | Effect of graded intervention on occurrence of dysphagia related complications | Intervention agent: Dysphagia therapist (SLP?) Dosage: Bi-weekly session monitoring for all groups | Group A–Patient-managed diet. One session-therapist recommended diet based on MBS results and compensatory swallowing techniques. Patient chose diet (regular vs. graded). Group B–Therapist-prescribed diet (MBS) and swallowing techniques, evaluated every other week. Group C-Therapist prescribed diet and daily reinforcement of swallowing techniques through mealtime dysphagia group. | Primary outcomes: Dysphagia related complications: Pneumonia, dehydration, calorie-nitrogen deficit, recurrent upper airway obstruction, and death. | No significance between groups for time until end inpatient stay or to 1-year post. Only significance was patients in group B developed pneumonia sooner than group A. |
| Eom et al. [24] | Effect of resistance Expiratory Muscle Strength Training (EMST) on swallowing function | Intervention agent: NR Dosage: 5 days p/wk × 4 wks, 5 sets of 5 breaths on device × 25 p/day. Both groups treatment 30 min × 5 days/wk × 4 wk | Experimental group (EMST + Conventional treatment): Portal Expiratory Muscle Strength Trainer (EMST150). Patients opened mouth after inhalation, EMST mouthpiece between lips. Blew strongly and rapidly until pressure release valve within EMST device opens. Pressure release set to open if pressure target exceeded. < 1-min break after each session, for muscle fatigue and dizziness. Placebo group (Sham EMST + Conventional treatment): Trained using a sham non-functional EMST device with no loading device. Conventional treatment. | Primary outcomes: VDS and PAS based on a VFSS to analyse oropharyngeal swallowing function. | Experimental significant in VDS pharyngeal phase (p = 0.02 and 0.01) and PAS vs. placebo (p = 0.01). Both significant VDS all phases (all p < 0.05). Experimental only significant in PAS (p = 0.01 vs. 0.102). |
| Gao and Zhang [25] | Effects of rehabilitation training on dysphagia and psychological state | Intervention agent: NR Dosage: 3 sessions/actions performed morning, midday and evening. 7 days p/wk × 42 days | All patients received routine treatment including internal medicine, traditional rehabilitation and routine nursing. Control: Traditional tongue and mouth exercises. Each movement repeated 10 times as one session. Shaker exercise: Supine position, single action raised head to look at feet. 30 reps = set of actions. Perform 3 sets of actions-continuously or with 1-min relaxation until complete. (Denoted as ‘Goa & Zhang, 2017a’ in Figure 5.) Chin Tuck Against Resistance (CTAR) exercise: Patients seated tucking chin to compress inflatable rubber ball for 30 reps = set of actions. Perform 3 sets, continuously or with relaxation. (Denoted as ‘Goa & Zhang, 2017b’ in Figure 5.) | Primary outcomes: Dysphagia: VFSS at baseline, 2, 4, 6 wks post. Swallowing function, PAS Psychological state: Self-Rating Depression Scale (SDS) baseline, 6 wks post. | Degrees of dysphagia improvement, between 2–4 wks in CTAR and Shaker. Significantly higher in CTAR (87%) and Shaker (77%) vs. control (43%) (all p < 0.05). Significantly lower SDS in CTAR vs. Shaker/control 6 wks post (all p < 0.05). |
| Guillén-Solà et al. [26] | Effectiveness of inspiratory/expiratory muscle training (IEMT) and neuromuscular electrical stimulation (NMES) | Intervention agent: Occupational, speech, physical therapist Dosage: Control- 3 hrs p/day × 5 days wk × 3 wks. Group 2-2 × p/day, 5 days × 3 wks. Group 3–40-min daily sessions (5 days per wk × 3 wks) | Control/SST: Multidisciplinary inpatient rehabilitation for mobility, activities of daily living, swallowing and communication. Education self-management of dysphagia, oral exercises and compensatory techniques based on VFSS. EMST + SST: Inspiratory/Expiratory Muscle Training (EMST)-respiratory training, 5 sets of 10 respirations, 1 min unloaded recovery breathing, with therapist. Pressure 30% of maximal expiratory pressures increased weekly. NMES + Sham EMST + SST: Sham respiratory muscle training, fixed at 10 cmH2O. Neuromuscular electrical stimulation using VitalStim device. Supervision by speech therapist, electrodes on suprahyoid muscles 80 Hz of transcutaneous electrical stimulus, patients to swallow when felt muscle contraction. | Primary outcomes: Dysphagia severity by PAS. Respiratory muscle strength (maximal inspiratory and expiratory pressures). Post- and 3-month follow-up. | Maximal respiratory pressures most improved Group 2: treatment effect 12.9 (CI 4.5–21.2) and 19.3 (CI 8.5–30.3) for maximal inspiratory and expiratory pressures. Swallowing security improved in Groups 2 and 3. PAS and complications -no between group difference 3-months. |
| Hägglund et al. [27] | Effect of oral neuromuscular training among older people in intermediate care with impaired swallowing | Intervention agent: Dental hygienists and speech pathologist Dosage: NR | Intervention (IQoro® + Usual care): The device IQoro® was used for oral neuromuscular training. The device is designed to stimulate sensory input and strengthen the facial, oral, and pharyngeal muscles. Professionals provided training instructions. If participants had difficulties performing training, staff or family members were instructed on how to assist. Control (Usual care): Usual care with adjustments in food consistencies and posture instructions. | Primary outcomes: Swallowing rate (timed water swallow test) Secondary outcomes: Signs of aspiration during water swallow, swallowing related quality of life (QOL). | Swallowing rate significant improvement, intervention vs. controls post (p = 0.01), 6 months following (p = 0.03). Aspiration significantly reduced in intervention vs. controls (p = 0.01). QoL no between-group differences |
