Clinical Significance of Multiparameter Intracranial Pressure Monitoring in the Prognosis Prediction of Hypertensive Intracerebral Hemorrhage
Abstract
:1. Introduction
2. Data and Methods
2.1. Patient Information and Grouping
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
3.1. Comparison of Baseline Information between the Two Groups
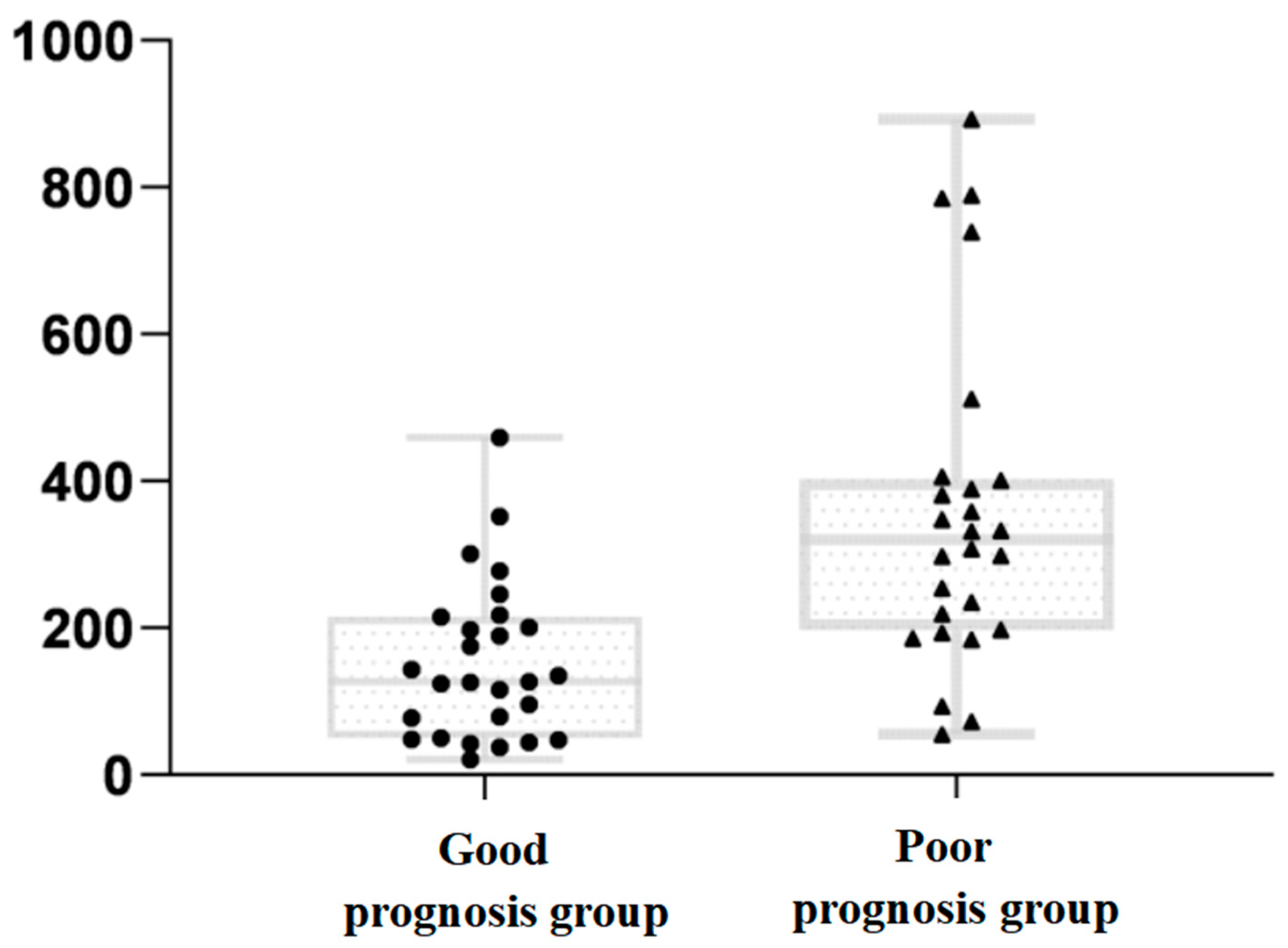
3.2. Comparison of DICP20 mmHg × h Data between the Two Groups
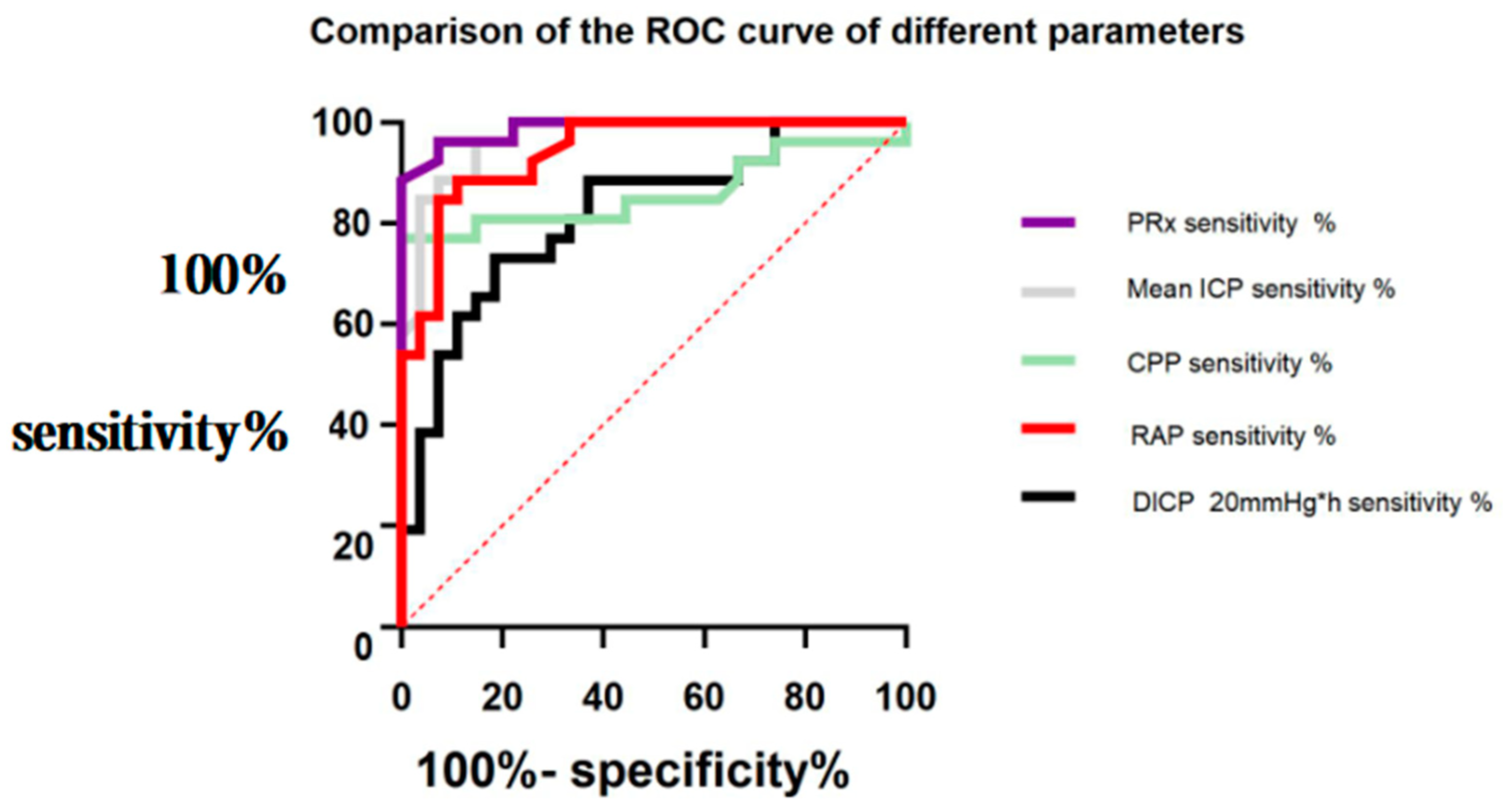
3.3. Comparison of ROC Curves of the Significance of Different Parameters in Predicting a Poor Prognosis in Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ICP | intracranial pressure |
| HICH | hypertensive intracerebral hemorrhage |
| PRx | pressure reactivity index |
| DICP | ICP dose |
| RAP | correlation coefficient between AMP amplitude and mean ICP |
| CPP | cerebral perfusion pressure |
| GOS | Glasgow outcome scale |
| GCS | Glasgow coma scale |
| CBF | cerebral blood flow |
| CVR | cerebrovascular reactivity |
| MAP | mean arterial pressure |
| AUC | area under curve |
| ROC | receiver operating characteristic |
| M | median |
| IQR | inter-quartile range |
References
- Zhou, M.; Wang, H.; Zeng, X.; Yin, P.; Zhu, J.; Chen, W.; Li, X.; Wang, L.; Wang, L.; Liu, Y.; et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 394, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.-Y. Head trauma in China. Injury 2013, 44, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Y.; Gao, G.-Y.; Feng, J.-F.; Mao, Q.; Chen, L.-G.; Yang, X.-F.; Liu, J.-F.; Wang, Y.-H.; Qiu, B.-H.; Huang, X.-J. Traumatic brain injury in China. Lancet Neurol. 2019, 18, 286–295. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Tian, D.; Long, W.; Zhang, S. Clinical significance of dynamic monitoring by transcranial doppler ultrasound and intracranial pressure monitor after surgery of hypertensive intracerebral hemorrhage. Int. J. Clin. Exp. Med. 2015, 8, 11456. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Diedler, J.; Sykora, M.; Orakcioglu, B.; Kentar, M.; Czosnyka, M.; Unterberg, A.; Sakowitz, O.W. Low-frequency sampling for PRx calculation does not reduce prognostication and produces similar CPPopt in intracerebral haemorrhage patients. Acta Neurochir. 2011, 153, 2189–2195. [Google Scholar] [CrossRef]
