Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Electrocardiography
2.3. Right Heart Catheterization and Pulmonary Angiography
2.4. Echocardiography
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Hemodynamic and Echocardiographic Results
3.3. Electrocardiographic Results
3.4. Discriminatory Performance of Electrocardiography in CTEPH Localization
3.5. Correlations between Electrocardiographic and Hemodynamic Data in Proximal CTEPH
3.6. Changes in Electrocardiographic Parameters after Interventional Treatment of CTEPH
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [Green Version]
- Pepke-Zaba, J.; Delcroix, M.; Lang, I.; Mayer, E.; Jansa, P.; Ambroz, D.; Treacy, C.; Monforte, A.D.; Morsolini, M.; Snijder, R.; et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation 2011, 124, 1973–1981. [Google Scholar] [CrossRef] [Green Version]
- Kopeć, G.; Dzikowska-Diduch, O.; Mroczek, E.; Mularek-Kubzdela, T.; Chrzanowski, Ł.; Skoczylas, I.; Tomaszewski, M.; Peregud-Pogorzelska, M.; Karasek, D.; Lewicka, E.; et al. Characteristics and outcomes of patients with chronic thromboembolic pulmonary hypertension in the era of modern therapeutic approaches: Data from the Polish multicenter registry (BNP-PL). Ther. Adv. Chronic Dis. 2021, 12, 20406223211002961. [Google Scholar] [CrossRef]
- Kim, N.H.; Delcroix, M.; Jenkins, D.P.; Channick, R.; Dartevelle, P.; Jansa, P.; Lang, I.; Madani, M.M.; Ogino, H.; Pengo, V.; et al. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, D92–D99. [Google Scholar] [CrossRef]
- Kataoka, M.; Inami, T.; Kawakami, T.; Fukuda, K.; Satoh, T. Balloon Pulmonary Angioplasty (Percutaneous Transluminal Pulmonary Angioplasty) for Chronic Thromboembolic Pulmonary Hypertension: A Japanese Perspective. JACC Cardiovasc. Interv. 2019, 12, 1382–1388. [Google Scholar] [CrossRef]
- Araszkiewicz, A.; Darocha, S.; Pietrasik, A.; Pietura, R.; Jankiewicz, S.; Banaszkiewicz, M.; Sławek-Szmyt, S.; Biederman, A.; Mularek-Kubzdela, T.; Lesiak, M.; et al. Balloon pulmonary angioplasty for the treatment of residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int. J. Cardiol. 2019, 278, 232–237. [Google Scholar] [CrossRef]
- Darocha, S.; Roik, M.; Kopeć, G.; Araszkiewicz, A.; Furdal, M.; Lewandowski, M.; Jacheć, W.; Grabka, M.; Banaszkiewicz, M.; Piertrasik, A.; et al. Balloon Pulmonary Angioplasty in Chronic Thromboembolic Pulmonary Hypertension: A Multicenter Registry. EuroIntervention, 2021; Epub ahead of print. [Google Scholar] [CrossRef]
