Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
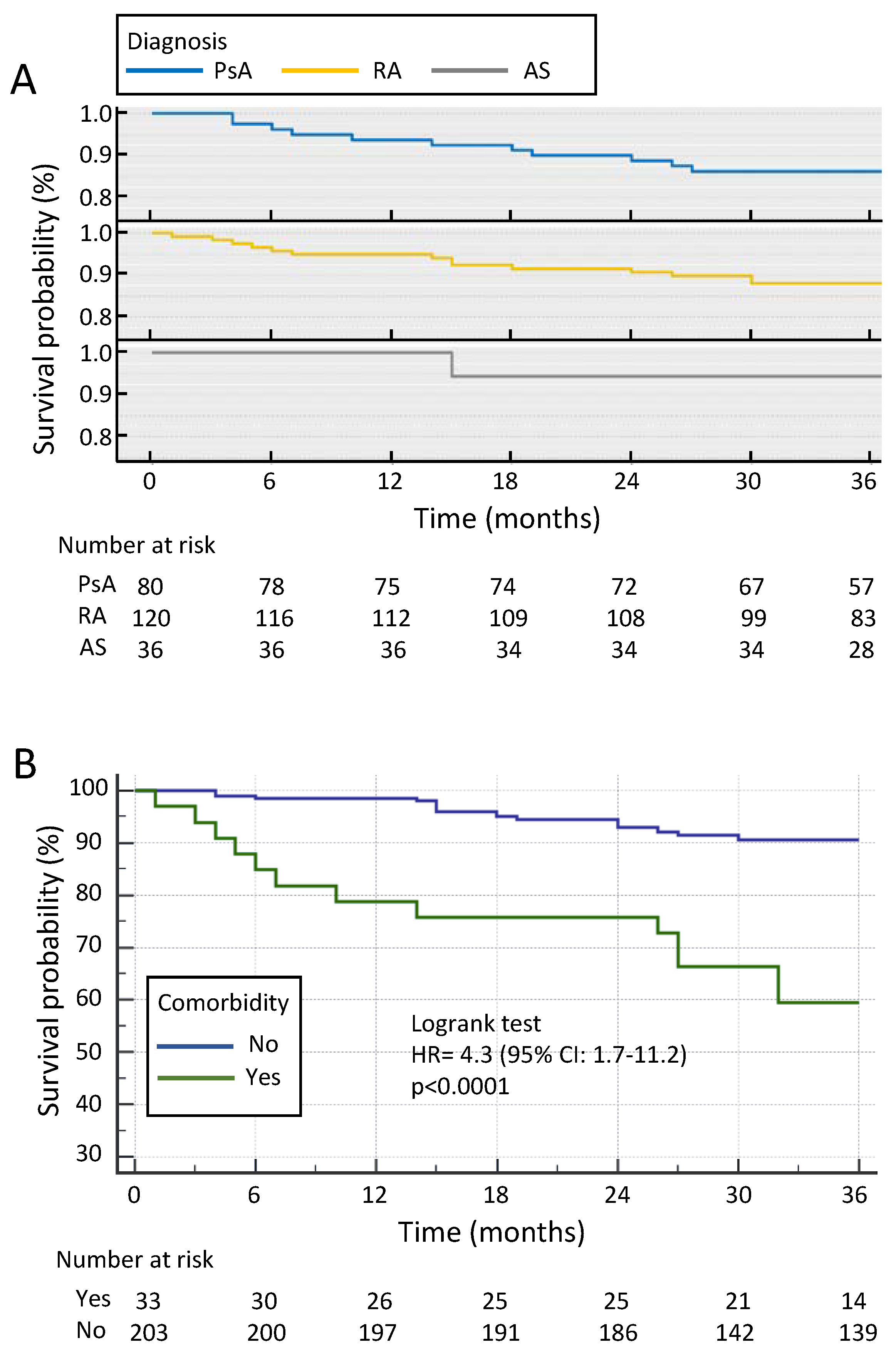
3.2. Drug Survival
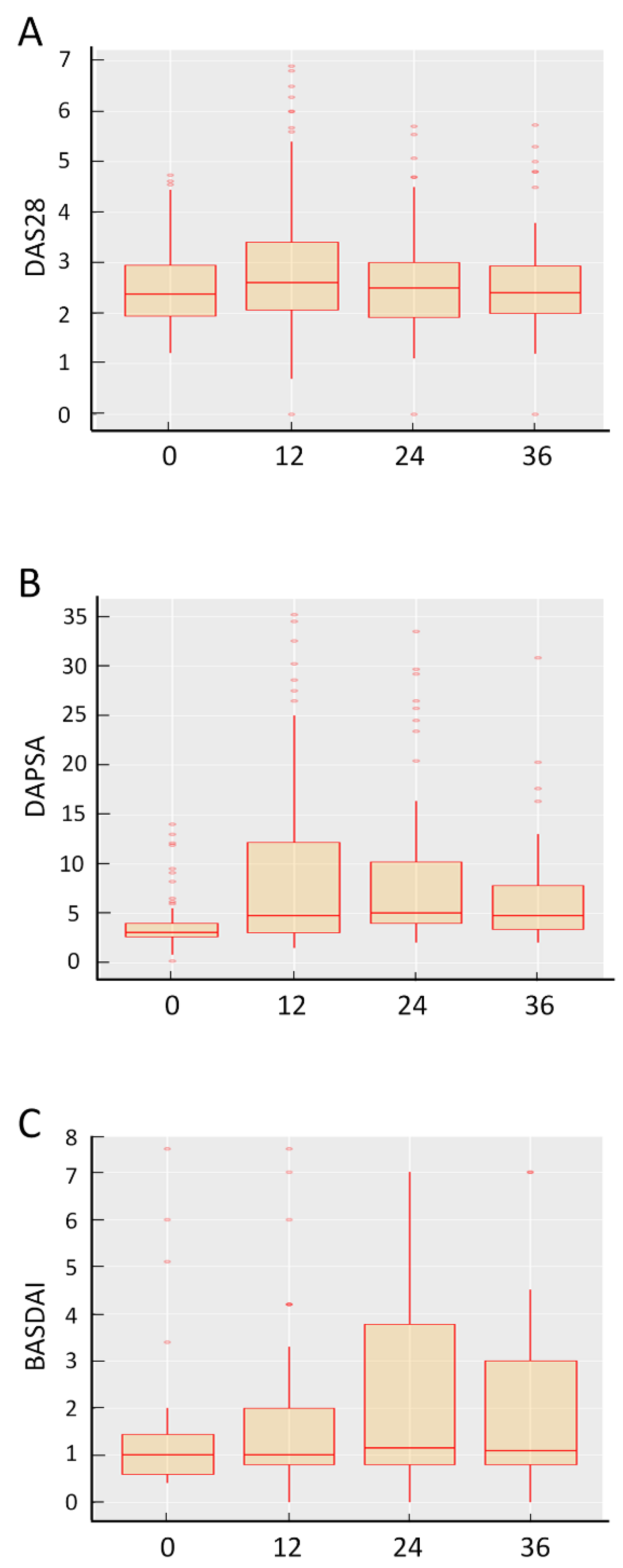
3.3. Effectiveness of SB4
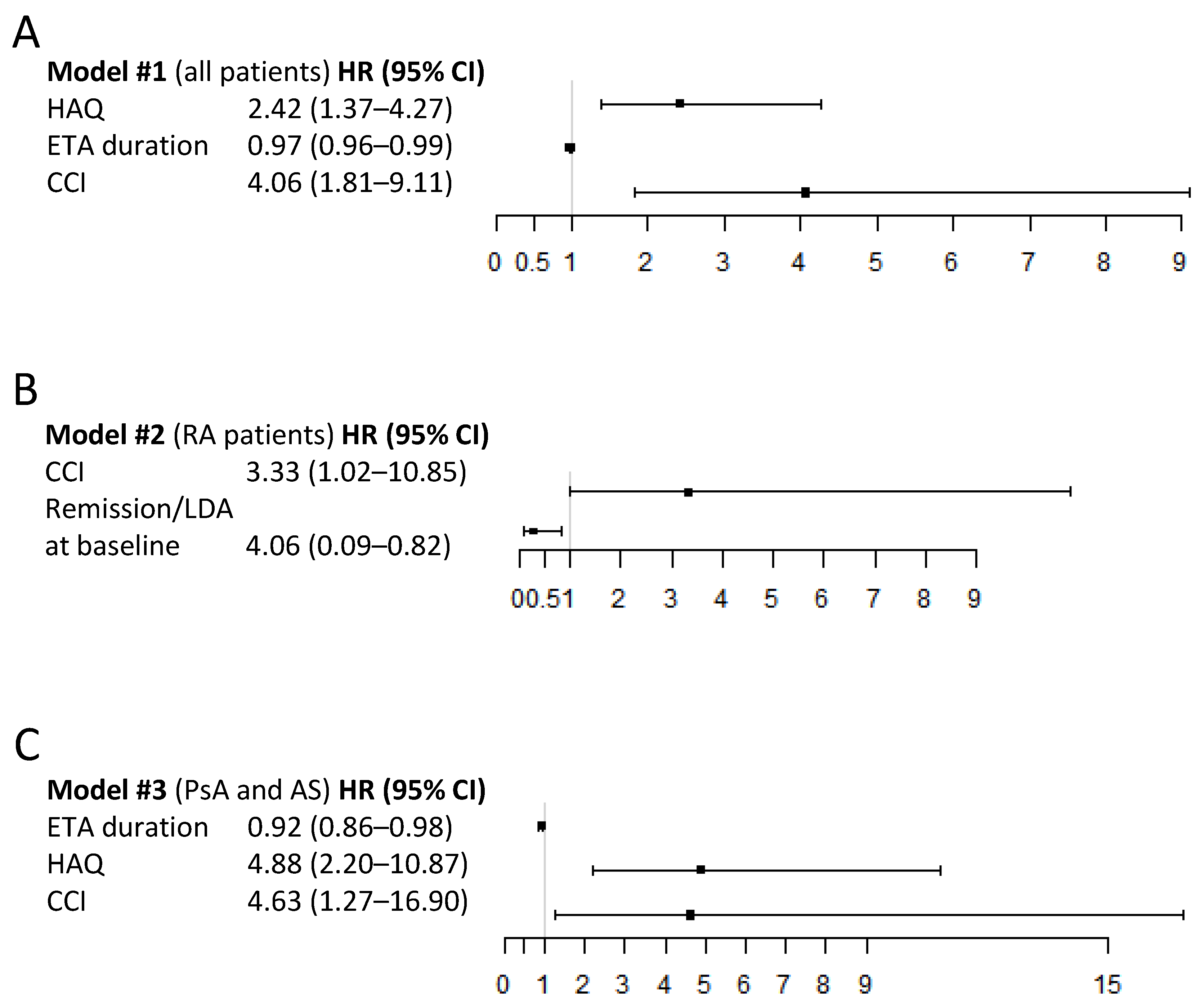
3.4. Predictors of SB4 Discontinuation
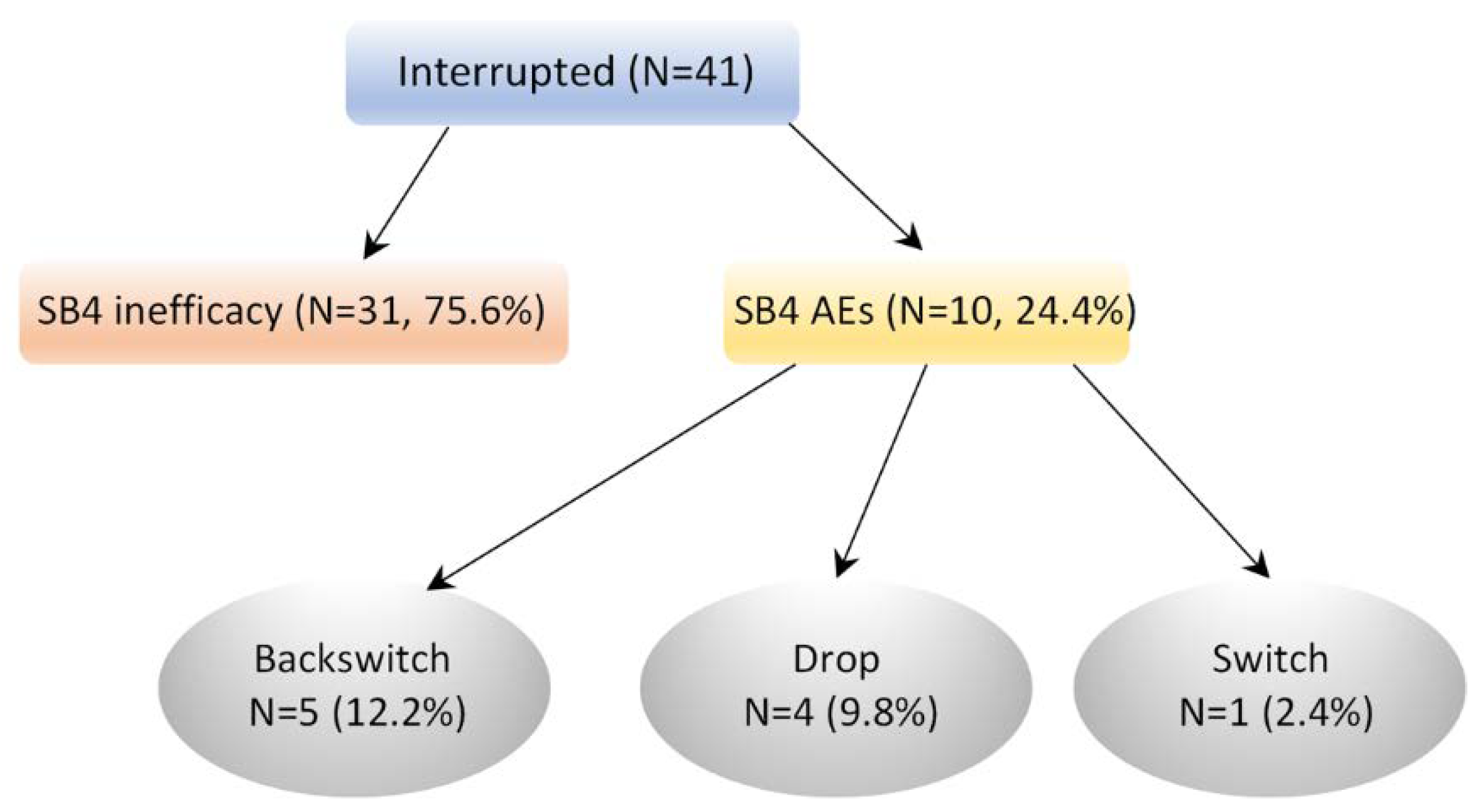
3.5. Reasons for Discontinuation
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Braun, J.; Dougados, M.; Emery, P.; Fitzgerald, O.; Helliwell, P.; Kavanaugh, A.; Kvien, T.K.; Landewé, R.; Luger, T.; et al. Treating Spondyloarthritis, Including Ankylosing Spondylitis and Psoriatic Arthritis, to Target: Recommendations of an International Task Force. Ann. Rheum. Dis. 2014, 73, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid Arthritis. Lancet Lond. Engl. 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Manara, M.; Prevete, I.; Marchesoni, A.; D’Angelo, S.; Cauli, A.; Zanetti, A. The Italian Society for Rheumatology Recommendations for the Management of Axial Spondyloarthritis. Reumatismo 2021, 73, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E.; Michaud, K. Epidemiological Studies in Incidence, Prevalence, Mortality, and Comorbidity of the Rheumatic Diseases. Arthritis Res. Ther. 2009, 11, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolwijk, C.; van Onna, M.; Boonen, A.; van Tubergen, A. Global Prevalence of Spondyloarthritis: A Systematic Review and