The Influence of Parathyroidectomy on Osteoporotic Fractures in Kidney Transplant Recipients: Results from a Retrospective Single-Center Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Analyzed Markers and Parameters
2.3. Statistical Analysis
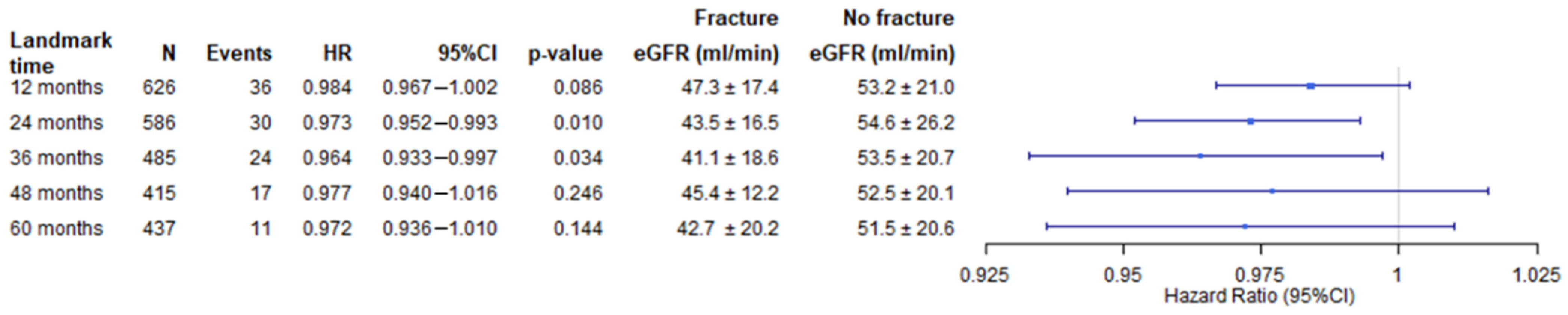
3. Results
3.1. Baseline Characteristics
3.2. Outcome Data
3.3. Fracture Events after KTx
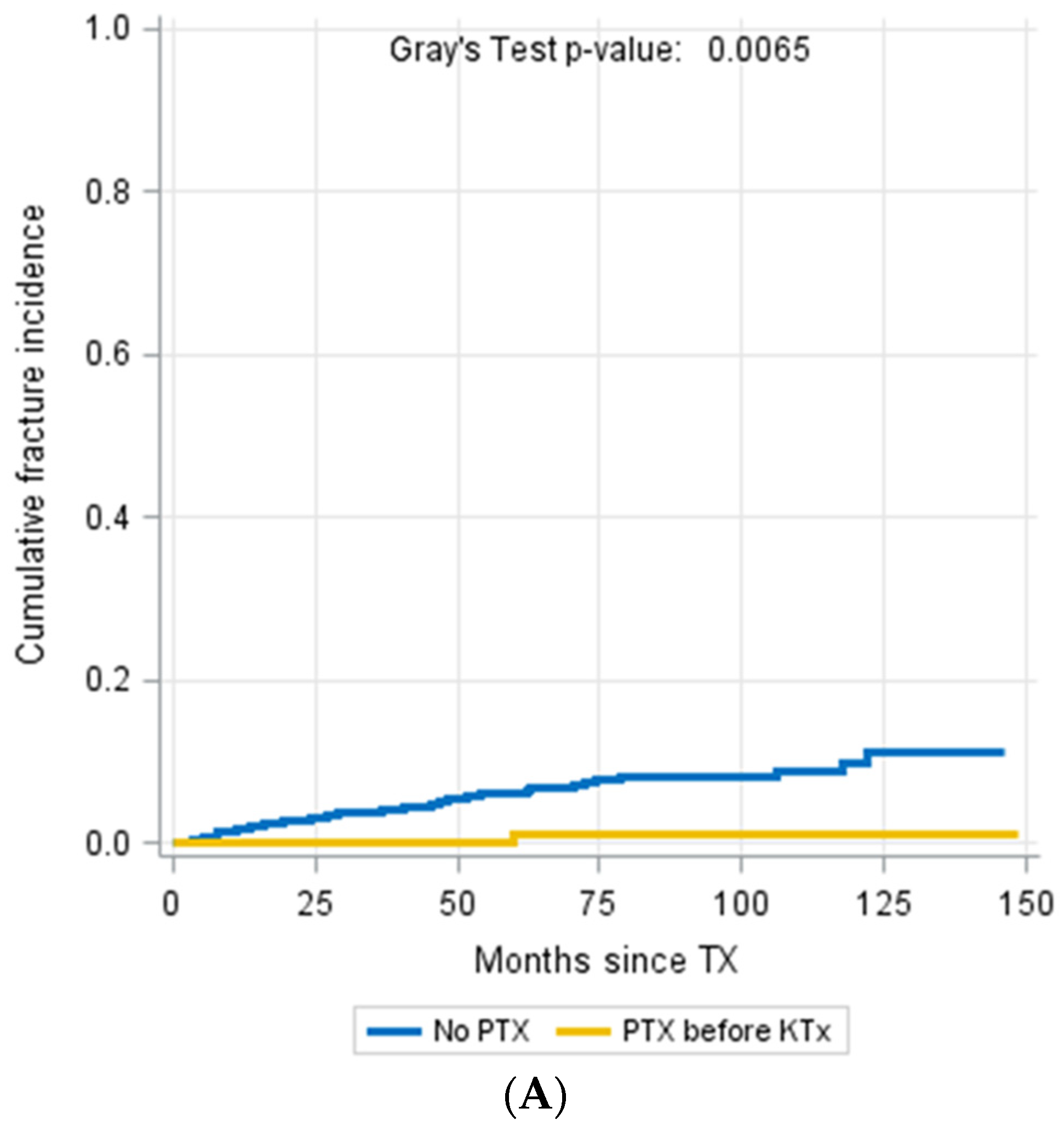
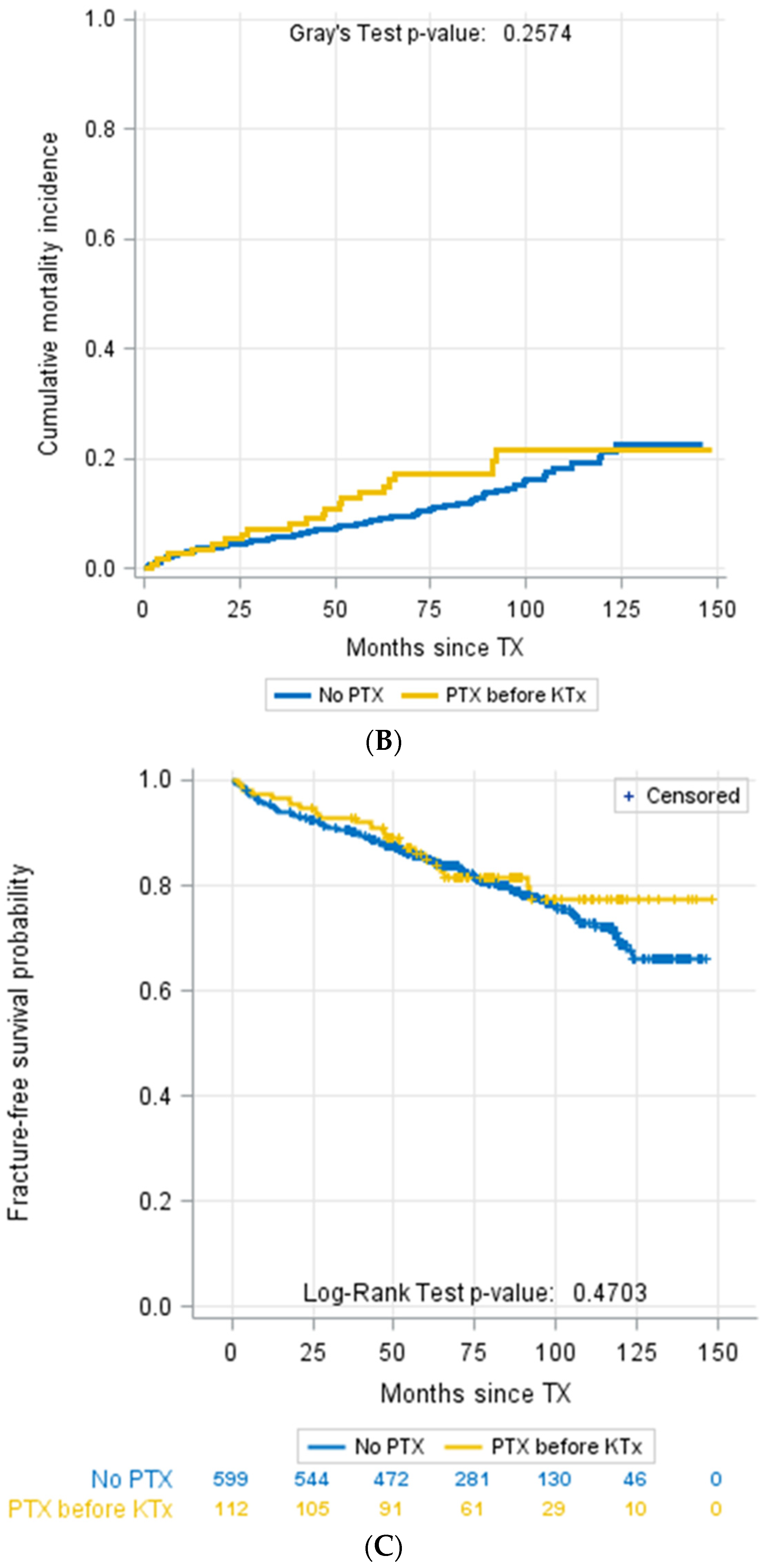
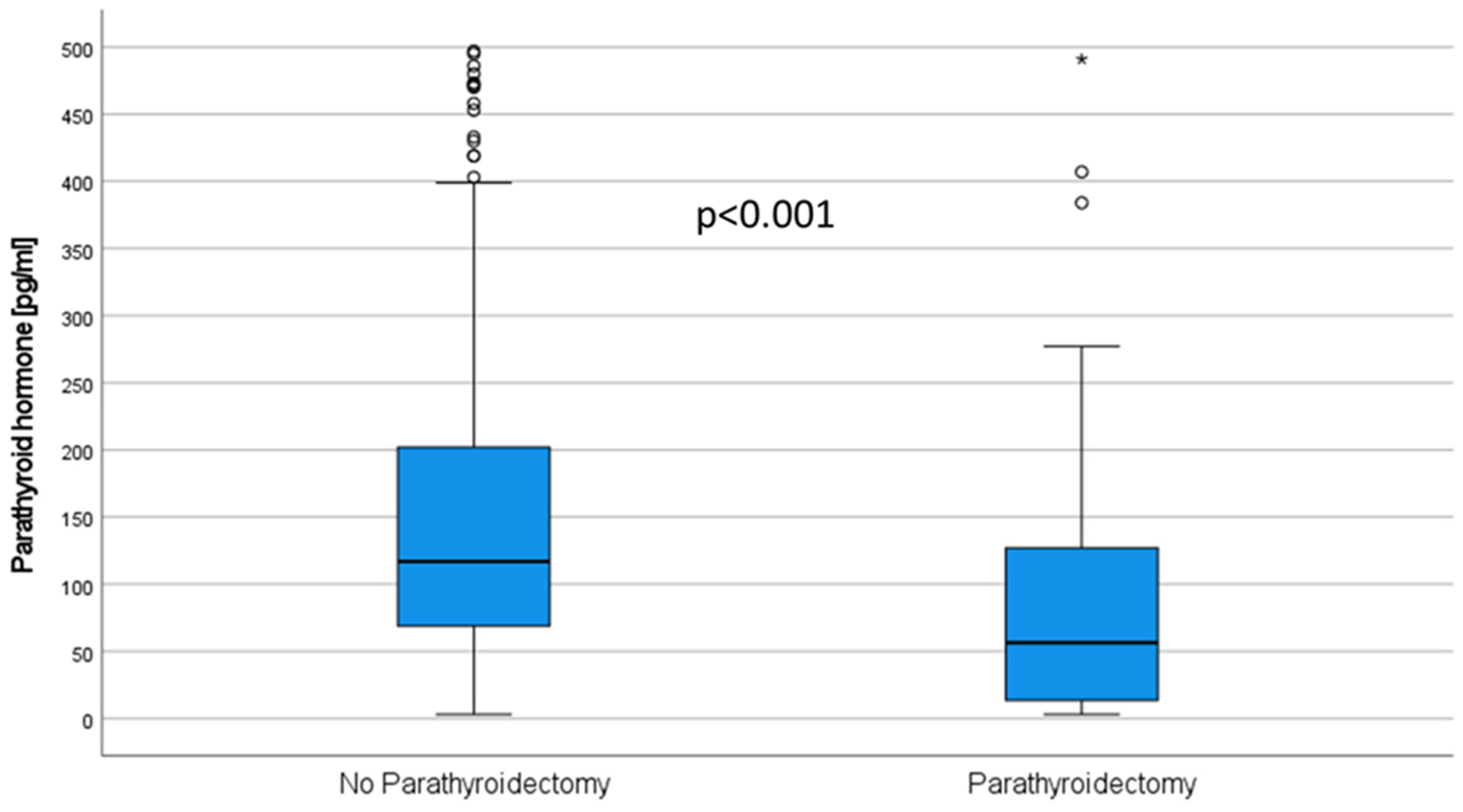
3.4. Parathyroidectomy
3.5. Medication with Calcimimetics
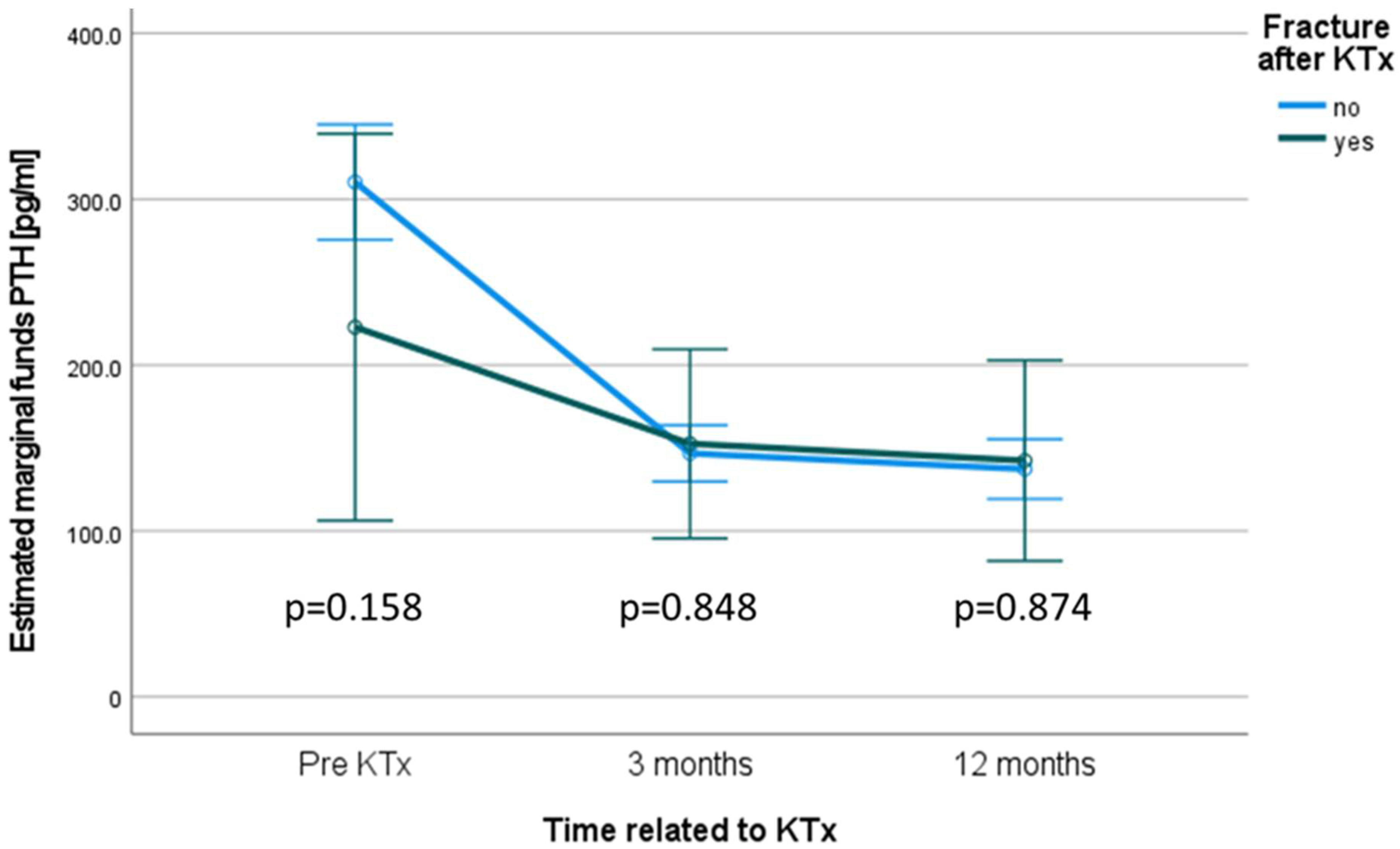
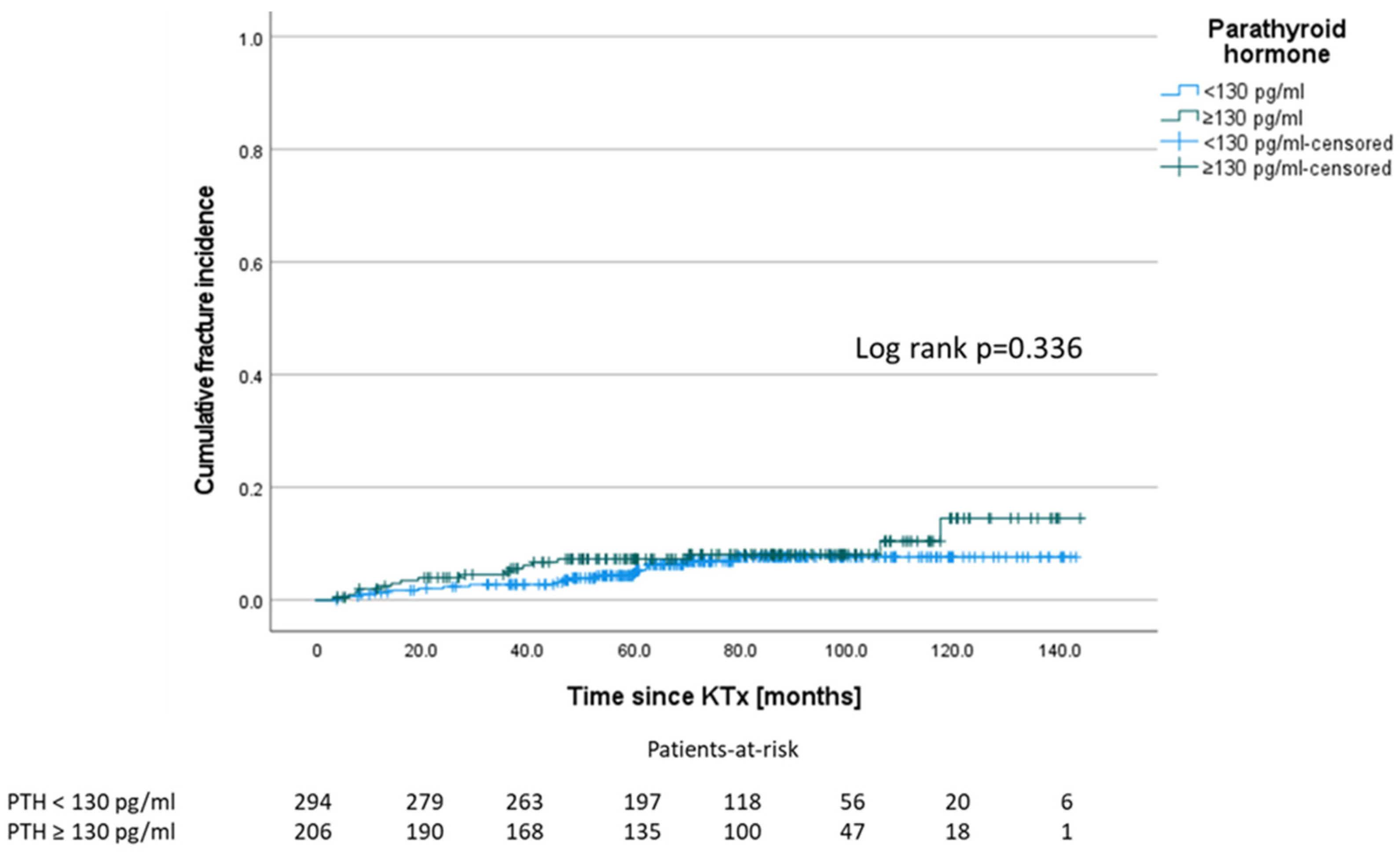
3.6. Levels of Parathyroid Hormone
3.7. Calcium Levels
3.8. Vitamin D Preparations and Bisphosphonates
3.9. Immunosuppressive Medication
3.10. Multivariate Cox Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center, J.R.; Nguyen, T.V.; Schneider, D.; Sambrook, P.N.; Eisman, J.A. Mortality after all major types of osteoporotic fracture in men and women: An observational study. Lancet 1999, 353, 878–882. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Lu, C.-L.; Lu, K.-C. Mineral bone disorders in chronic kidney disease. Nephrology 2018, 23 (Suppl. 4), 88–94. [Google Scholar] [CrossRef] [PubMed]
- Bozkaya, G.; Nart, A.; Uslu, A.; Onman, T.; Aykas, A.; Doğan, M.; Karaca, B. Impact of calcineurin inhibitors on bone metabolism in primary kidney transplant patients. Transplant. Proc. 2008, 40, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Yajima, A.; Tsuchiya, K. Management of Osteoporosis in Chronic Kidney Disease. Intern. Med. 2017, 56, 3271–3276. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Bilha, S.C.; Constantinescu, D.; Pavel-Tanasa, M.; Cianga, P.; Covic, A.; Branisteanu, D.D. Body composition, adipokines, FGF23-Klotho and bone in kidney transplantation: Is there a link? J. Nephrol. 2021. Ahead of print. [Google Scholar] [CrossRef]
- KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [CrossRef]
- Tominaga, Y.; Matsuoka, S.; Uno, N.; Sato, T. Parathyroidectomy for secondary hyperparathyroidism in the era of calcimimetics. Ther. Apher. Dial. 2008, 12 (Suppl. 1), S21–S26. [Google Scholar] [CrossRef]
- Yeh, M.W.; Zhou, H.; Adams, A.L.; Ituarte, P.H.G.; Li, N.; Liu, I.-L.A.; Haigh, P.I. The Relationship of Parathyroidectomy and Bisphosphonates with Fracture Risk in Primary Hyperparathyroidism: An Observational Study. Ann. Intern. Med. 2016, 164, 715–723. [Google Scholar] [CrossRef]