- Darocha, S.; Araszkiewicz, A.; Kurzyna, M.; Banaszkiewicz, M.; Jankiewicz, S.; Dobosiewicz, A.; Sławek-Szmyt, S.; Janus, M.; Grymuza, M.; Pietrasik, A.; et al. Balloon Pulmonary Angioplasty in Technically Operable and Technically Inoperable Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2021, 10, 1038. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef] [Green Version]
- Ghofrani, H.-A.; D’Armini, A.M.; Grimminger, F.; Hoeper, M.; Jansa, P.; Kim, N.H.; Mayer, E.; Simonneau, G.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013, 369, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Simonneau, G.; D’armini, A.M.; Ghofrani, A.; Grimminger, F.; Hoeper, M.; Jansa, P.; Kim, N.H.; Wang, C.; Wilkins, M.R.; Fritsch, A.; et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: A long-term extension study (CHEST-2). Eur. Respir. J. 2014, 45, 1293–1302. [Google Scholar] [CrossRef]
- Klok, F.A.; Barco, S.; Konstantinides, S.; Dartevelle, P.; Fadel, E.; Jenkins, D.; Kim, N.H.; Madani, M.; Matsubara, H.; Mayer, E.; et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: Results from the European CTEPH Registry. Eur. Respir. J. 2018, 52, 1801687. [Google Scholar] [CrossRef]
- Piłka, M.; Darocha, S.; Banaszkiewicz, M.; Florczyk, M.; Wieteska, M.; Dobosiewicz, A.; Mańczak, M.; Mańczak, R.; Pietrasik, A.; Pietura, R.; et al. The evolution of electrocardiographic signs of right ventricular overload after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Pol. Arch. Intern. Med. 2019, 129, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Ghio, S.; Turco, A.; Klersy, C.; Scelsi, L.; Raineri, C.; Crescio, V.; Viscardi, A.; Grazioli, V.; Sciortino, A.; Visconti, L.O.; et al. Changes in surface electrocardiogram in patients with chronic thromboembolic pulmonary hypertension undergoing pulmonary endarterectomy. Correlations with hemodynamic and echocardiographic improvements after surgery. J. Electrocardiol. 2016, 49, 223–230. [Google Scholar] [CrossRef]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar]
- Hancock, E.W.; Deal, B.J.; Mirvis, D.M.; Okin, P.; Kligfield, P.; Gettes, L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part V: Electrocardiogram changes associated with cardiac chamber hypertrophy: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 992–1002. [Google Scholar]
- Surawicz, B.; Childers, R.; Deal, B.J.; Gettes, L.S.; Bailey, J.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; Kors, J.A.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part III: Intraventricular conduction disturbances: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 976–981. [Google Scholar]