| Hägglund et al. [28] | To determine the effects of neuromuscular training on swallowing function in patients with stroke and dysphagia. | Intervention agent: Discipline NR Dosage: Neuromuscular training = 3 times per session and 3 times daily before eating Orofacial sensory vibration stimulation was performed 3 times daily before meals. 5 weeks of training in total. | Group A-Orofacial sensory-vibration stimulation: Patients received 5 weeks of continued oro-facial sensory vibration stimulation using an Oral B® electric toothbrush. Instructions given on how to stimulate the buccinator mechanism, lips, external floor, tongue. Group B-Orofacial sensory-vibration stimulation + oral neuromuscular training (Muppy®): Patients received oral neuromuscular training for 5 weeks + oro-facial sensory vibration stimulation 1) Oral device (Muppy®) was used for oral neuromuscular training that aims to stimulate sensory input and strengthen facial, oral, pharyngeal muscles. Muppy® is placed pre-dentally behind closed lips and pt sits in upright position. Patients hold device against a gradually increasing horizontal pulling force for 5–10 s whilst trying to resist the force by tightening the lips (2) oro-facial sensory stimulation of buccinator using electric toothbrush. Verbal, practical and written instructions about training given. Patient/caregiver reported training in a log-book. All patients in both groups self-administered or were assisted by relatives or ward staff in oro-facial sensory vibratory stim. | Primary outcome: Changes in swallowing rate measured by the Timed Water Swallow Test (TWST). Secondary outcomes: changes in lip force measured by lip-force test + swallowing dysfunction as measured by VFS (in lateral projection). | Swallowing rate: After intervention, both groups had improved significantly (Group B, p< 0.001; Group A, p = 0.0001) in TWST, but no significant between-group difference in swallowing rate. At 12 month follow-up, Group2 had improved significantly in swallowing rate compared to Group A (p = < 0.032) Lip force: Significant improvement in lip force in Group 2 (p < 0.001) compared to non-significant improvement in Group 1 (p = 0.079). Improvement in Group 2 maintained at 12 month follow up. |
| Hwang et al. [29] | Effect of tongue stretching exercises (TSE) on tongue motility and oromotor function in patients with dysphagia after stroke. | Intervention agent: TDT/TSE by occupational therapists. Dosage: TSE–5 × p/wk × 4 wks. Stretching 20 × p/ day. | Control group: Traditional Dysphagia Treatment (TDT)- oral facial massage, thermal-tactile stimulation, compensatory skill straining. Both groups received TDT. Experimental group: +Tongue Stretching Exercise (TSE); dynamic/static stretching exercises (20 reps each). Dynamic-therapist pulled patient’s tongue to end feel point of ROM and held for 2–3 s before guiding back to mouth. Static-therapist pulled tongue to end feel point, held 20 s. | Primary outcomes: Oromotor function-Oral phase events of VDS, VFSS Tongue motility-Distance from lower lip to tip of tongue during maximum protrusion of the tongue. | Experimental significant differences in tongue motility, bolus formation, tongue to palate, bolus loss, oral transit time-oral VDS phase (p < 0.05 for all). Control significant for lip closure only (p < 0.05). |
| Jakobsen et al. [30] | Effect of the intensification of the nonverbal facilitation of swallowing on dysphagia. | Intervention agent: Occupational therapist Dosage: 30 sessions (10-min rest, 20-min session, 10-min rest), 3 wks (2 × day). | Experimental treatment: Facial Oral Tract Therapy (F.O.T.T.) concept-rehabilitation intervention using structured tactile input and nonverbal facilitation techniques (to allow for effective function in meaningful daily life activities). Control group: Treatment comprised stimulating activities in the facial oral tract similar to those of the intervention group but without facilitation of swallowing or verbal request to swallow. | Primary outcomes: FOIS, PAS, and electrophysiological swallowing specific parameters (EMBI). | Intervention feasible. PAS and FOIS improved in both groups, no group differences. Swallowing specific parameters reflected clinically observed changes. |
| Jang et al. [31] | Effects of Mechanical Inspiration and Expiration (MIE) exercise using mechanical cough assist on velopharyngeal incompetence | Intervention agent: NR Dosage: 20 sessions Both groups, 30 min 2 × day, 5 × wk × 2 wks. | Study group MIE exercise: CNS-100 Cough Assist® and conventional swallowing rehabilitation. Inspiration- positive pressure 15–20 cm H2O, increased to 40 cm H2O for 2 s. Expiration–similar pressure 10–20 cm H2O above positive pressure; held 3–6 s, simulating airflow during cough. Patient coordinated respiratory rhythm to cough assist machine. Control: Conventional dysphagia rehabilitation of oral motor and sensory stimulation, NMES, oral exercises for safe swallow. | Primary outcomes: Swallowing function American Speech-Language-Hearing association scale, functional dysphagia score, and PAS, VFSS. Coughing function-peak cough flow. | Study group significant improvement in functional dysphagia score- nasal penetration degree. Nasal penetration degree and peak cough flow showed greater improvement in study vs. control group. |