Meta-Regression Analysis. Arthritis Care Res. 2016, 68, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Scotti, L.; Franchi, M.; Marchesoni, A.; Corrao, G. Prevalence and Incidence of Psoriatic Arthritis: A Systematic Review and Meta-Analysis. In Seminars in Arthritis and Rheumatism; WB Saunders: Philadelphia, PA, USA, 2018. [Google Scholar] [CrossRef]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The Global Prevalence of Rheumatoid Arthritis: A Meta-Analysis Based on a Systematic Review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, M.A.; Cicala, G.; Cutroneo, P.M.; Gerratana, E.; Palleria, C.; De Sarro, C.; Vero, A.; Iannone, L.; Manti, A.; Russo, E.; et al. Safety Profile of Biologics Used in Rheumatology: An Italian Prospective Pharmacovigilance Study. J. Clin. Med. 2020, 9, 1227. [Google Scholar] [CrossRef]
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; Sarro, C.D.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety Profile of Biologic Drugs for Psoriasis in Clinical Practice: An Italian Prospective Pharmacovigilance Study. PLoS ONE 2020, 15, e0241575. [Google Scholar] [CrossRef]
- Ma, X.; Xu, S. TNF Inhibitor Therapy for Rheumatoid Arthritis. Biomed. Rep. 2013, 1, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Urquhart, L. Top Product Forecasts for 2019. Nat. Rev. Drug Discov. 2019, 18, 91. [Google Scholar] [CrossRef]