- Schütte-Nütgen, K.; Thölking, G.; Steinke, J.; Pavenstädt, H.; Schmidt, R.; Suwelack, B.; Reuter, S. Fast Tac Metabolizers at Risk—It is Time for a C/D Ratio Calculation. J. Clin. Med. 2019, 8, 587. [Google Scholar] [CrossRef]
- Perrin, P.; Caillard, S.; Javier, R.M.; Braun, L.; Heibel, F.; Borni-Duval, C.; Muller, C.; Olagne, J.; Moulin, B. Persistent hyperparathyroidism is a major risk factor for fractures in the five years after kidney transplantation. Am. J. Transplant. 2013, 13, 2653–2663. [Google Scholar] [CrossRef]
- Bliuc, D.; Nguyen, N.D.; Milch, V.E.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009, 301, 513–521. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.R.; Bammens, B.; Naesens, M.; Sprangers, B.; Pottel, H.; Cavalier, E.; Kuypers, D. Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients. Kidney Int. 2019, 95, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Chung, E.Y.; McGregor, D.O.; Bachmann, F.; Strippoli, G.F. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst. Rev. 2019, 10, CD005015. [Google Scholar] [CrossRef] [PubMed]
- Rudser, K.D.; de Boer, I.H.; Dooley, A.; Young, B.; Kestenbaum, B. Fracture risk after parathyroidectomy among chronic hemodialysis patients. J. Am. Soc. Nephrol. 2007, 18, 2401–2407. [Google Scholar] [CrossRef] [PubMed]
- Grotz, W.H.; Mundinger, F.A.; Gugel, B.; Exner, V.; Kirste, G.; Schollmeyer, P.J. Bone fracture and osteodensitometry with dual energy X-ray absorptiometry in kidney transplant recipients. Transplantation 1994, 58, 912–915. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, E.A.; Dahl, D.C.; Smith, C.L.; Kasiske, B.L. Risk factors for fractures in kidney transplantation. Tranplantation 2002, 74, 362–366. [Google Scholar] [CrossRef][Green Version]
- Haarhaus, M.; Evenepoel, P. Differentiating the causes of adynamic bone in advanced chronic kidney disease informs osteoporosis treatment. Kidney Int. 2021, 100, 546–558. [Google Scholar] [CrossRef]
- Zu, Y.; Lu, X.; Song, J.; Yu, L.; Li, H.; Wang, S. Cinacalcet Treatment Significantly Improves All-Cause and Cardiovascular Survival in Dialysis Patients: Results from a Meta-Analysis. Kidney Blood Press. Res. 2019, 44, 1327–1338. [Google Scholar] [CrossRef]