- Godoy, D.A.; Núñez-Patiño, R.A.; Vaca, A.Z.; Ziai, W.C.; Hemphill, J.C. Intracranial Hypertension After Spontaneous Intracerebral Hemorrhage: A Systematic Review and Meta-analysis of Prevalence and Mortality Rate. Neurocritical Care 2019, 31, 176–187. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Shi, S.X.; Yao, N.; Cheng, X.; Guo, A.; Zhu, Z.; Zhang, X.; Liu, Q. Brain transforms natural killer cells that exacerbate brain edema after intracerebral hemorrhage. J. Exp. Med. 2020, 217, e20200213. [Google Scholar] [CrossRef]
- Wu, X.; Gao, G.; Feng, J.; Mao, Q.; Jiang, J. A detailed protocol for physiological parameters acquisition and analysis in neurosurgical critical patients. J. Vis. Exp. 2017, 128, e56388. [Google Scholar] [CrossRef]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the management of spontaneous intracerebral hemorrhage. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [Green Version]
- Schramm, P.; Klein, K.U.; Pape, M.; Berres, M.; Werner, C.; Kochs, E.; Engelhard, K. Serial measurement of static and dynamic cerebrovascular autoregulation after brain injury. J. Neurosurg. Anesthesiol. 2011, 23, 41–44. [Google Scholar] [CrossRef]
- Donnelly, J.; Czosnyka, M.; Adams, H.; Cardim, D.; Kolias, A.G.; Zeiler, F.A.; Lavinio, A.; Aries, M.; Robba, C.; Smielewski, P.; et al. Twenty-five years of intracranial pressure monitoring after severe traumatic brain injury: A retrospective, single-center analysis. Neurosurgery 2018, 85, E75–E82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand Suppl. 1960, 36, 1–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, L.; Beqiri, E.; Younsi, A.; Czosnyka, M.; Smielewski, P. Predictive and Discriminative Power of Pressure Reactivity Indices in Traumatic Brain Injury. Neurosurgery 2020, 87, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Zweifel, C.; Lavinio, A.; Steiner, L.A.; Radolovich, D.; Smielewski, P.; Timofeev, I.; Hiler, M.; Balestreri, M.; Kirkpatrick, P.J.; Pickard, J.D.; et al. Continuous monitoring of cerebrovascular pressure reactivity in patients with head injury. Neurosurg. Focus 2008, 25, E2. [Google Scholar] [CrossRef]
