Abstract
(1) Background: Deep hypothermic circulatory arrest (DHCA) with selective antegrade cerebral perfusion (ACP) is an established cerebral protection technique for the conduction of complex surgical procedures involving the aortic arch. It is controversial whether the duration of DHCA is associated with adverse outcome in patients with acute type A aortic dissection (AAAD). Our goal was to investigate whether DHCA time was associated with surgical outcome in patients undergoing a surgical treatment of AAAD. (2) Methods: A total of 410 patients were divided into two groups based on the DHCA time less than 60 min and equal to or longer than 60 min. (3) Results: Patients with longer DHCA times were significantly younger (p = 0.001). Intraoperatively, complex procedures with aortic arch surgery were more common in patients with longer DHCA times (p < 0.001). Accordingly, cardiopulmonary bypass (p < 0.001), cross-clamping (p < 0.001) and DHCA times (p < 0.001) were significantly longer in this group. Postoperatively, only the duration of mechanical ventilation (p < 0.001) and the rate of tracheotomy were significantly higher in these patients. Thirty-day mortality was satisfactory for both groups (p = 0.746). (4) Conclusions: Our results showed that improvements in perioperative management including ACP allow for the successful performance of surgical treatment of AAAD under DHCA with a duration of even longer than 60 min.
1. Introduction
Cerebral complications are a predominant cause of mortality and morbidity after thoracic aortic surgery [1]. In the mid-1970s, the first experience with applying deep hypothermic circulatory arrest (DHCA) to protect the central nervous system during a complex surgical procedure for the replacement of the aortic arch was reported [2]. DHCA with adjunctive selective antegrade cerebral perfusion (SACP) supports the protection of the central nervous system during a prolonged period of circularly arrest (more than 30 min) [3]. Moreover, cerebral perfusion using antegrade and retrograde cerebral perfusion strategies during hypothermic circulatory arrest are associated with reduced death and stroke risk [4].
Despite the benefits of DHCA with SACP for vital organ support during complex cardiac surgery, some centers do not prefer its use to avoid the potential adverse impact of prolonged duration of circulatory arrest on postoperative renal function [5]. It is controversial if only the grade of hypothermia, the duration of DHCA or a combination of pre-, intra- and postoperative factors is associated with mortality in patients [6,7].
The aim of the present study was to investigate whether DHCA time is associated with the clinical outcome of patients undergoing surgical treatment of acute type A aortic dissection (AAAD).
2. Materials and Methods
2.1. Patients and Study Design
Between January 2001 and May 2019, a total of 410 consecutive patients underwent a surgical treatment of an AAAD using DHCA and ACP. Patients were divided into two groups: those with a DHCA time less than 60 min (n = 337; 82.2%) and those with a DHCA time equal to or longer than 60 min (n = 73; 17.8%).
Preoperatively, contrast enhanced computed tomography (CT) was performed to detect the exact location and extension of the dissection membrane. In a few cases, the aortic dissection was discovered incidentally during magnetic resonance imaging (MRI) or coronary angiography examination or was detected during coronary angiography in patients with iatrogenic dissections. Postoperatively, patients were examined for neurological symptoms and questioned at admission for any history of neurological events. Neurological complications were consulted directly by a neurologist and categorized according to neurological assessment, followed by head and neck CT as well as, in many cases, CT angiography for the carotid arteries to estimate the extent of stroke and brain ischemia.
The primary endpoints were 30-day mortality and postoperative neurological events. Secondary endpoints were pre- and intraoperative variables, as well as the postoperative courses such as blood loss and transfusion of blood products.
2.2. Operative Technique
After a standard median sternotomy, a cardiopulmonary bypass (CPB) was performed with DHCA with a nasopharyngeal temperature between 20–24 °C. Venous cannulation was performed either through the femoral vein or the right atrium. Until 2010, depending on the pathology and the extent of the dissection, the arterial cannulation was performed either through the distal ascending aorta or the femoral artery. Since 2010, the standard approach in our center is the cannulation of the left ventricle transatrial via the right upper pulmonary [8]. Retrograde blood cardioplegic solution was used for myocardial protection. Antegrade cerebral perfusion with oxygenated cold blood (18 °C) was introduced through a balloon catheter inserted bilateral in arch vessels with a pressure control of 50–60 mmHg. Once the distal anastomosis was completed, de-airing was performed by restarting retrograde perfusion through the venous cannula, followed by slow antegrade perfusion of the newly cannulated prosthesis. During rewarming, appropriate procedures for the aortic root and the aortic valve were performed. Transfusion trigger was defined as that value of hemoglobin (Hb) below 10 gm/dL. Perioperatively, neuromonitoring with near-infrared spectroscopy (NIRS) was applied. The operative technique has been described in more detail in previous papers [9,10].