- Moreno, P.; Coloma, A.; Torregrosa, J.V.; Montero, N.; Francos, J.; Codina, S.; Manonelles, A.; Bestard, O.; García-Barrasa, A.; Melilli, E.; et al. Long-term results of a randomized study comparing parathyroidectomy with cinacalcet for treating tertiary hyperparathyroidism. Clin. Transplant. 2020, 34, e13988. [Google Scholar] [CrossRef]
- Isaksson, E.; Ivarsson, K.; Akaberi, S.; Muth, A.; Sterner, G.; Karl-Göran, P.; Clyne, N.; Almquist, M. The Effect of Parathyroidectomy on Risk of Hip Fracture in Secondary Hyperparathyroidism. World J. Surg. 2017, 41, 2304–2311. [Google Scholar] [CrossRef]
- Cruzado, J.M.; Moreno, P.; Torregrosa, J.V.; Taco, O.; Mast, R.; Gómez-Vaquero, C.; Polo, C.; Revuelta, I.; Francos, J.; Torras, J.; et al. A Randomized Study Comparing Parathyroidectomy with Cinacalcet for Treating Hypercalcemia in Kidney Allograft Recipients with Hyperparathyroidism. J. Am. Soc. Nephrol. 2016, 27, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Claes, K.; Kuypers, D.; Maes, B.; Vanrenterghem, Y. Impact of parathyroidectomy on renal graft function, blood pressure and serum lipids in kidney transplant recipients: A single centre study. Nephrol. Dial. Transplant. 2005, 20, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.L.; Obi, Y.; Kalantar-Zadeh, K. Parathyroidectomy in the Management of Secondary Hyperparathyroidism. Clin. J. Am. Soc. Nephrol. 2018, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Sutton, W.; Ahn, J.B.; Prescott, J.D.; Zeiger, M.A.; Segev, D.L.; McAdams-DeMarco, M. Association Between Treatment of Secondary Hyperparathyroidism and Posttransplant Outcomes. Transplantation 2021, 105, e366–e374. [Google Scholar] [CrossRef]
- Koh, E.Y.; van der Plas, W.Y.; Dulfer, R.R.; Pol, R.A.; Kruijff, S.; Rotmans, J.I.; Appelman-Dijkstra, N.; Schepers, A.; de Borst, M.H.; Hoorn, E.J.; et al. Outcomes of parathyroidectomy versus calcimimetics for secondary hyperparathyroidism and kidney transplantation: A propensity-matched analysis. Langenbecks Arch. Surg. 2020, 405, 851–859. [Google Scholar] [CrossRef]
- Dulfer, R.R.; Koh, E.Y.; van der Plas, W.Y.; Engelsman, A.F.; van Dijkum, E.J.M.N.; Pol, R.A.; Vogt, L.; de Borst, M.H.; Kruijff, S.; Schepers, A.; et al. Parathyroidectomy versus cinacalcet for tertiary hyperparathyroidism; a retrospective analysis. Langenbecks Arch. Surg. 2019, 404, 71–79. [Google Scholar] [CrossRef]