- Tóth, P.J.; Szarka, N.; Farkas, E.; Ezer, E.; Czeiter, E.; Amrein, K.; Ungvari, Z.; Hartings, J.; Buki, A.; Koller, A. Traumatic brain injury-induced autoregulatory dysfunction and spreading depression-related neurovascular uncoupling: Pathomechanisms, perspectives, and therapeutic implications. Am. J. Physiol. Circ. Physiol. 2016, 311, H1118–H1131. [Google Scholar] [CrossRef] [Green Version]
- Steiner, L.A.; Czosnyka, M.; Piechnik, S.K.; Smielewski, P.; Chatfield, D.; Menon, D.K.; Pickard, J.D. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 2002, 30, 733–738. [Google Scholar] [CrossRef]
- Barton, C.W.; Hemphill, J.C.; Morabito, D.; Manley, G. A Novel Method of Evaluating the Impact of Secondary Brain Insults on Functional Outcomes in Traumatic Brain-injured Patients. Acad. Emerg. Med. 2005, 12, 1–6. [Google Scholar] [CrossRef]
- Czosnyka, M.; Czosnyka, Z.; Smielewski, P. Pressure reactivity index: Journey through the past 20 years. Acta Neurochir. 2017, 159, 2063–2065. [Google Scholar] [CrossRef] [Green Version]
- Lang, E.W.; Kasprowicz, M.; Smielewski, P.; Santos, E.; Pickard, J.; Czosnyka, M. Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. J. Neurosurg. 2015, 122, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Vik, A.; Nag, T.; Fredriksli, O.A.; Skandsen, T.; Moen, K.G.; Schirmer-Mikalsen, K.; Manley, G.T. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J. Neurosurg. 2008, 109, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Fraser, C.D.; Brady, K.M.; Rhee, C.J.; Easley, R.B.; Kibler, K.; Smielewski, P.; Czosnyka, M.; Kaczka, D.W.; Andropoulos, D.B.; Rusin, C.; et al. The frequency response of cerebral autoregulation. J. Appl. Physiol. 2013, 115, 52–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howells, T.; Johnson, U.; McKelvey, T.; Enblad, P. An optimal frequency range for assessing the pressure reactivity index in patients with traumatic brain injury. Int. J. Clin. Monit. Comput. 