| Jeon et al. [32] | To investigate the effects of NMES plus upper spine cervical mobilisation on forward head posture, and swallowing in stroke patients with dysphagia. | Intervention agent: Joint mobilisation was performed by a physical therapist (with over 160 h of manual therapy education. NMES was delivered by 3 experienced OTs. Dosage: once a day, 3 × times a week, for 4 weeks; both groups received NMES for 30 min; experimental group received 10 min of upper cervical spine mobilisation; control group received 10 min of sham mobilisation. | All interventions were performed in sitting position. NMES: Intervention group received upper cervical spine (C1–2) mobilisation with NMES. Mobilisation: Therapist used one hand to hold the subject’s C1(atlas); other hand placed on subject’s occiput. Mobilisation force could not be standardised. NMES was applied to the suprahyoid using VitalStim®. Electrodes attached to the motor point of the suprahyoid muscles (digastric) to induce anterior excursion and vertical elevation movements of hyoid bone during normal swallowing. Stimulation was applied by gradually increasing the intensity to the level that patients felt a grabbing sensation in the neck without pain or laryngospasm. Control group: Patients received upper cervical spine sham mobilisation combined with NMES. | Primary outcome: Forward head posture measured by CCFT (Stabilizer TM Pressure Biofeedback) and craniovertebral angle (CVA). Swallowing function measured by VFS and PAS. | The intervention group showed significantly better scores in CCFT (p = 0.05) and in CVA (p = 0.05) than in control group. PAS scores were significantly better in the intervention group compared to control group (p = <0.05). Significant increase in VFS total score and PAS than in the control group (p = <0.05) |
| Kim et al. [33] | The effects of Proprioceptive Neuromuscular Facilitation (PNF) on swallowing function of stroke pts with dysphagia | Intervention agent: NR Dosage: PNF-based short neck exercises 3 times a week for 30 min each time for 6 weeks | Experimental group: PNF
1. Isometric exercises: Patients lay on bed and raised their heads without moving shoulders off the bed, looked at ends of their feet for 60 s, and then lowered heads back on the bed. If patient had difficulty raising his/her head, they were asked to perform same exercise for 3 times for as long as they could. Isotonic exercises: Patients raised their head in same posture and looked at the ends of their feet 30 consecutive times. | Primary outcome: New VFSS and ASHA NOMS Scales. | Statistically significant improvements in: premature bolus loss, residue in the valleculae, laryngeal evaluation, epiglottic closure, residue in pyriform sinuses, coating of pharyngeal wall after swallowing, pharyngeal transit time and aspiration on both new VFSS scale and ASHA NOMS scale (p < 0.05). Control group also demonstrated statistically significant improvements in premature bolus loss, residue in the valleculae, laryngeal evaluation, epiglottic closure, residue in pyriform sinuses, pharyngeal transit time and aspiration (p < 0.05). No statistically significant differences between the groups were found in new VFSS scale and ASHA NOMS scale. |
| Kim and Park [34] | Effect of modified chin tuck against resistance (mCTAR) exercise on patients with post-stroke dysphagia. | Intervention agent: Occupational therapist Dosage: 30 min × 5 days a week, for 6 weeks | Experimental group mCTAR exercise: PhagiaFLEX-HF device. Subject seated, fixed part of device to desk, firmly attach chin surface under chin. Exercise performed in isotonic/isometric. Isometric- holding chin down for 10 s against resistance (10 s, 3 times). Isotonic-30 × reps chin-down against resistance. Traditional dysphagia treatment (TDT): Oral facial massage, thermal-tactile stimulation and compensatory training. | Primary outcomes: Aspiration and oral diet -PAS and FOIS. Secondary outcomes: Rate of nasogastric tube removal was analysed. | Experimental statistically significant improvement in PAS and FOIS vs. control (p < 0.001). Rates of nasogastric tube removal were 25% (experimental) vs. 15% (control). |
| Koyama et al. [35] | Feasibility and effectiveness newly developed Modified Jaw Opening Exercise (MJOE) in poststroke patients with pharyngeal residue. | Intervention agent: Speech pathologist/physician Dosage: 4 × sets daily, 5 × p/wk × 6 wks. (6 s × 5 reps = 1 set) | Intervention MJOE: Surface electrodes mandibular midline. Participants closed mouth, sitting position, pressed tongue against hard palate. Trainer hand under participant’s chin and applied upward vertical resistance. Visual feedback given. Maintained 80% Maximum Voluntary Contraction (MVC). Control sham exercise isometric jaw closing exercise: Surface electrodes to masseter, visual feedback, 20% MVC. | Primary outcomes: VFSS was performed before and after exercise. The distance between the mental spine and the hyoid bone (DMH) and hyoid displacement (HD) were measured. | No temporomandibular joint or neck pain. Intervention group, DMH decrease where anterior HD ended and an increase in anterior HD were seen. Control, no changes. |