- Finnerty, B.M.; Chan, T.W.; Jones, G.; Khader, T.; Moore, M.; Gray, K.D.; Beninato, T.; Watkins, A.C.; Zarnegar, R.; Fahey, T.J. Parathyroidectomy versus Cinacalcet in the Management of Tertiary Hyperparathyroidism: Surgery Improves Renal Transplant Allograft Survival. Surgery 2019, 165, 129–134. [Google Scholar] [CrossRef]
- Delos Santos, R.; Rossi, A.; Coyne, D.; Maw, T.T. Management of Post-transplant Hyperparathyroidism and Bone Disease. Drugs 2019, 79, 501–513. [Google Scholar] [CrossRef]
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, 76, S1–S2. [CrossRef]
- Kramer, H.; Berns, J.S.; Choi, M.J.; Martin, K.; Rocco, M.V. 25-Hydroxyvitamin D testing and supplementation in CKD: An NKF-KDOQI controversies report. Am. J. Kidney Dis. 2014, 64, 499–509. [Google Scholar] [CrossRef]
- Buckley, L.; Humphrey, M.B. Glucocorticoid-Induced Osteoporosis. N. Engl. J. Med. 2018, 379, 2547–2556. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Shi, Y.; Bai, Y.; Zou, Y.; Cai, B.; Tao, Y.; Lin, T.; Wang, L. Impact of tacrolimus on bone metabolism after kidney transplantation. Int. Immunopharmacol. 2012, 13, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Kanda, J.; Izumo, N.; Furukawa, M.; Shimakura, T.; Yamamoto, N.; Takahashi, H.E.; Asakura, T.; Wakabayashi, H. Effects of the calcineurin inhibitors cyclosporine and tacrolimus on bone metabolism in rats. Biomed. Res. 2018, 39, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Schutte-Nutgen, K.; Tholking, G.; Suwelack, B.; Reuter, S. Tacrolimus—Pharmacokinetic Considerations for Clinicians. Curr. Drug Metab. 2018, 19, 342–350. [Google Scholar] [CrossRef]
- Evenepoel, P.; Claes, K.; Meijers, B.; Laurent, M.R.; Bammens, B.; Naesens, M.; Sprangers, B.; Cavalier, E.; Kuypers, D. Natural history of mineral metabolism, bone turnover and bone mineral density in de novo renal transplant recipients treated with a steroid minimization immunosuppressive protocol. Nephrol. Dial. Transplant. 2020, 35, 697–705. [Google Scholar] [CrossRef]
- Gitomer, B.; Pereira, R.; Salusky, I.B.; Stoneback, J.W.; Isakova, T.; Cai, X.; Dalrymple, L.S.; Ofsthun, N.; You, Z.; Malluche, H.H.; et al. Mineral bone disease in autosomal dominant polycystic kidney disease. Kidney Int. 2021, 99, 977–985. [Google Scholar] [CrossRef]