- Food and Drug Administration. Biosimilar and Interchangeable Biologics: More Treatment Choices; Food and Drug Administration: Silver Spring, MD, USA, 2021.
- Anonymous. Benepali. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/benepali (accessed on 15 October 2021).
- Lee, Y.J.; Shin, D.; Kim, Y.; Kang, J.; Gauliard, A.; Fuhr, R. A Randomized Phase I Pharmacokinetic Study Comparing SB4 and Etanercept Reference Product (Enbrel®) in Healthy Subjects. Br. J. Clin. Pharmacol. 2016, 82, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, P.; Vencovský, J.; Sylwestrzak, A.; Leszczyński, P.; Porawska, W.; Baranauskaite, A.; Tseluyko, V.; Zhdan, V.M.; Stasiuk, B.; Milasiene, R.; et al. A Phase III Randomised, Double-Blind, Parallel-Group Study Comparing SB4 with Etanercept Reference Product in Patients with Active Rheumatoid Arthritis despite Methotrexate Therapy. Ann. Rheum. Dis. 2017, 76, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, P.; Vencovský, J.; Sylwestrzak, A.; Leszczynski, P.; Porawska, W.; Baranauskaite, A.; Tseluyko, V.; Zhdan, V.M.; Stasiuk, B.; Milasiene, R.; et al. 52-Week Results of the Phase 3 Randomized Study Comparing SB4 with Reference Etanercept in Patients with Active Rheumatoid Arthritis. Rheumatol. Oxf. Engl. 2017, 56, 2093–2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, P.; Vencovský, J.; Sylwestrzak, A.; Leszczyński, P.; Porawska, W.; Stasiuk, B.; Hilt, J.; Mosterova, Z.; Cheong, S.Y.; Ghil, J. Long-Term