| Krajczy et al. [36] | Effects of dysphagia therapy in patients in the early post-stroke period. | Intervention agent: Physiotherapist Dosage: Physiotherapy program average 60 min × day, × 15 days | Control/both groups: Safe food education and neurological physiotherapy depending on patient dysfunction. Therapy included passive, assisted, supported and respiration exercises, erect posture, walking re-education, and training on NDT Bobath and PNF methods. Study group: +original dysphagia treatment, restoring chewing and swallowing functionality–Strengthening and breathing exercises and thermal stimulation. | Primary outcomes: Swallowing function - Timed test of swallowing Swallowing reflux – Controlled swallowing after swallowing blended food. Reflex categorised as good or delayed. | Swallowing reflux, Cough and voice quality and swallowing time, number of swallows and SpO2 All Statistically significant differences between groups after therapy (p = <0.01). |
| Kyodo et al. [37] | To evaluate the effectiveness of puree diets containing a gelling agent for the prevention of aspiration pneumonia in elderly patients with moderate to severe dysphagia. | Intervention agent: Gastroenterologists experienced in transnasal endoscopy along with a speech therapist evaluated swallowing. Discipline who created gelling agent (intervention) NR. Dosage (average): NR | Patients underwent endoscopic swallowing evaluation while sitting in a chair/sitting up in bed. Images of oropharynx and larynx were displayed on a monitor and recorded on digital video recorder. Pureed diet without gelling agent was made by mixing 100 g of white rice and 50 mL of water with a blender for one minute. Texture characteristics (IDDSI Level 4) were: hardness, 1760 ± 125 N/m2; cohesiveness, 0.59 ± 0.03; adhesiveness, 224 ± 56 J/m3. Pureed diet with gelling agent was made by mixing 100 g of rice porridge at > 70 degrees with 0.5 g of the gelling agent with a blender for one minute. Texture characteristics (IDDSI Level 4) were: hardness, 312 ± 11.3 N/m2; cohesiveness, 0.81 ± 0.02; adhesiveness, 108 ± 5.8 J/m3. | Primary outcome: Presence of material in throat using endoscopic cyclic ingestion score (0 to 4) Secondary outcomes: Sense of material remaining in the throat after swallowing of pureed rice and/or test jelly; degree of dysphagia using Hyodo-Komagane score (0 to 12: mild 0–3; moderate 4–7; severe 8–9) | Residuals in throat were significantly less likely with pureed rice with than without the gelling agent (median cyclic ingestion score (range); 1 (0–4) vs. 2 (0–4); p = 0.001. Irrespective of presence or absence of the gelling agent, the sense of materials in the throat was significantly less frequent in older patients (p = <0.01). No adverse events occurred. |
| Logemann et al. [38] | 3 treatments for aspiration on thin liquids—chin-down posture, nectar-thickened liquids, or honey-thickened Liquids. | Intervention agent: Speech pathologist Dosage: NR | Chin-down intervention: chin to the front of the neck, three swallows of 3 mL of thin liquid from a spoon and three swallows of the same liquid from an 8-oz cup filled with 6 oz of liquid. Nectar or Honey-thickened liquids: on the two thickened liquid interventions, three swallows of 3 mL of thickened liquid from a spoon and three self-regulated swallows, performed as separate swallows, each from an 8-oz cup filled with 6 oz of the thickened liquid. | Primary outcomes: Swallowing function-VFSS | 49% aspirated all interventions, 25% not any. More on thin liquids despite chin-down posturing vs. using nectar-(p < 0.01) or honey-thickened (p < 0.01). More on nectar- vs. honey thickened (p < 0.01). |
| Manor et al. [39] | Effectiveness of visual information while treating swallowing disturbances in patients with PD. | Intervention agent: Speech and swallowing therapist Dosage: Each group 5 × 30 min sessions, during 2-wk period and a 6th session 4 wks after the 5th one | Control–conventional therapy: Both interventions swallowing exercises and compensatory therapy based on FEES. Compensatory strategies carried out with different food and liquid consistencies in clinic, patient practiced at home. VAST: video-assisted tool during each session, for educating and assisting understanding structure of swallowing. Patients observed a normal swallowing process and their distorted one. After learning compensatory technique, patient practiced it during drinking and eating in the clinic after observing video then at home. During next four sessions patients observed video with suitable compensatory swallowing technique while eating and drinking focusing on the new swallowing behaviour. | Primary outcomes: Swallowing function-by fiberoptic endoscopic evaluation of swallowing (FEES). Quality of life-quality of care and degree of pleasure from eating assessed by questioners | Significant improvement in swallowing functions both groups. FEES significantly greater reduction in food residues in pharynx in VAST vs. conventional treatment group. SWAL-QOL scores significant between groups favour of VAST: burden, eating desire, social functioning, mental health, symptom frequency (p < 0.01). |