2.3. Statistical Analysis
The statistical analysis was performed with IBM SPSS statistics (version 28.0, IBM Corp., Armonk, NY, USA). The frequency distribution of the sample data was examined for deviations from the normal distribution using the Kolmogorov–Smirnov–Lilliefors test. The mean ± standard deviation was given for normally distributed, continuous variables, while non-normally distributed continuous variables were displayed as median with associated quartiles. Categorical variables were presented as an absolute number of affected patients (n) and the corresponding percentage (%). The chi-square test and, if necessary, the exact Fisher test were used to compare categorial variables of the two groups examined, while the Mann–Whitney U test was applied to compare non-normally distributed continuous and ordinal variables. Survival was estimated on the right-censored data of 30-day survivors using the Kaplan–Meier method and compared for differences using the log-rank test. All p-values ≤ 0.05 were rated as a significant difference between the two groups. Missing values were excluded pairwise and missing data>5% are indicated in the tables.
3. Results
3.1. Demographics and Clinical Characteristics of the Study Population
Relevant demographics and preoperative data of the study participants are presented in Table 1. Patients with longer DHCA times were significantly younger (58.0 ± 12.9 vs. 63.2 ± 12.8 years, p = 0.001) and frequently belonged to DeBakey type I (233 vs. 69, p < 0.001). A lower percentage of these patients were female (23.3% vs. 37.7%, p = 0.019). Otherwise, there were no between-group differences concerning preoperative data.

Table 1.
Demographic and clinical characteristics of the study population.
3.2. Intraoperative Data
Intraoperatively, complex procedures with total aortic arch replacement were significantly more common in patients with longer DHCA times (64.4% vs. 3.6%; p < 0.001). Accordingly, cardiopulmonary bypass time [245 vs. 154 min; p < 0.001], cross-clamping time [145 vs. 83 min; p < 0.001] and DHCA times [88 vs. 30 min; p < 0.001] were significantly longer due to the complexity of the performed surgical procedures (Table 2).

Table 2.
Intraoperative data.
Moreover, the total number of received packed red blood cells (6 vs. 3, p = 0.001), fresh frozen plasma (4 vs. 0, p = 0.002), and platelet concentrate (2 vs. 0, p < 0.001) was administered significantly more often in this patient group.
3.3. Postoperative Data and Outcomes
Also postoperatively, the total number of received packed red blood cells (6 (2; 16) vs. 3 (0; 7) unit, p = 0.001), fresh frozen plasma (4 (0; 12) vs. 0 (0; 6) unit, p = 0.002), and platelet concentrate (2 (0; 3) vs. 0 (0; 2) unit; p < 0.001) was significantly higher in patients with longer DHCA time (Table 3). These patients required a significantly higher rate of re-intubation (26.0% vs. 15.4%, p = 0.030) and tracheotomy (43.8% vs. 20.2%, p < 0.001) with higher duration of mechanical ventilation (134 vs. 50 min, p < 0.001) and duration of stay in ICU (9 (4; 19) vs. 5 (2; 10) days). Patients undergoing longer DHCA presented a non-significant higher rate of postoperative neurological deficits (31.5% vs. 21.4%, p = 0.06). Moreover, there was no difference between both groups concerning the postoperative complications and 30-day mortality (15.1% vs. 16.6%; p = 0.746).

Table 3.
Postoperative data and outcomes.
Short- and long-term survival was satisfactory in both groups. The 1-year (94% vs. 86%), 3-year (90% vs. 86%), 5-year (85% vs. 82%) and 10-year (61% vs. 50%) survival rates were in the same range in both groups (p = 0.784) (Figure 1).
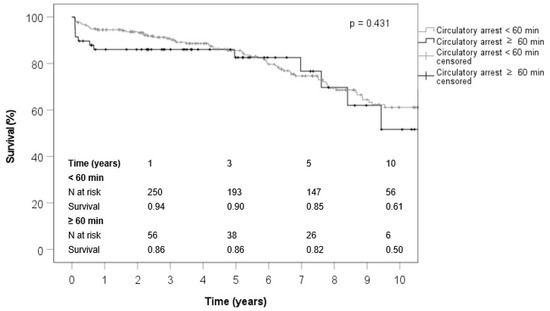
Figure 1.