Efficacy and Safety in Patients with Rheumatoid Arthritis Continuing on SB4 or Switching from Reference Etanercept to SB4. Ann. Rheum. Dis. 2017, 76, 1986–1991. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; Feldman, S.R.; Emery, P.; Ghil, J.; Keum, J.W.; Cheong, S.Y.; Hong, E. Comparison of Injection-Site Reactions between the Etanercept Biosimilar SB4 and the Reference Etanercept in Patients with Rheumatoid Arthritis from a Phase III Study. Br. J. Dermatol. 2018, 178, e215–e216. [Google Scholar] [CrossRef] [Green Version]
- Glintborg, B.; Loft, A.G.; Omerovic, E.; Hendricks, O.; Linauskas, A.; Espesen, J.; Danebod, K.; Jensen, D.V.; Nordin, H.; Dalgaard, E.B.; et al. To Switch or Not to Switch: Results of a Nationwide Guideline of Mandatory Switching from Originator to Biosimilar Etanercept. One-Year Treatment Outcomes in 2061 Patients with Inflammatory Arthritis from the DANBIO Registry. Ann. Rheum. Dis. 2019, 78, 192–200. [Google Scholar] [CrossRef]
- Giunta, A.; Manfreda, V.; Esposito, M.; Del Duca, E.; Bianchi, L. Etanercept Biosimilar SB4 in the Treatment of Plaque-Type Psoriasis and Psoriatic Arthritis: A Single-Centre, Observational, Retrospective, Real-Life Study. Br. J. Dermatol. 2019, 181, 1078–1079. [Google Scholar] [CrossRef]