| Mepani et al. [40] | Effect of the Shaker exercise on thyrohyoid muscle Shortening improve pharyngeal dysphagia | Intervention agent: Speech pathologist Dosage: Biweekly 45-min therapy sessions for 6 weeks. | Traditional therapy: 5 times daily. Laryngeal and tongue ROM exercises and swallowing manoeuvres (Super-Supraglottic Swallow, Mendelsohn Manoeuvre, Effortful Swallow). Shaker Exercise: 3 times per day for 6 weeks. Isometric and isokinetic head-lift in supine position. Patients raised head high and forward to observe toes. Isometric–3 times head lifts held 60 s, 60-s rest period. Isokinetic-30 head lifts at constant velocity, performed without holding or rest periods. | Primary outcomes: Change in thyrohyoid muscle shortening by Videofluoroscopy | After therapy, the percent change in thyrohyoid distance in the Shaker Exercise group was significantly greater vs. traditional therapy (p = 0.034). |
| Moon et al. [41] | Effects of Tongue pressure strength and accuracy training (TPSAT) on tongue pressure strength, swallowing function, and quality of life in stroke patients with dysphagia. | Intervention agent: Occupational therapist Dosage: TPSAT and traditional dysphagia therapy 30 min × day; Only traditional therapy performed 30 min × twice daily. Both groups, daily 5× times wk × 8 wks. | Both groups received standardized physical/occupational therapies. Traditional dysphagia therapy: thermal tactile stimulation, Mendelsohn manoeuvre, effortful swallow, diet modification. TPSAT with traditional dysphagia treatment: TPSAT consisted of an anterior and posterior isometric tongue strength exercise and an isometric tongue accuracy exercise. The protocol involved five sets of tongue-to-palate presses, 6 reps per set for each session. Isometric tongue accuracy exercise, amplitudes were set at 50, 75, 100% of maximum pressure from first isometric strength. Participants generated precise pressures within 10 kPa error for each amplitude. | Primary outcomes: Tongue pressure strength -maximum isometric tongue pressures (MIPs) of anterior, posterior tongue using Iowa Oral Performance Instrument. Swallowing function-MASA; QoL-SWAL-QOL | TPSAT with traditional dysphagia significantly improved MASA, SWAL-QOL, and MIPs. Traditional dysphagia significantly increased MASA, SWAL-QOL, and MIPs anteriorly (p < 0.05). TPSAT significant in anterior, posterior MIPs, tongue movement MASA, vs. controls (p < 0.05). |
| Park et al. [42] | Effects of EMST on the activity of suprahyoid muscles, aspiration and dietary stages in stroke patients with dysphagia. | Intervention agent: Occupational therapist Dosage: 5 days × wk × 4 wks. 5 sets × 5 breaths on device, 25 breaths per day. | Experimental group: resistance set at 70% range of MEP (Maximal Expiratory Pressure). Subjects open mouth following maximum inhalation, EMST mouthpiece between lips, close mouth. Blow strong and fast until pressure release valve in EMST device opens- expiratory pressure exceeded set target. Placebo group: training using sham device-non-functional device, little effect of physiologic load on targeted muscles. | Primary outcomes: Activity in the suprahyoid muscle group -using surface electromyography (sEMG). PAS used to assess VFSS results. Dietary stages-FOIS. | Experimental significantly more in suprahyoid muscle activity (p = 0.01), liquid PAS (p = 0.03) and FOIS (p = 0.06), but not semisolid type PAS (p = 0.32), vs. placebo. |
| Park et al. [43] | Effect of chin tuck against resistance exercise (CTAR) on the swallowing function in patients with dysphagia following subacute stroke. | Intervention agent: Occupational therapist Dosage: 30 min × day, × 5/wk, × 4 wks | CTAR: Isometric CTAR, patients chin tuck against device 3 × 60 s no repetition. Isotonic CTAR, patient 30 reps by strongly pressing against resistance of the device and releasing it. Therapist demonstrated exercise methods. Conventional dysphagia treatment: Both groups -orofacial muscle exercises, thermal tactile stimulation, and therapeutic or compensatory manoeuvres. | Primary outcomes: Swallowing function -Functional Dysphagia Scale (FDS) and PAS, based on VFSS | Experimental more improvement in oral cavity, laryngeal elevation/epiglottic closure, residue in valleculae, and residue in pyriform sinuses of FDS and PAS compared vs. controls (p < 0.05, all). |
| Park et al. [44] | Effects of Effortful Swallowing Training (EST) on tongue strength and swallowing function in patients with stroke. | Intervention agent: Occupational therapist Dosage: Training 30 min, 5× days per wk × 4 wks. Both groups conventional dysphagia treatment 30 min/day, 5 days/wk × 4 wks. | Experimental EST: Patients pushed tongue onto palate, squeezing neck muscles, swallow forcefully. Performed 10 times p/session, 3 sessions p/day. Effortful swallowing confirmed by therapist through visual observation and palpation. Control group: Swallow naturally without intentional force. Patients given small spray of water to induce swallowing, and rest. Both groups received conventional dysphagia therapy (compensatory techniques -chin tuck, head tilting, rotation; therapeutic techniques -orofacial muscle exercises, thermal tactile stimulation using ice sticks, expiratory training). | Primary outcomes: Tongue strength-Iowa Oral Performance Instrument. Oropharyngeal swallowing function VDS, based on VFSS. | Experimental group greater improvements in anterior and posterior tongue strength vs. control (p = 0.05 and 0.04), and greater improvement in oral phases of VDS (p = 0.02). |