The estimated survival curves by Kaplan–Meier method.
4. Discussion
In our study, the association between DHCA duration and clinical outcomes of 410 patients with AAAD was investigated. In total, 73 patients (17.8%) were identified as those with a DHCA time equal to or longer than 60 min and 337 patients (82.2%) as those with a DHCA time less than 60 min.
Both groups were compared concerning their demographic, preoperative data. However, there were significant differences between both groups concerning the intra- and postoperative data. Although the patients with longer DHCA have more complex aortic surgical procedures and correspondingly significant longer surgical duration, ICU time and postoperative in-hospital days, there was no significant difference between both groups concerning the 7-day and 30-day mortality and also long-term outcome.
In the literature, there are several studies and review articles about investigating the effect of the DHCA duration on clinical outcomes of patients during aortic surgery [3,11,12,13].
Our analysis demonstrated that patients undergoing longer DHCA presented a non-significant higher rate of postoperative neurological events (31.5% vs. 21.4%, p = 0.06). In a large study of a national clinical registry, O’Hara et al. [4] suggested that cerebral perfusion using antegrade and retrograde cerebral perfusion strategies are associated with reduced death and stroke risk compared with hypothermic circulatory arrest without cerebral perfusion. The impact of DHCA duration and the potential effects of ACP on mid-term quality of life (QoL) was assessed in a retrospective study of Immer et al. in 2004 [14]. In total, 363 patients with a thoracic aortic surgery were included in this study. A total of 176 patients (48.5%) of the cohort presented with an AAAD. The patients were divided into three groups according to the duration of DHCA (<20 min, 20–29 min and ≥30 min). In-hospital data comparable to our study were assessed in this study, but a much shorter follow-up (2.4 ± 1.2 years) was performed in all survivors. Immer et al. demonstrated that a DHCA duration longer than 20 min, especially longer than 35 min, adversely affected mid-term QoL in their study patients. However, the use of ACP improved the average QoL score and allowed DHCA to be extended up to 30 min, without impairment in mid-term QoL. The median DHCA is 33 min in our patients. Although we did not assess the QoL in our study, the comparison between the survivors within our patient groups during a 10-year follow-up confirm the mentioned success of DHCA with adjunctive selective ACP by Immer et al.
In a retrospective study by Mazzeffi et al. [12] in 2012, the relationship between DHCA time and perioperative bleeding and coagulopathy was investigated in a cohort of 507 consecutive patients who had thoracic aortic surgery. Mazzeffi et al. estimated the degree of bleeding and coagulopathy using perioperative transfusion. In accordance with our study, they reported a significant association between DHCA time and RBC transfusion (p = 0.001). In contrast to our study, they could not find any significant association between DHCA time and FFP and platelet transfusion (p = 0.18 and p = 0.06). Mazzeffi et al. reported a dependency of the association between DHCA time and the amount of bleeding (RBC transfusion) on cardiopulmonary bypass (CPB) time. They stated a positive relationship between DHCA duration and bleeding for CPB time up to 180 min but no such relationship for considerably longer CPB times (300 to 360 min). In contrast to Mazzeffi et al. our data confirm an association between longer CPB time and the amount of bleeding.
The varying evidence on deep hypothermic circulatory arrest was dealt with in a review article by Gupta et al. in 2018 [15]. In total, 17 original articles from 1999 to 2013 were reviewed in this article, which had various main foci such as neuropsychological outcome after DHCA, cerebral protection during aortic surgery, and quality of life after DHCA with ACP. Gupta et al. demonstrated that there are controversial discussions about the safe duration of DHCA. Some authors have suggested that DHCA durations in a range of 20 to 25 min affect the surgical outcomes and the quality of life [14,16], while other authors, in contrast, reported that DHCA durations under 50 min are safe for performing aortic arch interventions [17]. In a retrospective study with 656 patients, an increased incidence of postoperative stroke was reported as a result of DHCA durations of longer than 40 min [18]. In contrast, DHCA less than 60 min was demonstrated as an adequate cerebral protection technique in a comparably homogeneous and smaller study with 200 patients [19]. In a further review article, the authors associated DHCA duration of longer than 49 min with a higher rate of stroke compared to the duration of 40 to 49 min [11]. Based on a multivariable analysis in this article, DHCA times equal to and longer than 50 min significantly correlated with adverse outcomes and early death [11]. In comparison with some studies in the literature, our study included a homogenous and relatively large cohort of 410 patients with AAAD. Therefore, the surgical outcome of patients in two different DHCA times of under 60 min and equal to or above 60 min could be investigated within one study with a similar surgical setup. Our patients with longer DHCA times were reintubated significantly more frequently and underwent a common tracheotomy. They also had a significantly longer ventilation time and ICU stay time. Nevertheless, we could not observe any effect of DHCA time on cardiac and neurologic outcomes after DHCA in terms of postoperative myocardial infarction, delirium and TIA/stroke.