- Kurzyna, M.; Araszkiewicz, A.; Błaszczak, P.; Grabka, M.; Hawranek, M.; Kopeć, G.; Mroczek, E.; Zembala, M.; Torbicki, A.; Ochała, A. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Kardiol. Polska 2015, 73, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Thistlethwaite, P.A.; Mo, M.; Madani, M.M.; Deutsch, R.; Blanchard, D.; Kapelanski, D.P.; Jamieson, S.W. Operative classification of thromboembolic disease determines outcome after pulmonary endarterectomy. J. Thorac. Cardiovasc. Surg. 2002, 124, 1203–1211. [Google Scholar] [CrossRef] [Green Version]
- Kopec, G.; Tyrka, A.; Miszalski-Jamka, T.; Sobien, M.; Waligóra, M.; Brózda, M.; Podolec, P. Electrocardiogram for the Diagnosis of Right Ventricular Hypertrophy and Dilation in Idiopathic Pulmonary Arterial Hypertension. Circ. J. 2012, 76, 1744–1749. [Google Scholar] [CrossRef] [Green Version]
- Waligóra, M.; Tyrka, A.; Podolec, P.; Kopeć, G. ECG Markers of Hemodynamic Improvement in Patients with Pulmonary Hypertension. Biomed Res. Int. 2018, 2018, 4606053. [Google Scholar] [CrossRef] [Green Version]
- Piłka, M.; Darocha, S.; Banaszkiewicz, M.; Wieteska-Miłek, M.; MSc, M.M.; Mańczak, R.; Kędzierski, P.; Florczyk, M.; Dobosiewicz, A.; Torbicki, A.; et al. Assessment of electrocardiographic markers of acute and long-term hemodynamic improvement in patients with pulmonary hypertension. Ann. Noninvasive Electrocardiol. 2020, 25, e12758. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, A.R.; Baumgärtner, M.; AlKukhun, L.; Minai, O.A.; Dweik, R.A. Electrocardiography at diagnosis and close to the time of death in pulmonary arterial hypertension. Ann. Noninvasive Electrocardiol. 2013, 19, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Lewis, C.; Lambiase, P. The electrocardiogram in pulmonary hypertension. Br. J. Hosp. Med. 2005, 66, M62–M63. [Google Scholar] [CrossRef]
- Al-Naamani, K.; Hijal, T.; Nguyen, V.; Andrew, S.; Nguyen, T.; Huynh, T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int. J. Cardiol. 2008, 127, 214–218. [Google Scholar] [CrossRef]
- Nishiyama, T.; Takatsuki, S.; Kawakami, T.; Katsumata, Y.; Kimura, T.; Kataoka, M.; Tsuruta, H.; Itabashi, Y.; Murata, M.; Yuasa, S.; et al. Improvement in the electrocardiograms associated with right ventricular hypertrophy after balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. IJC Hear. Vasc. 2018, 19, 75–82. [Google Scholar] [CrossRef]