- Bonifati, C.; De Felice, C.; Lora, V.; Morrone, A.; Graceffa, D. Effectiveness of Etanercept Biosimilar SB4 in Maintaining Low Disease Activity in Patients with Psoriatic Arthritis Switched from Etanercept Originator: An Open-Label One Year Study. J. Dermatol. Treat. 2020, 31, 687–691. [Google Scholar] [CrossRef]
- Pescitelli, L.; Lazzeri, L.; Di Cesare, A.; Tripo, L.; Ricceri, F.; Prignano, F. Clinical Experience with the Etanercept Biosimilar SB4 in Psoriatic Patients. Int. J. Clin. Pharm. 2019, 41, 9–12. [Google Scholar] [CrossRef]
- Bruni, C.; Gentileschi, S.; Pacini, G.; Baldi, C.; Capassoni, M.; Tofani, L.; Bardelli, M.; Cometi, L.; Cantarini, L.; Nacci, F.; et al. The Switch from Etanercept Originator to SB4: Data from a Real-Life Experience on Tolerability and Persistence on Treatment in Joint Inflammatory Diseases. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20964031. [Google Scholar] [CrossRef]
- AIFA. Secondo Position Paper AIFA Sui Farmaci Biosimilari. 2018. Available online: https://www.aifa.gov.it/sites/default/files/pp_biosimilari_27.03.2018.pdf (accessed on 21 June 2021).
- Regione Piemonte. Farmaci Biosimilari. 2017. Available online: https://www.regione.piemonte.it/web/sites/default/files/media/documenti/2019-03/farmaci_biosimilari_linee_indirizzo2.docx.pdf (accessed on 21 June 2021).