- Iseri, K.; Carrero, J.J.; Evans, M.; Felländer-Tsai, L.; Berg, H.E.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Fractures after kidney transplantation: Incidence, predictors, and association with mortality. Bone 2020, 140, 115554. [Google Scholar] [CrossRef]
| Variable | All ** (n = 711) | Fracture (n = 47) | No Fracture (n = 664) | p-Value |
|---|---|---|---|---|
| Age at Tx * (years), median (IQR) | 53.0 (21.7) | 60.8 (11.7) | 52.39 (21.9) | 0.000 a |
| Sex male, n (%) | 430 (60.5%) | 22 (46.8%) | 406 (61.5%) | 0.063 b |
| mismatch-HLA-A, n (%) | 1.000 b | |||
| none | 250 (35.3%) | 16 (34.0%) | 232 (35.3%) | |
| 1 | 338 (47.7%) | 23 (48.9%) | 313 (47.6%) | |
| 2 | 120 (16.9%) | 8 (17.0%) | 112 (17.0%) | |
| mismatch-HLA-B, n (%) | 0.152 b | |||
| none | 163 (23.0%) | 12 (25.5%) | 151 (23.0%) | |
| 1 | 339 (47.9%) | 27 (57.4%) | 309 (47.0%) | |
| 2 | 206 (29.0%) | 8 (17.0%) | 197 (30.0%) | |
| mismatch-HLA-DR, n (%) | 0.607 b | |||
| none | 178 (25.1%) | 12 (25.5%) | 164 (25.0%) | |
| 1 | 340 (48.0%) | 20 (42.6%) | 320 (48.7%) | |
| 2 | 190 (26.8%) | 15 (31.9%) | 173 (26.3%) | |
| PRA > 85%, n (%) | 12 (1.7%) | 1 (2.1%) | 11 (1.7%) | 0.566 b |
| PRA > 5%, n (%) | 84 (11.8%) | 4 (8.5%) | 80 (12.2%) | 0.641 b |
| Living donor Tx *, n (%) | 204 (28.7%) | 10 (21.3%) | 194 (29.4%) | 0.317 b |
| ABO incompatible Tx *, n (%) | 40 (5.6%) | 0 (0.0%) | 40 (6.1%) | 0.101 b |
| Cold ischemia time (hours), median (IQR) | 7.93 (9.18) | 7.67 (7.03) | 7.96 (9.21) | 0.779 a |
| Warm ischemia time (minutes), mean ± SD | 32.94 ± 8.33 | 34.20 ± 8.93 | 32.82 ± 8.29 | 0.130 a |
| Dialysis prior to Tx *, n (%) | 662 (93.1%) | 45 (95.7%) | 612 (92.7%) | 0.764 b |
| Dialysis vintage (months) median (IQR) | 54.59 (65.23) | 54.59 (51.44) | 53.82 (66.35) | 0.622 a |
| Previous Tx *, n (%) | 93 (13.1%) | 6 (12.8%) | 87 (13.2%) | 1.000 b |
| CMV mismatch D/R, n (%) | 0.489 b | |||
| D−/R− | 122 (17.2%) | 6 (13.0%) | 116 (17.6%) | |
| D−/R+ | 123 (17.3%) | 5 (10.9%) | 118 (17.9%) | |
| D+/R− | 165 (23.2%) | 12 (26.1%) | 152 (23.0%) | |
| D+/R+ | 300 (42.2%) | 23 (50.0%) | 274 (41.5%) | |
| Initial immunosuppression, n (%) | ||||
| Initial steroid use | 700 (98.5%) | 47 (100%) | 649 (98.3%) | 1.000 b |
| Initial MMF * use | 683 (96.1%) | 44 (93.6%) | 635 (96.2%) | 0.423 b |
| Initial CyA * use | 24 (3.4%) | 2 (4.3%) | 22 (3.3%) | 0.670 b |