- Yokokawa, T.; Sugimoto, K.; Nakazato, K.; Misaka, T.; Oikawa, M.; Kobayashi, A.; Yoshihisa, A.; Yamaki, T.; Kunii, H.; Ishida, T.; et al. Electrocardiographic Criteria of Right Ventricular Hypertrophy in Patients with Chronic Thromboembolic Pulmonary Hypertension after Balloon Pulmonary Angioplasty. Intern. Med. 2019, 58, 2139–2144. [Google Scholar] [CrossRef] [Green Version]
- Kanemoto, N. Electrocardiographic and Hemodynamic Correlations in Primary Pulmonary Hypertension. Angiology 1988, 39, 781–787. [Google Scholar] [CrossRef]
- Bossone, E.; Paciocco, G.; Iarussi, D.; Agretto, A.; Iacono, A.; Gillespie, B.W.; Rubenfire, M. The prognostic role of the ECG in primary pulmonary hypertension. Chest 2002, 121, 513–518. [Google Scholar] [CrossRef]
- Henkens, I.R.; Gan, C.T.-J.; van Wolferen, S.A.; Hew, M.; Boonstra, A.; Twisk, J.W.R.; Kamp, O.; van der Wall, E.E.; Schalij, M.J.; Noordegraaf, A.V.; et al. ECG Monitoring of Treatment Response in Pulmonary Arterial Hypertension Patients. Chest 2008, 134, 1250–1257. [Google Scholar] [CrossRef]
- Cheng, X.-L.; He, J.-G.; Liu, Z.-H.; Gu, Q.; Ni, X.-H.; Zhao, Z.-H.; Luo, Q.; Xiong, C.-M. The Value of the Electrocardiogram for Evaluating Prognosis in Patients with Idiopathic Pulmonary Arterial Hypertension. Lung 2016, 195, 139–146. [Google Scholar] [CrossRef]
- Asano, R.; Ogo, T.; Ohta-Ogo, K.; Fukui, S.; Tsuji, A.; Ueda, J.; Konagai, N.; Fukuda, T.; Morita, Y.; Noguchi, T.; et al. Prolonged QRS duration as a predictor of right ventricular dysfunction after balloon pulmonary angioplasty. Int. J. Cardiol. 2019, 280, 176–181. [Google Scholar] [CrossRef] [PubMed]
| ECG Sign | Definition—Amplitude |
|---|---|
| R-wave in lead V1 | >6 mm |
| R-wave in lead V1 + S-wave in lead V5 (V6) | >10.5 mm |
| R-wave: S-wave ratio in lead V1 | >1.0 |
| R-wave in lead aVR | >4 mm |
| R-wave in lead V5 (V6) | <3 mm |
| S-wave in V1 | <2 mm |
| S-wave in V5 | >10 mm |
| S-wave in V6 | >3 mm |
| R-wave: S-wave ratio in lead V5 | <0.75 |
| R-wave: S-wave ratio in lead V6 | <0.4 |
| R-wave: S-wave ratio in lead V5 to R-wave: S-wave ratio in lead V1 | <0.04 |
| (R-wave in lead I + S-wave in lead III) minus (S-wave in lead I + R-wave in lead III) | <15 mm |
| R-wave peak in lead V1 (QRS < 120 ms) | >0.035 s |
| QR complex in lead V1 | present |
| Supportive criteria | |
| RSR’ complex in lead V1 (QRS duration > 0.12 sec) | present |
| S wave > R wave in lead I, II, III | present |
| S-wave in lead I and Q-wave in lead III | present |
| Negative T-wave in leads V1–V4 | present |
| R-wave: S-wave ratio in lead V1 > R-wave: S-wave ratio in lead V3 (V4) | present |
| P-wave in lead II | >2.5 mm |