| Park et al. [45] | Effects of game-based Chin Tuck against resistance exercise (gbCTAR) and head-lift exercise on swallowing function and compliance in dysphagia post-stroke | Intervention agent: Occupational therapist Dosage: 5 × wk × 4 weeks. Traditional dysphagia treatment (TDT) 30 min per day | Experimental group: performed gbCTAR exercise LES 100 device. Before gbCTAR exercise, 1-RM measured for resistance values. 1-RM, resistance bar placed directly beneath jaw, and chin tuck directed against resistance. gbCTAR exercise at threshold of 70% 1-RM, divided into isometric and isotonic exercises, combined with the game. Control group: head lift exercises in supine (isometric and isotonic). Both groups TDT- oral facial massage, thermal-tactile stimulation and compensatory training. | Primary outcomes: Swallowing function-VDS and PAS. Dietary assessment-FOIS Compliance with the 2 exercises-(motivation, interest, physical effort, fatigue), numerical rating self-report scale. | No significant between group difference in VDS, PAS, FOIS. Compliance, motivation and interest Scores significantly higher, and scores for physical effort needed and fatigue significantly lower, in experimental vs. control. |
| Park et al. [46] | Effect of Resistive Jaw Opening Exercise (RJOE) on hyoid bone movement, aspiration, and oral intake level in stroke patients. | Intervention agent: Occupational therapist Dosage: 30 min × 5 times wk × 4 wks. | Experimental group: RJOE device to provide resistance to suprahyoid muscles. Isometric exercise, 30 s with device resistors pressed downward (3 times, 30–60 s of rest). Isotonic exercise repeatedly depressed by RJOE by holding device resistance down for 2–3 s then returned to original state (10 reps, 3 sets) with 30 s rest. Placebo group: RJOE using 1-mm thick device with almost no resistance to suprahyoid muscles. Exercise type and frequency of RJOE same as experimental group. Both groups received conventional dysphagia therapy after intervention, which involved orofacial muscle exercises, thermal tactile stimulation and therapeutic or compensatory manoeuvres. | Primary outcomes: Hyoid bone movement -by two-dimensional analysis of anterior and superior motion on VFSS. Aspiration-PAS Oral intake level-FOIS. | Both groups significant differences in hyoid movement, PAS, FOIS (p< 0.05). No significant difference between groups except for liquid type, PAS. Effect sizes (Cohen’s d) 0.6–1.1 for anterior, superior movement of hyoid bone, semisolid and liquid type of PAS, and FOIS respectively. |
| Ploumis et al. [47] | Evaluate cervical isometric exercises in dysphagic patients with cervical spine alignment disorders due to hemiparesis after stroke. | Intervention agent: Allied health Dosage: inpatient 12 wks, speech 30 min daily. Experimental-4× reps 10 min, 3× day, 12 wks. | All patients -inpatient program including physiotherapy, occupational and speech therapy. Speech included deglutition muscle strengthening, compensatory techniques. Experimental group: +plus cervical isometric strengthening exercises contract neck muscles under resistance forward-backward-sidewards). Control group: Regular speech therapy plus sitting balance. | Primary outcomes: Cervical spine radiographs in erect (sitting/standing) position coronal, sagittal C2-C7 Cobb angle, VFSS to evaluate deglutition. | Experimental group- more pronounced correction (p < 0.01) of cervical alignment in both planes and greater improvement (p < 0.05) of deglutition too, than control group. |
| Sayaca et al. [48] | Whether combined isotonic technique of Proprioceptive Neuromuscular Facilitation (PNF) is superior to Shaker exercises in improving function of swallowing muscles. | Intervention agent: Shaker ‘CS’ (?). PNF physiotherapist Dosage: Each exercise set 1 x per day, 3x wk x 6 wks. | Shaker exercises: isometric (3 reps) and isotonic contractions (30 reps) neck flexor muscles. Patients raised head to observe toes without raising shoulders. Isometric- lifted head, held for 1-min 3 times, 1-min rest. Isotonic- lifted head 30 reps, no holding. PNF: Combined isotonic technique- concentric, stabilizing and eccentric contraction without relaxation. Stabilizing contractions to improve control, force, coordination, and eccentric contraction. Moved head against resistance with open mouth- kept position for 6 s against resistance in seated position; kept position while physiotherapist moved back to initial position. 30 reps per day. | Primary outcomes: Swallowing difficulties Turkish Eating Assessment Tool (T-EAT-10). Capacity, volume, and speed of swallowing-100 mL-water swallow test. Contraction amplitude changes-motor unit activity, by superficial electromyography. | T-EAT-10 decreased both groups (p < 0.001). Water swallowing capacity and volume improved both groups (p < 0.001). No change in swallowing speed both groups (p > 0.05). Maximal voluntary contraction of suprahyoid muscles higher in PNF vs. Shaker (p < 0.05). |