| Initial tacrolimus use | 687 (96.6%) | 45 (95.7%) | 638 (96.7%) | 0.670 b |
| Initial mTOR * inhibitor use | 29 (4.1%) | 3 (6.4%) | 26 (3.9%) | 0.433 b |
| Diagnosis of ESRD, n (%) | 0.004 c | |||
| Hypertension | 55 (7.7%) | 1 (2.1%) | 54 (8.2%) | |
| Diabetes | 43 (6.0%) | 3 (6.4%) | 40 (6.1%) | |
| Polycystic kidney disease | 104 (14.6%) | 15 (31.9%) | 88 (13.3%) | |
| Obstructive Nephropathy | 35 (4.9%) | 2 (4.3%) | 33 (5.0%) | |
| Glomerulonephritis | 228 (32.1%) | 6 (12.8%) | 221 (33.5%) | |
| FSGS * | 32 (4.5%) | 3 (6.4%) | 29 (4.4%) | |
| Interstitial nephritis | 36 (5.1%) | 2 (4.3%) | 33 (5.0%) | |
| Vasculitis | 23 (3.2%) | 4 (8.5%) | 19 (2.9%) | |
| Other | 102 (14.3%) | 6 (12.8%) | 96 (14.5%) | |
| Unknown | 53 (7.5%) | 5 (10.6%) | 47 (7.1%) |
| All (n = 711) | Fracture (n = 47) | No Fracture (n = 664) | p-Value | |
|---|---|---|---|---|
| Alkaline phosphatase at M3 (u/L) median (IQR) | 83.50 (43) | 84.50 (41) | 83.00 (44) | 0.696 a |
| Whole serum calcium at prior to KTX (mmol/L) median (IQR) | 2.29 (0.29) | 2.33 (0.3) | 2.29 (0.29) | 0.539 a |
| Ionized calcium at prior to KTX (mmol/L) median (IQR) | 1.18 (0.12) | 1.19 (0.09) | 1.18 (0.12) | 0.264 a |
| Whole serum calcium at M3 (mmol/L) median (IQR) | 2.39 (0.24) | 2.38 (0.29) | 2.40 (0.24) | 0.685 a |
| Ionized calcium at M3 (mmol/L) median (IQR) | 1.27 (0.14) | 1.25 (0.14) | 1.27 (0.13) | 0.323 a |
| Serum phosphate at M3 (mg/dL) median (IQR) | 2.70 (1.1) | 2.70 (1.2) | 2.70 (1.1) | 0.502 a |
| Calcium phosphate product at M3 ((mg/dL)/(mmol/L)) | 6.55 (2.23) | 6.48 (2.35) | 6.55 (2.22) | 0.579 a |
| Parathyroid hormone prior to KTX (pg/mL) median (IQR) | 217.0 (329.5) | 188.0 (209.8) | 222.0 (333.6) | 0.244 a |
| Parathyroid hormone at M 3 (pg/mL) median (IQR) | 111.0 (130.5) | 121.5 (174.1) | 108.0 (126.6) | 0.322 a |
| Parathyroid hormone at M 12 (pg/mL) median (IQR) | 99.4 (104.0) | 95.2 (114.4) | 99.7 (102.6) | 0.962 a |
| Use of calcimimetics within Y1-3, n (%) | 47 (7.2%) | 3 (7.0%) | 44 (7.2%) | 1.000 b |
| Use of bisphosphonates within Y1-3, n (%) | 22 (3.3%) | 7 (16.3%) | 15 (2.5%) | <0.001 b |
| Use of nutritional Vitamin D within Y1-3, n (%) | 355 (54.0%) | 26 (60.5%) | 327 (53.5%) | 0.430 b |
| Use of VDR activators within Y1-3, n (%) | 259 (39.4%) | 15 (34.9%) | 243 (39.8%) | 0.629 b |
| Parathyroidectomy prior to KTX, n (%) | 112 (15.8%) | 1 (2.1%) | 111 (16.7%) | 0.003 b |