| Right ventricular strain | |
| ST-T segment depression by at least 1 mm in leads V1–V3 | present |
| ST-T segment depression by at least 1 mm in leads: II, III, aVF | present |
| negative T wave in leads: II, III, aVF | present |
| Parameter | All (n, (%) | Proximal CTEPH (n = 58) | Distal CTEPH (n = 70) | p Value |
|---|---|---|---|---|
| Age (years), median (IQR) | 62 (52–70.5) | 59.5 (47–67) | 67 (55–73) | 0.0021 |
| Sex | 0.053 | |||
| (female/ male) | 65 (50.8)/ | 35 (60.3)/ | 30 (42.9)/ | |
| 63 (49.2) | 23 (39.7) | 40 (57.1) | ||
| BMI (kg/m2) | 29 (25.5–33.1) | 27.7 (24.2–32.3) | 29.5 (26–33.3) | 0.64 |
| BSA | 1.9 (1.7–2.1) | 2.0 (1.7–2.2) | 1.9 (1.7–2.1) | 0.013 |
| WHO FC | 0.22 | |||
| I | - | - | - | |
| II | 28 (21.9%) | 11 (19%) | 17 (24.3%) | |
| III | 74 (57.8%) | 32 (55.1%) | 42 (60%) | |
| IV | 26 (20.3%) | 15 (25.9%) | 11 (15.7%) | |
| Previous symptomatic pulmonary embolism | 97 (75.8%) | 52 (89.7%) | 45 (64.3%) | 0.00087 |
| Chronic obstructive pulmonary disease | 16 (12.5%) | 4 (6.9%) | 12 (17.1%) | 0.1 |
| Diabetes mellitus | 20 (15.6%) | 6 (10.3%) | 14 (20%) | 0.15 |
| Systemic arterial hypertension | 85 (66.4%) | 37 (63.8%) | 48 (68.6%) | 0.58 |
| Known thrombophilia | 6 (4.7%) | 4 (6.9%) | 2 (2.9%) | 0.29 |
| Chronic renal insufficiency | 30 (23.4%) | 13 (22.4%) | 17 (24.3%) | 0.83 |
| Anticoagulation therapy | ||||
| DOAC | 80 (64.5%) | 34 (58.6%) | 46 (67.7%) | 0.35 |
| VKA | 42 (33.9%) | 22 (37.9%) | 20 (30.3%) | 0.45 |
| 6MWD (m), median (IQR) | 310 (250–390) | 298.5 (260–405) | 320 (240–390) | 0.47 |
| NT-proBNP (pg/mL), median (IQR) | 1020 (286–2177) | 1038 (355–1977) | 1002 (286–3436) | 0.59 |
| Parameter | All n = 128 | Proximal CTEPH (n = 58) | Distal CTEPH (n = 70) | p Value |
|---|---|---|---|---|
| Hemodynamic data | ||||
| sSAP (mmHg) | 139.5 (128–155) | 134 (125–142) | 144 (128–166) | 0.001 |
| dSAP (mmHg) | 86.5 (80–92) | 76 (56–95) | 87 (82–92) | 0.34 |
| mRAP (mmHg) | 7 (5–11.5) | 6.5 (5–13) | 8 (5–10) | 0.97 |
| sRVP (mmHg) | 78.5 (63.5–89) | 82 (70–92) | 70.5 (62–83) | 0.0018 |
| dRVP (mmHg) | 5.8 (2–8) | 3 (5–6) | 2 (5–8) | 0.62 |
| edRVP (mmHg) | 11 (8–16) | 11.5 (8–16) | 11 (8–15) | 0.62 |
| sPAP (mmHg) | 78.5 (63.5–89) | 82 (70–92) | 70.5 (62–83) | 0.0018 |
| mPAP (mmHg) | 45 (40–52) | 48 (44–57) | 43 (39–50) | 0.0004 |
| PAWP (mmHg) | 7 (10–12) | 10 (7–12) | 10 (7.5–10) | 0.49 |
| PVR (Wood units) | 6.9 (5.4–8.6) | 7.3 (5.6–10.9) | 6.2 (4.6–8.1) | 0.0038 |
| SVR (Wood units) | 16.4 (13.8–20.1) | 16.7 (14.4–20.1) | 16.3 (13.3–19.9) | 0.8 |