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Porras, R.; Santos, E.; Czosnyka, M.; Zheng, Z.; Unterberg, A.W.; Sakowitz, O.W. ‘Long’ pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir. 2012, 154, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Diedler, J.; Kasprowicz, M.; Budohoski, K.P.; Haubrich, C.; Smielewski, P.; Outtrim, J.G.; Manktelow, A.; Hutchinson, P.J.; Pickard, J.D.; et al. Critical Thresholds for Cerebrovascular Reactivity After Traumatic Brain Injury. Neurocrit. Care 2012, 16, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Castilla, L.; Gasco, J.; Nauta, H.J.W.; Okonkwo, D.O.; Robertson, C.S. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg. Focus 2008, 25, E7. [Google Scholar] [CrossRef]
- Lee, J.K.; Kibler, K.K.; Benni, P.B.; Easley, R.B.; Czosnyka, M.; Smielewski, P.; Koehler, R.C.; Shaffner, D.H.; Brady, K.M. Cerebrovascular Reactivity measured by near-infrared spectroscopy. Stroke 2009, 40, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Koskinen, L.-O.D.; Sundström, N.; Hägglund, L.; Eklund, A.; Olivecrona, M. Prostacyclin Affects the Relation between Brain Interstitial Glycerol and Cerebrovascular Pressure Reactivity in Severe Traumatic Brain Injury. Neurocritical Care 2019, 31, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Lazaridis, C.; DeSantis, S.M.; Smielewski, P.; Menon, D.K.; Hutchinson, P.; Pickard, J.D.; Czosnyka, M. Patient-specific thresholds of intracranial pressure in severe traumatic brain injury. J. Neurosurg. 2014, 120, 893–900. [Google Scholar] [CrossRef]
- Dias, C.; Silva, M.J.; Pereira, E.; Monteiro, E.; Maia, I.; Barbosa, S.; Silva, S.; Honrado, T.; Cerejo, A.; Aries, M.J.H.; et al. Optimal Cerebral Perfusion Pressure Management at Bedside: A Single-Center Pilot Study. Neurocritical Care 2015, 23, 92–102. [Google Scholar] [CrossRef]
- Czosnyka, M. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Czosnyka, M.; Hutchinson, P.J.; Balestreri, M.; Hiler, M.; Smielewski, P.; Pickard, J.D. Monitoring and interpretation of intracranial pressure after head injury. Acta Neurochir. Suppl. 2006, 96, 114–118. [Google Scholar] [CrossRef] [PubMed]