| Death censored allograft survival, n (%) | 630 (88.6%) | 37 (78.7%) | 589 (89.2%) | 0.053 b |
| Overall Graft Survival, n (%) | 568 (79.9%) | 30 (63.8) | 535 (81.1) | 0.008 b |
| NODAT, n (%) | 116 (16.3%) | 8 (17.0%) | 107 (16.2%) | 0.839 b |
| BK viremia, n (%) | 165 (23.2%) | 11 (23.4%) | 154 (23.3%) | 1.000 b |
| CMV viremia, n (%) | 236 (33.2%) | 13 (28.3%) | 221 (33.5%) | 0.520 b |
| Rejection yes, n (%) | 280 (39.4%) | 24 (51.1%) | 255 (38.6%) | 0.122 b |
| Tacrolimus use at M12, n (%) | 331 (76.8%) | 12 (42.9%) | 317 (79.1%) | <0.001 b |
| Tacrolimus C/D ratio at M3, median (IQR) | 1.30 (1.08) | 1.43 (1.48) | 1.30 (1.09) | 0.187 a |
| Tacrolimus trough levels (ng/mL) at M3, mean ± SD | 7.86 ± 2.64 | 7.91 ± 2.65 | 7.85 ± 2.63 | 0.881 a |
| Steroid use at M12 | 406 (57.1%) | 27 (96.4%) | 378 (94.0%) | 1.000 b |
| Fracture Localisation | ||||
| Extremity | 25 (53.2%) | |||
| Femoral neck | 6 (12.8%) | |||
| Pelvis | 5 (10.6%) | |||
| Vertebrae | 8 (17.0%) | |||
| Thorax (rips, sternum, clavicle) | 3 (6.4%) |
| Patients with Parathyroidectomy before KTX (n = 112) | Patients without Parathyroidectomy before KTX (n = 599) | p-Value | |
|---|---|---|---|
| PTX with partial autotransplantation, n (%) | 80 (71.4 %) | - | - |
| Parathyroid hormone at KTx (pg/mL), median (IQR) | 37.7 (169.5) | 232.0 (353.5) | <0.001 a |
| Parathyroid hormone at M3 (pg/mL) median (IQR) | 56.0 (111.1) | 117.0 (132.8) | <0.001 a |
| Parathyroid hormone at Y1 (pg/mL) median (IQR) | 57.6 (118.1) | 104.0 (92.2) | <0.001 a |
| Alkaline phosphatase at M3 (u/L) median (IQR) | 77.0 (41) | 85.0 (45) | 0.035 a |
| eGFR at Y1 (mL/min/1.73 m2) (±SD) | 53.6 (±20.0) | 55.5 (±20.7) | 0.763 a |
| eGFR at Y3 (mL/min/1.73 m2) (±SD) | 54.6 (±20.6) | 55.2 (±20.3) | 0.747 a |
| eGFR at Y5 (mL/min/1.73 m2), mean (± SD) | 50.9 (±22.0) | 51.5 (±20.4) | 0.868 a |
| Whole serum calcium at prior to KTX (mmol/L) median (IQR) | 2.26 (0.38) | 2.29 (0.28) | 0.379 a |
| Ionized calcium at prior to KTX (mmol/L) median (IQR) | 1.18 (0.11) | 1.18 (0.11) | 0.010 a |
| Whole serum calcium at M3 (mmol/L), median (IQR) | 2.26 (0.32) | 2.41 (0.22) | <0.001 a |
| Ionized calcium at M3 (mmol/L), median (IQR) | 1.22 (0.26) | 1.28 (0.13) | <0.001 a |
| Serum phosphate at M3 (mg/dL), median (IQR) | 2.95 (1.5) | 2.70 (1.0) | 0.001 a |