| CO (L/min) | 5.8 (4.65–6.6) | 5.8 (5.0–6.6) | 5.8 (4.6–6.6) | 0.8 |
| CI (L/min/m2) | 2.9 (2.7–3.5) | 2.9 (2.7–4.5) | 3.1 (2.4–3.6) | 0.64 |
| SV (mL) | 76.5 (63–98) | 81 (66–101) | 72 (60–98) | 0.56 |
| SvO2 (%) | 67.8 | 68.7 (64.5–72.8) | 67.4 (62.6–74) | 0.7 |
| SaO2 (%) | 92.3 (91–94) | 92.7 (91.4–94) | 92.2 (90.9–93.9) | 0.35 |
| Echocardiographic data | ||||
| RAA (cm2) | 24.1 (21–32) | 27 (21–36) | 23 (20–28) | 0.0278 |
| RV free wall thickness (mm) | 5.5 (4.5–6) | 5.9 (4.8–6) | 5.3 (4.5–5.8) | 0.3 |
| RV end-diastolic diameter (4 chamber) (mm) | 47 (43–53) | 47 (43–53) | 45 (42–52) | 0.39 |
| TAPSE (mm) | 19 (15–24) | 19 (14–22) | 19 (16–25) | 0.17 |
| TRV max (m/s) | 4.4 (3.9–4.7) | 4.5 (4.2–4.8) | 4.4 (3.6–4.6) | 0.06 |
| TVPG (mmHg) | 84 (60–95) | 90 (70–95) | 76 (55–90) | 0.014 |
| Pulmonary trunk (mm) | 31 (26–36) | 32 (28–37) | 29 (25–35) | 0.24 |
| S’ (cm/s) | 12 (9–15) | 11 (9–14) | 12.5 (9–15) | 0.095 |
| Parameter | All | Proximal CTEPH (n = 58) | Distal CTEPH (n = 70) | p Value |
|---|---|---|---|---|
| HR (bpm), median, (IQR) | 78 (70–90) | 80 (72–98) | 76 (67–82) | 0.64 |
| Sinus tachycardia, n (%) | 18 (14) | 11 (19) | 7 (10) | 0.2 |
| Axis | 0.61 | |||
| Normal, n (%) | 62 (48.4) | 28 (48.3) | 34 (48.6) | |
| Right axis deviation, n (%) | 58 (45.3) | 24 (41.4) | 34 (48.6) | |
| Left axis deviation, n (%) | 8 (6.3) | 6 (10.3) | 2 (2.9) | |
| RV1, median, (IQR) | 5 (3–8) | 8 (5–9.5) | 4 (2–6) | <0.0001 |
| RV1 > 6 mm, n (%) | 55 (43) | 41 (70.7) | 14 (20) | <0.0001 |
| RV1 + SV5 (V6), median, (IQR) | 11 (8–13.8) | 12 (9–18) | 9.5 (7–12) | 0.002 |
| RV1 + SV5 (V6) > 10.5 mm, n (%) | 70 (54.7) | 40 (69) | 30 (42.9) | 0.004 |
| RV1:SV1 > 1.0, n (%) | 78 (60.9) | 45 (77.6) | 33 (47.1) | 0.0005 |
| RaVR (mm), median, (IQR) | 3 (2–4) | 4.5 (2.5–5) | 2 (1–3) | <0.0001 |
| RaVR > 4 mm, n (%) | 36 (28.1) | 31 (53.5) | 5 (7.1) | <0.0001 |
| RV5 (V6) (mm), median, (IQR) | 6 (5–8) | 6 (5–8) | 6 (5–8) | 0.69 |
| RV5 (V6) < 3 mm, n (%) | 6 (5) | 2 (3) | 4 (6) | 0.69 |
| SV1(mm), median, (IQR) | 1 (0.8–4) | 1 (1–2) | 1 (0.8–5) | 0.28 |
| SV1 < 2 mm, n (%) | 69 (53.9) | 39 (67.2) | 30 (42.9) | 0.024 |
| SV5 > 10 mm, median, (IQR) | 5 (3–7.5) | 6 (5–10) | 4 (3–6.5) | 0.0054 |
| SV5 >10 mm, n (%) | 15 (12) | 11 (19) | 4 (6) | 0.027 |
| SV6 (mm), median, (IQR) | 5 (3–7) | 7 (5–8) | 3 (2–4) | <0.0001 |
| SV6 > 3 mm n (%) | 85 (66) | 51 (88) | 34 (48.6) | <0.0001 |
| RV5:SV5 < 0.7, n (%) | 19 (15) | 10 (17) | 9 (13) | 0.62 |
| RV6:SV6 < 0.4, n (%) | 6 (5) | 2 (3) | 4 (6) | 0.69 |
| RV5:SV5 to RV1:SV1 < 0.04 | 3 (2.34) | 3 (5.2) | 0 | 0.9 |