| Poor Prognosis Group | Good Prognosis Group | Current Result | p | |
|---|---|---|---|---|
| Age (mean ± standard deviation, years) | 64.58 ± 13.62 | 63.67 ± 11.84 | t = 0.260 | 0.796 |
| Gender (male/female, patient) | 13:13 | 14:13 | x2 = 0.018 | 0.893 |
| GCS score on admission (M(IQR), points) | 6 (7) | 7 (7) | U = 326 | 0.650 |
| Hematoma volume (mL) | 37.38 ± 6.40 | 35.41 ± 6.63 | t = 1.10 | 0.275 |
| Mean ICP (mean ± standard deviation, mmHg) | 27.68 ± 13.17 | 10.12 ± 4.37 | t = 6.566 | <0.001 |
| PRx (mean ± standard deviation) | 0.34 ± 0.12 | 0.05 ± 0.98 | t = 9.869 | <0.001 |
| RAP (mean ± standard deviation) | 0.37 ± 0.12 | 0.14 ± 0.10 | t = 7.619 | <0.001 |
| CPP (mean ± standard deviation, mmHg) | 55.88 ± 14.68 | 73.65 ± 8.27 | t = 5.454 | <0.001 |
| DICP20 (M (IQR), mmHg × h) | 320.36 (403.775) | 127.25 (215.64) | U = 574.00 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Pan, Y.; Chen, C.; Zhao, P.; Hang, C. Clinical Significance of Multiparameter Intracranial Pressure Monitoring in the Prognosis Prediction of Hypertensive Intracerebral Hemorrhage. J. Clin. Med. 2022, 11, 671. https://doi.org/10.3390/jcm11030671
Yang Y, Pan Y, Chen C, Zhao P, Hang C. Clinical Significance of Multiparameter Intracranial Pressure Monitoring in the Prognosis Prediction of Hypertensive Intracerebral Hemorrhage. Journal of Clinical Medicine. 2022; 11(3):671. https://doi.org/10.3390/jcm11030671
Chicago/Turabian StyleYang, Yongbo, Yuchun Pan, Chunlei Chen, Penglai Zhao, and Chunhua Hang. 2022. "Clinical Significance of Multiparameter Intracranial Pressure Monitoring in the Prognosis Prediction of Hypertensive Intracerebral Hemorrhage" Journal of Clinical Medicine 11, no. 3: 671. https://doi.org/10.3390/jcm11030671
APA StyleYang, Y., Pan, Y., Chen, C., Zhao, P., & Hang, C. (2022). Clinical Significance of Multiparameter Intracranial Pressure Monitoring in the Prognosis Prediction of Hypertensive Intracerebral Hemorrhage. Journal of Clinical Medicine, 11(3), 671. https://doi.org/10.3390/jcm11030671