| Steele et al. [49] | Compare outcomes of two tongue resistance training protocols | Intervention agent: Speech pathologist Dosage: 24 sessions (TPPT or TPSAT), 2–3× wk, 8–12 wks. 60 tongue-pressure tasks per session. | Tongue-pressure profile training (TPPT): emphasized pressure-timing patterns that are typically seen in healthy swallows by focusing on gradual pressure release and saliva swallowing tasks. Tongue- pressure strength and accuracy training (TPSAT): emphasized strength and accuracy in tongue-palate pressure generation and did not include swallowing tasks. | Primary outcomes: Posterior tongue strength, oral bolus control, penetration– aspiration and vallecular residue- VFS, PAS | Both groups significant tongue strength and post-swallow vallecular residue with thin liquids. Stage transition duration (bolus control), PAS no significant differences. |
| Tang et al. [50] | Effect of rehabilitation therapy on radiation-induced dysphagia and trismus in nasopharyngeal carcinoma (NPC) patients after radiotherapy. | Intervention agent: Therapists, assistants Dosage: Rehabilitation group, exercises 3× per day, each 15 cycles, 45 cycles per day. | Both groups routine treatment. Rehabilitation group: training by therapists at hospital, continued at home post-discharge by exercise booklet, guardian oversight and calendar Exercises: Tongue-range of motion exercises included passive and active movement exercises. Pharynx and Larynx-exercises changing body position to maximize swallow function and minimize aspiration. Swallow manoeuvres included effortful swallow and Mendelsohn manoeuvre. Sensory procedures utilizing pharyngeal cold stimulation performed by therapists. Exercise for Trismus- Active jaw movements- opening/closing mouth repeatedly, opening mouth slightly, moving lower mandible to left and right, stretched chin downward and forward and a range of passive jaw movements. Control group: No rehabilitation exercises Both groups received routine treatment (e.g., anti inflammatory treatment for aspiration pneumonia) | Primary outcomes: Severity of dysphagia- water swallow test Trismus-LENT/SOMA score and the interincisor distance (IID). | Rehabilitation group only significant improvement in swallowing function. Percentage of patients with effective results in rehabilitation higher than control (p = 0.02). Control IID significantly decreased at Post (p = 0.001), both groups decreased at 3 months, rehabilitation group less than controls (p = 0.004). Trismus in rehabilitation higher vs. control (p = 0.02). |
| Tarameshlu et al. [51] | Effects of Traditional Dysphagia Therapy (TDT) on swallowing function in Multiple Sclerosis (MS) patients with dysphagia. | Intervention agent: Therapist Dosage: both groups 6 weeks, 18 sessions, 3 × per week, every other day. | Traditional Dysphagia Therapy (TDT): Includes oral motor control, range of motion exercises, swallowing manoeuvres, strategies to heighten sensory input. Usual care (UC): postural changes, modifying volume and speed of food presentation, changing food consistency and viscosity, and improving sensory oral awareness. | Primary outcomes: Swallowing ability- Mann Assessment of Swallowing Ability (MASA) Secondary outcomes: PAS and PRRS. | Groups improved MASA, PAS and PRRS (p < 0.001). All significantly greater in TDT vs. UC group. Large effect size MASA in TDT (d = 3.9) and UC (d = 1.1). |
| Troche et al. [52] | Treatment outcome of device-driven EMST on swallow safety, physiologic mechanisms through measures of swallow timing and hyoid displacement. | Intervention agent: Clinician Dosage: EMST, 4 weeks, 5 days per week, for 20 min per day, using a calibrated or sham, handheld device. | Expiratory muscle strength training (EMST): device set to 75% of participant’s average MEP. Visited weekly by clinician-instructed to wear nose clips, deep breath, hold cheeks lightly, blow hard into device, identify air was flowing freely through device (once reached threshold pressure). Sham: Sham device identical to EMST device, pressure release valve non-functional and to 75% of participants’ average MEP-no physiologic load to muscles. | Primary outcomes: Swallow function-judgments of swallow safety, PAS scores, swallow timing, and hyoid movement from VFS images. | EMST improved swallow safety, PA scores vs. sham. EMST improvement of hyolaryngeal function during swallowing, findings not evident for sham group. |
| Wakabayashi et al. [53] | Effects of resistance training of swallowing muscles in community dwelling older individuals with dysphagia. | Intervention agent: Research co-workers Dosage: intervention exercises for 10 s; 1 set = 10 reps. 2 sets per day 3× per wk × 3 months | Control/both groups: dysphagia brochure (about oral hygiene, tongue resistance exercise, head flexion exercise against manual resistance, nutrition, and food modifications). Intervention: resistance exercises for swallowing muscles involving tongue resistance exercise and head flexion against manual resistance. Research co-workers instructed participants once how to perform resistance training. | Primary outcomes: Improvement in dysphagia -Eating Assessment Tool (EAT-10) score. Secondary outcomes: Tongue pressure | Percentage of participants with EAT-10 scores <3 not statistically significantly different between groups p = 0.6). Post intervention EAT-10 (p = 0.7) and mean tongue pressure (p = 0.4). |