| (R I + S III) − (S I + RIII) < 15 mm, n, (%) | 101 (78.9) | 42 (72.4) | 59 (84.3) | 0.128 |
| RV1 peak > 0.035 s (QRS < 120 ms), n, (%) | 60 (46.9) | 37 (63.8) | 23 (32.9) | 0.0007 |
| qRV1, n (%) | 9 (7) | 5 (9) | 4 (6) | 0.73 |
| Supportive criteria | ||||
| RSR’ complex in lead V1 (QRS duration > 0.12 s), n (%) | 9 (7) | 5 (9) | 4 (6) | 0.73 |
| SI > RI wave, n (%) | 62 (48.4) | 39 (67.2) | 23 (32.9) | 0.00017 |
| SII > RII wave, n (%) | 31 (24.2) | 19 (32.8) | 12 (17.1) | 0.06 |
| SIII > RIII, n (%) | 24 (18.8) | 15 (25.9) | 9 (12.9) | 0.07 |
| SIQIII, n (%) | 83 (65) | 41 (71) | 42 (60) | 0.26 |
| Negative TV1–V4, n (%) | 85 (66) | 41 (71) | 44 (63) | 0.45 |
| RV1:SV1 > RV3(V4):SV3(V4), n (%) | 76 (75.3) | 45 (90) | 31 (60.8) | 0.001 |
| PII > 2.5 mm, median, (IQR) | 2 (1–2.5) | 2.0 (1.5–3.0) | 2.0 (0.5–2) | 0.0008 |
| PII > 2.5 mm, n (%) | 30 (24) | 19 (34) | 11 (16) | 0.02 |
| RV strain | ||||
| ST-T segment depression V1–V3, n (%) | 87 (68) | 43 (74) | 44 (43) | 0.18 |
| ST-T segment depression II, III, aVF, n (%) | 65 (51) | 32 (55) | 33 (47) | 0.38 |
| negative T II, III, aVF, n (%) | 65 (51) | 32 (55) | 33 (47) | 0.38 |
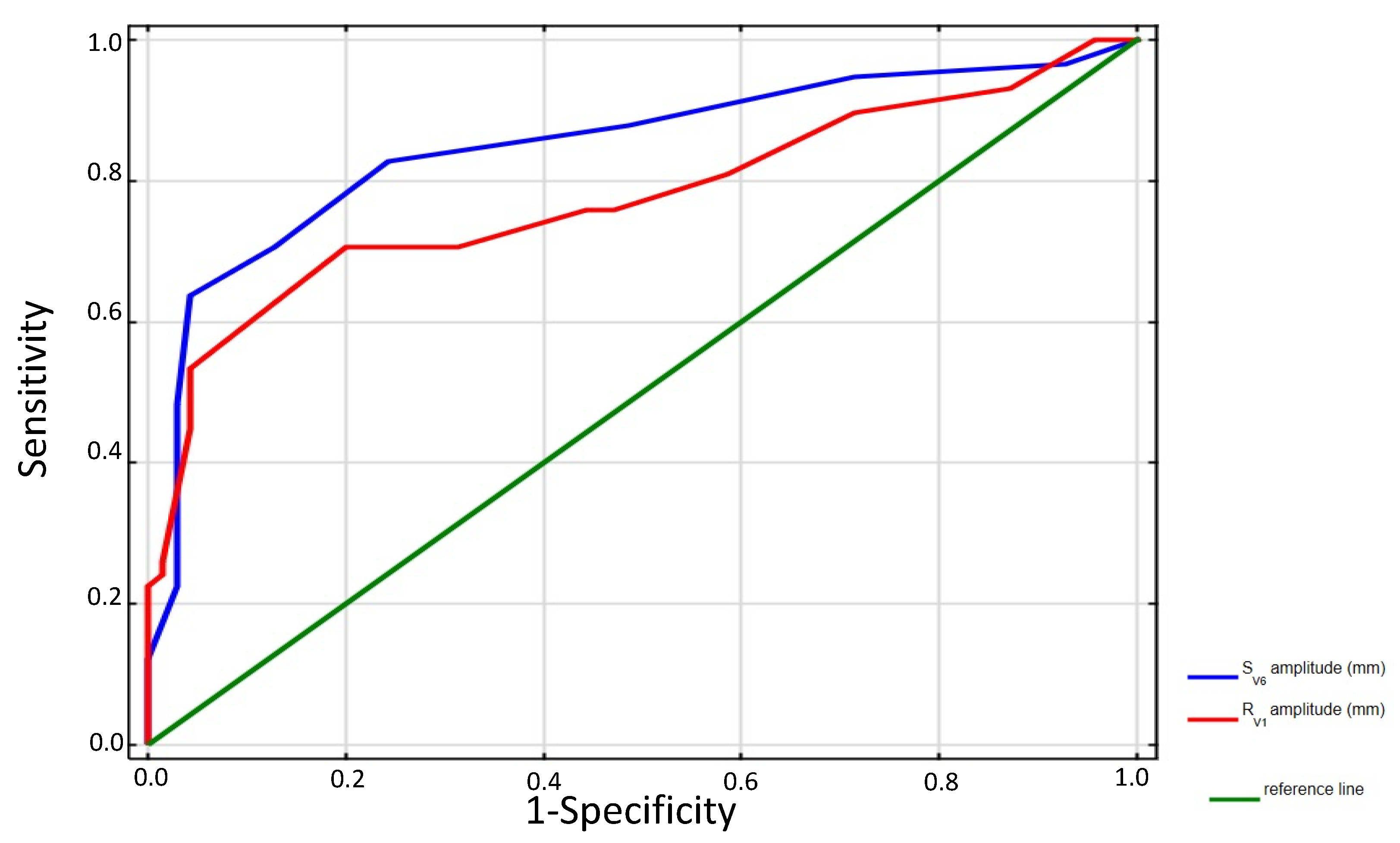
| ECG Signs | AUC | 95% CI | p Value | Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|---|---|
| RV1 > 6 mm | 0.75 | 0.66–0.84 | <0.00001 | 70 | 80 | 75.8 | 74.5 | 76.7 | 3.5 | 0.37 |
| RV1:SV1 > 1.0 | 0.66 | 0.57–0.76 | 0.0009 | 79.3 | 52.9 | 64.8 | 58.2 | 75.5 | 1.68 | 0.39 |
| RaVR > 4 mm | 0.62 | 0.52–0.71 | 0.02 | 65.5 | 74.3 | 61 | 71 | 70.7 | 1.7 | 0.75 |
| SV6 > 3 mm | 0.70 | 0.60–0.79 | <0.00001 | 88.0 | 51.4 | 68 | 60 | 83.7 | 1.8 | 0.24 |
| RV1 peak > 0.035 s (QRS < 120 ms) | 0.69 | 0.59–0.79 | 0.0002 | 80.4 | 58.2 | 68.9 | 61.7 | 69.1 | 0.31 | 0.34 |
| SI > RI | 0.67 | 0.58–0.77 | 0.0004 | 67.2 | 67.1 | 67.2 | 62.9 | 71.2 | 2.0 | 0.49 |
| RV1:SV1 > RV3(V4):SV3(V4) | 0.65 | 0.54–0.75 | 0.0081 | 90 | 39.2 | 64.4 | 59.2 | 80 | 1.49 | 2.55 |
| PII > 2.5 mm | 0.62 | 0.52–0.72 | 0.0162 | 41.4 | 82.9 | 64.1 | 66.7 | 63 | 2.41 | 0.71 |
| Proximal CTEPH | ||||
|---|---|---|---|---|
| ECG Signs | mPAP | PVR | ||
| r | p | r | p | |
| RV1 | 0.34 | 0.008 | 0.29 | 0.015 |
| RV1:SV1 | 0.11 | 0.42 | 0.24 | 0.07 |
| RaVR | 0.21 | 0.076 | 0.25 | 0.044 |
| SV6 | 0.53 | 0.000027 | 0.4 | 0.0024 |
| RV1 peak (QRS < 120 ms) | 0.24 | 0.075 | −0.06 | 0.66 |
| SI: RI | 0.31 | 0.009 | 0.2 | 0.093 |
| RV1:SV1/RV3(V4):SV3(V4) | 0.31 | 0.026 | −0.16 | 0.26 |
| PII | 0.44 | 0.00007 | 0.4 | 0.002 |