It is to be expected that longer DHCA time is associated with a significant higher morbidity and mortality [12,20,21]. However, the current advances in surgical techniques, circulatory management and postoperative care improve the early and late clinical outcome of patients with DHCA times of longer than 60 min [13].
The main limitations of our study were the retrospective design and the inhomogeneity of both patient groups without a propensity score matching analysis. Multivariable logistic regression analysis was performed based on this large sample size to adjust for known confounders, however, there remains a risk of unknown or not surveyed confounders.
5. Conclusions
Based on our 18-year single-center experience, we investigated the effect of DHCA duration on clinical outcomes in patients with AAAD. Our results confirmed that improvements in perioperative management including ACP allow for a successful performance of surgical treatment of AAAD under DHCA with a duration of even longer than 60 min. Further prospective, multicentric and randomized clinical studies with a larger group of patients are required to investigate in detail if, in consideration of improved peri- and intraoperative management, the duration of DHCA still has a strong effect on clinical outcomes in patients with AAAD.
Author Contributions
Conceptualization, C.F., J.C., J.S., L.H., G.E., M.S., G.L., T.P., J.C. and A.H.; methodology, C.F., J.C., J.S., L.H., G.E., M.S., G.L., T.P., J.C. and A.H.; software, not applicable; validation, not applicable; formal analysis, C.F.; investigation, M.S.R.; resources, not applicable.; data curation, C.F. and A.H.; writing—original draft preparation, M.S.R., C.F. and A.H.; writing—review and editing, M.S.R., C.F., J.C., J.S., L.H., G.E., M.S., G.L., T.P., J.C. and A.H.; visualization, C.F.; supervision, A.H.; project administration, A.H.; funding acquisition, not applicable. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This retrospective single-centre cohort study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the local ethics committee of Christian-Albrechts University of Kiel (No. D417/17).
Informed Consent Statement
Written informed consent for this retrospective analysis was waived.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- TTaylor, K.M. Brain damage during open-heart surgery. Thorax 1982, 37, 873–876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griepp, R.B.; Stinson, E.B.; Hollingsworth, J.F.; Buehler, D. Prosthetic replacement of the aortic arch. J. Thorac. Cardiovasc. Surg. 1975, 70, 1051–1063. [Google Scholar] [CrossRef]
- Tian, D.H.; Wan, B.; Bannon, P.G.; Misfeld, M.; LeMaire, S.A.; Kazui, T.; Kouchoukos, N.T.; Elefteriades, J.A.; Bavaria, J.E.; Coselli, J.S.; et al. A meta-analysis of deep hypothermic circulatory arrest alone versus with adjunctive selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2013, 2, 261–270. [Google Scholar] [PubMed]
- O’Hara, D.; McLarty, A.; Sun, E.; Itagaki, S.; Tannous, H.; Chu, D.; Egorova, N.; Chikwe, J. Type-A aortic dissection and cerebral perfusion: The society of thoracic surgeons database analysis. Ann. Thorac. Surg. 2020, 110, 1461–1467. [Google Scholar] [CrossRef]
- Cao, L.; Guo, X.; Jia, Y.; Yang, L.; Wang, H.; Yuan, S. Effect of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest in aortic arch surgery on postoperative renal function: A systematic review and meta-analysis. J. Am. Heart Assoc. 2020, 9, e017939. [Google Scholar] [CrossRef]
- Okita, Y.; Takamoto, S.; Ando, M.; Morota, T.; Matsukawa, R.; Kawashima, Y. Mortality and cerebral outcome in patients who underwent aortic arch operations using deep hypothermic circulatory arrest with retrograde cerebral perfusion: No relation of early death, stroke, and delirium to the duration of circulatory arrest. J. Thorac. Cardiovasc. Surg. 1998, 115, 129–138. [Google Scholar] [CrossRef]