| Woisard et al. [54] | Effect of a personalised transportable folding device for seating on dysphagia | Intervention agent: Occupational therapy Dosage: 1 x training session with device (D+ group) and without device (D- group). | D-/All groups: All patients training session: evaluation of needs, impact of head positioning on swallowing, adapted position of head through body positioning, practice using occupational therapy cushions or personalised transportable folding device for seating (DATP) according to randomisation. D+ group: in charge to determine characteristics of the device required so they could have them during the training session. Instruction for patients was to put the personalised instructions into practice by using the device. | Primary outcomes:quality of swallowing Secondary outcomes:posture, device acceptability, QoL. Measurement of hyoid bone movement during swallowing. VFSE and questionnaire. | Significantly better posture both groups (p < 0.001), more hyoid bone motion in D+ group. Significant mean difference for D+ group vs. D− group, for horizontal and vertical movement. Other swallowing markers not significant. |
| Zhang and Ju [55] | Clinical improvement of nursing intervention in swallowing dysfunction of elderly stroke patients. | Intervention agent: Nursing staff Dosage: NR | Control group: conventional nursing service that strictly conforms to the doctor’s advice. Nursing intervention: (1) Psychological intervention, nurses communication with patients/family, evaluates psychological state, encourages and comforts. (2) Health education, nurse introduces knowledge about swallowing dysfunction and effects through videos and images. (3) Rehabilitation exercises, pronunciation training, muscle training, mouth opening exercises, ingestion training. (4) Diet intervention, appropriate foods should be chosen according to specific conditions. | Primary outcomes: Swallowing dysfunction–30 mL water drink test Living quality-assessment questionnaire of living quality (GQOL-74), includes physical, psychological and social functions, and material life. Pulmonary infection–rate Nursing satisfaction–self-made questionnaire. | Improved swallowing dysfunction higher in intervention vs. control (p < 0.05). Scores of physical, psychological and social functions, and material life and nursing satisfaction higher in intervention vs. control (p < 0.05). Pulmonary infection lower in intervention vs. control p < 0.05). |
| Subgroup | Hedge’s g | Lower Limit CI | Upper Limit CI | Z-Value | p-Value |
|---|---|---|---|---|---|
| Intervention type | |||||
| Combined vs. CDT (Combined) (n = 5) | 0.610 | 0.263 | 0.957 | 3.446 | 0.001 * |
| Combined vs. CDT (Compensation) (n = 3) | 1.180 | 0.362 | 1.998 | 2.828 | 0.005 * |
| Rehabilitation vs. CDT (Combined) (n = 1) | 0.019 | −0.656 | 0.659 | 0.057 | 0.955 |
| Rehabilitation vs. CDT (Rehabilitation) (n = 3) | 0.178 | 0.304 | 1.133 | 3.395 | 0.001 * |
| Rehabilitation vs. No CDT (n = 3) | 0.842 | 0.440 | 1.244 | 4.110 | <0.001 * |
| Selected interventions | |||||
| Shaker vs. CDT (n = 2) | 1.038 | 0.300 | 1.776 | 2.756 | 0.006 * |
| CTAR vs. CDT (n = 3) | 1.045 | 0.427 | 1.663 | 3.316 | 0.001 * |
| EMST vs. no CDT (n = 2) | 0.819 | 0.389 | 1.250 | 3.733 | <0.001 * |
| Diagnostic groups | |||||
| Acquired Brain Injury (n = 1) | 0.947 | −0.247 | 2.141 | 1.554 | 0.120 |
| Parkinson’s disease (n = 1) | 0.792 | 0.273 | 1.311 | 2.898 | 0.003 * |
| Stroke (n = 13) | 0.731 | 0.474 | 0.988 | 5.573 | <0.001 * |
| Outcome measures | |||||
| Superior hyoid displacement (n = 1) | 0.994 | −0.124 | 2.112 | 1.743 | 0.081 |
| MASA (n = 2) | 0.512 | −0.574 | 1.599 | 0.925 | 0.355 |
| PAS (n = 11) | 0.804 | 0.572 | 1.036 | 6.789 | <0.001 * |
| Tongue motility oromotor function (n = 1) | 0.359 | −0.470 | 1.189 | 0.849 | 0.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speyer, R.; Cordier, R.; Sutt, A.-L.; Remijn, L.; Heijnen, B.J.; Balaguer, M.; Pommée, T.; McInerney, M.; Bergström, L. Behavioural Interventions in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Clin. Med. 2022, 11, 685. https://doi.org/10.3390/jcm11030685
Speyer R, Cordier R, Sutt A-L, Remijn L, Heijnen BJ, Balaguer M, Pommée T, McInerney M, Bergström L. Behavioural Interventions in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Journal of Clinical Medicine. 2022; 11(3):685. https://doi.org/10.3390/jcm11030685
Chicago/Turabian StyleSpeyer, Renée, Reinie Cordier, Anna-Liisa Sutt, Lianne Remijn, Bas Joris Heijnen, Mathieu Balaguer, Timothy Pommée, Michelle McInerney, and Liza Bergström. 2022. "Behavioural Interventions in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analysis of Randomised Clinical Trials" Journal of Clinical Medicine 11, no. 3: 685. https://doi.org/10.3390/jcm11030685
APA StyleSpeyer, R., Cordier, R., Sutt, A.-L., Remijn, L., Heijnen, B. J., Balaguer, M., Pommée, T., McInerney, M., & Bergström, L. (2022). Behavioural Interventions in People with Oropharyngeal Dysphagia: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. Journal of Clinical Medicine, 11(3), 685. https://doi.org/10.3390/jcm11030685