| ECG Sign | Proximal CTEPH | p Value | Distal CTEPH | p Value | ||
|---|---|---|---|---|---|---|
| On Diagnosis (n = 58) | 3–6 Months after PEA (n = 36)/BPA Treatment (n = 12) | On Diagnosis (n= 70) | 3–6 Months after BPA Treatment (n = 45) | |||
| RV1 > 6 mm, median, (IQR) | 8 (5–9.5) | 4 (1–8) | <0.00001 | 4 (2–6) | 4.5 (2–5) | <0.00001 |
| RV1 > 6 mm, n (%) | 41 (70.7) | 15 (31.2) | <0.0001 | 14 (20) | 5 (11.1) | 0.0078 |
| RV1:SV1 >1.0 | 45 (77.6) | 19 (39.6) | <0.0001 | 33 (47.1) | 10 (22.2) | <0.0001 |
| RaVR > 4 mm, median, (IQR) | 4.5 (2.5–5) | 5.2 (4.7–6) | <0.0044 | 2 (1–3) | - | - |
| RaVR > 4 mm, n, (%) | 31 (53.5) | 12 (25) | <0.0001 | 5 (7.1) | - | - |
| SV6 (mm), median, (IQR) | 7 (5–8) | 2 (2–2.5) | <0.0001 | 3 (2–4) | 2 (1–2) | 0.0025 |
| SV6 > 3 mm n (%) | 51 (88) | 9 (18.8) | <0.0001 | 34 (48.6) | 6 (13.3) | <0.0001 |
| RV1 peak > 0.035 s (QRS < 120 ms) | 37 (63.8) | 16 (33.3) | <0.0001 | 23 (32.9) | 6 (13.3) | 0.0001 |
| SI > RI, n (%) | 39 (67.2) | 11 (22.9) | <0.0001 | 23 (32.9) | 11 (24.4) | 0.0015 |
| RV1:SV1 > RV3(V4):SV3(V4) | 45 (90) | 14 (29.2) | <0.0001 | 31 (60.8) | 11 (24.4) | <0.0001 |
| PII > 2.5 mm, median, (IQR) | 2.0 (1.5–3.0) | 2.0 (1.5–2.0) | 0.0002 | 2.0 (0.5–2) | 2 (0.5–2) | 0.14 |
| PII > 2.5 mm, n, (%) | 19 (34) | 8 (16.7) | 0.0026 | 11 (16) | 8 (17.8) | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławek-Szmyt, S.; Araszkiewicz, A.; Jankiewicz, S.; Smukowska-Gorynia, A.; Grygier, M.; Janus, M.; Lesiak, M.; Mularek-Kubzdela, T. Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension. J. Clin. Med. 2022, 11, 625. https://doi.org/10.3390/jcm11030625
Sławek-Szmyt S, Araszkiewicz A, Jankiewicz S, Smukowska-Gorynia A, Grygier M, Janus M, Lesiak M, Mularek-Kubzdela T. Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine. 2022; 11(3):625. https://doi.org/10.3390/jcm11030625
Chicago/Turabian StyleSławek-Szmyt, Sylwia, Aleksander Araszkiewicz, Stanisław Jankiewicz, Anna Smukowska-Gorynia, Marek Grygier, Magdalena Janus, Maciej Lesiak, and Tatiana Mularek-Kubzdela. 2022. "Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension" Journal of Clinical Medicine 11, no. 3: 625. https://doi.org/10.3390/jcm11030625
APA StyleSławek-Szmyt, S., Araszkiewicz, A., Jankiewicz, S., Smukowska-Gorynia, A., Grygier, M., Janus, M., Lesiak, M., & Mularek-Kubzdela, T. (2022). Association of Electrocardiographic Signs of Right Ventricular Hypertrophy and Clot Localization in Chronic Thromboembolic Pulmonary Hypertension. Journal of Clinical Medicine, 11(3), 625. https://doi.org/10.3390/jcm11030625