- Kumral, E.; Yüksel, M.; Büket, S.; Yagdi, T.; Atay, Y.; Güzelant, A. Neurologic complications after deep hypothermic circulatory arrest: Types, predictors, and timing. Tex. Heart Inst. J. 2001, 28, 83–88. [Google Scholar] [PubMed]
- Schoeneich, F.; Rahimi-Barfeh, A.; Grothusen, C.; Cremer, J. Transatrial left-ventricular cannulation in acute aortic dissection type A: A novel cannulation technique. Eur. J. Cardio. Surg. 2015, 48, e51–e52. [Google Scholar] [CrossRef][Green Version]
- Ravesh, M.S.; Salem, M.; Lutter, G.; Friedrich, C.; Walter, V.; Puehler, T.; Cremer, J.; Haneya, A. Comparison of outcomes in DeBakey type I versus DeBakey type II aortic dissection: A 17-year single center experience. J. Thorac. Dis. 2021, 13, 6769. [Google Scholar] [CrossRef]
- Salehi Ravesh, M.; Rusch, R.; Friedrich, C.; Teickner, C.; Berndt, R.; Haneya, A.; Cremer, J.; Pühler, T. Impact of patients’ age on short and long-term outcome after carotid endarterectomy and simultaneous coronary artery bypass grafting. J. Cardiothorac. Surg. 2019, 14, 109. [Google Scholar] [CrossRef]
- Ziganshin, B.A.; Elefteriades, J.A. Deep hypothermic circulatory arrest. Ann. Cardiothorac. Surg. 2013, 2, 303–315. [Google Scholar] [PubMed]
- Mazzeffi, M.; Marotta, M.; Lin, H.-M.; Fischer, G. Duration of deep hypothermia during aortic surgery and the risk of perioperative blood transfusion. Ann. Card. Anaesth. 2012, 15, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Moeller, E.; Nores, M.; Stamou, S.C. Repair of acute Type-A aortic dissection in the present era: Outcomes and controversies. Aorta 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Immer, F.F.; Lippeck, C.; Barmettler, H.; Berdat, P.A.; Eckstein, F.S.; Kipfer, B.; Saner, H.; Schmidli, J.; Carrel, T.P. Improvement of quality of life after surgery on the thoracic aorta: Effect of antegrade cerebral perfusion and short duration of deep hypothermic circulatory arrest. Circulation 2004, 110, II250–II255. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Harky, A.; Jahangeer, S.; Adams, B.; Bashir, M. Varying evidence on deep hypothermic circulatory arrest in thoracic aortic aneurysm surgery. Tex. Heart Inst. J. 2018, 45, 70–75. [Google Scholar] [CrossRef]
- Ergin, M.A.; Uysal, S.; Reich, D.L.; Apaydin, A.; Lansman, S.L.; McCullough, J.N.; Griepp, R.B. Temporary neurological dysfunction after deep hypothermic circulatory arrest: A clinical marker of long-term functional deficit. Ann. Thorac. Surg. 1999, 67, 1887–1890; discussion 1891–1894. [Google Scholar] [CrossRef]
- Elefteriades, J.A. What is the best method for brain protection in surgery of the aortic arch? Straight DHCA. Cardiol. Clin. 2010, 28, 381–387. [Google Scholar] [CrossRef]
- Svensson, L.G.; Crawford, E.S.; Hess, K.R.; Coselli, J.S.; Raskin, S.; Shenaq, S.A.; Safi, H.J. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J. Thorac. Cardiovasc. Surg. 1993, 106, 19–28; discussion 28–31. [Google Scholar] [CrossRef]
- Ergin, M.A.; Galla, J.D.; Lansman, S.L.; Quintana, C.; Bodian, C.; Griepp, R.B. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J. Thorac. Cardiovasc. Surg. 1994, 107, 788–797; discussion 797–799. [Google Scholar] [CrossRef]
- Cooper, W.A.; Duarte, I.G.; Thourani, V.H.; Nakamura, M.; Wang, N.-P.; Brown, W.; Gott, J.P.; Vinten-Johansen, J.; Guyton, R.A. Hypothermic circulatory arrest causes multisystem vascular endothelial dysfunction and apoptosis. Ann. Thorac. Surg. 2000, 69, 696–702. [Google Scholar] [CrossRef]
- Harrington, D.K.; Lilley, J.P.; Rooney, S.J.; Bonser, R.S. Nonneurologic morbidity and profound hypothermia in aortic surgery. Ann. Thorac. Surg. 2004, 78, 596–601. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).