| Calcium phosphate product ((mg/dL)/(mmol/L)) at M3, median (IQR) | 6.62 (2.39) | 6.54 (2.20) | 0.237 a |
| Use of calcimimetics within Y1-3, n (%) | 2 (1.7%) | 45 (7.5%) | 0.011 b |
| Use of bisphosphonates within Y1-3, n (%) | 4 (3.6%) | 17 (2.8%) | 0.76 b |
| Use of nutritional Vitamin D within Y1-3, n (%) | 58 (51.7%) | 297 (49.6%) | 0.749 b |
| Use of VDR activators within Y1-3, n (%) | 62 (55.4%) | 197 (32.9%) | <0.001 b |
| Variable | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Age at KTx | 1.051 | 1.023–1.079 | <0.001 |
| Dialysis vintage | 0.999 | 0.991–1.007 | 0.851 |
| Female sex | 1.692 | 0.919–3.115 | 0.091 |
| Underlying renal disease | - | - | 0.111 |
| Parathyroidectomy | 0.134 | 0.018–0.991 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jehn, U.; Kortenhorn, A.; Schütte-Nütgen, K.; Thölking, G.; Westphal, F.; Strauss, M.; Wennmann, D.-O.; Pavenstädt, H.; Suwelack, B.; Görlich, D.; et al. The Influence of Parathyroidectomy on Osteoporotic Fractures in Kidney Transplant Recipients: Results from a Retrospective Single-Center Trial. J. Clin. Med. 2022, 11, 654. https://doi.org/10.3390/jcm11030654
Jehn U, Kortenhorn A, Schütte-Nütgen K, Thölking G, Westphal F, Strauss M, Wennmann D-O, Pavenstädt H, Suwelack B, Görlich D, et al. The Influence of Parathyroidectomy on Osteoporotic Fractures in Kidney Transplant Recipients: Results from a Retrospective Single-Center Trial. Journal of Clinical Medicine. 2022; 11(3):654. https://doi.org/10.3390/jcm11030654
Chicago/Turabian StyleJehn, Ulrich, Anja Kortenhorn, Katharina Schütte-Nütgen, Gerold Thölking, Florian Westphal, Markus Strauss, Dirk-Oliver Wennmann, Hermann Pavenstädt, Barbara Suwelack, Dennis Görlich, and et al. 2022. "The Influence of Parathyroidectomy on Osteoporotic Fractures in Kidney Transplant Recipients: Results from a Retrospective Single-Center Trial" Journal of Clinical Medicine 11, no. 3: 654. https://doi.org/10.3390/jcm11030654
APA StyleJehn, U., Kortenhorn, A., Schütte-Nütgen, K., Thölking, G., Westphal, F., Strauss, M., Wennmann, D.-O., Pavenstädt, H., Suwelack, B., Görlich, D., & Reuter, S. (2022). The Influence of Parathyroidectomy on Osteoporotic Fractures in Kidney Transplant Recipients: Results from a Retrospective Single-Center Trial. Journal of Clinical Medicine, 11(3), 654. https://doi.org/10.3390/jcm11030654