- Ursula, A. Updating Position Statement from the European League Against Rheumatism (EULAR) Standing Committee of People with Arthritis/Rheumatism in Europe (PARE) August 2018. Biosimilars—Position Paper. 2018. Available online: https://www.eular.org/myUploadData/files/biosimilars_paper_updated_2018_09_14_dw.pdf (accessed on 25 June 2021).
- Kay, J.; Schoels, M.M.; Dörner, T.; Emery, P.; Kvien, T.K.; Smolen, J.S.; Breedveld, F.C. Task Force on the Use of Biosimilars to Treat Rheumatological Diseases Consensus-Based Recommendations for the Use of Biosimilars to Treat Rheumatological Diseases. Ann. Rheum. Dis. 2018, 77, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, P.L.C.M. The Development of the Disease Activity Score (DAS) and the Disease Activity Score Using 28 Joint Counts (DAS28). Clin. Exp. Rheumatol. 2014, 32, S65–S74. [Google Scholar]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A New Approach to Defining Disease Status in Ankylosing Spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- Bruce, B.; Fries, J.F. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol. 2005, 23, S14–S18. [Google Scholar]
- Ramiro, S.; Landewé, R.; van der Heijde, D.; Harrison, D.; Collier, D.; Michaud, K. Discontinuation Rates of Biologics in Patients with Rheumatoid Arthritis: Are TNF Inhibitors Different from Non-TNF Inhibitors? RMD Open 2015, 1, e000155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prior-Español, A.; Sánchez-Piedra, C.; Campos, J.; Manero, F.J.; Pérez-García, C.; Bohórquez, C.; Busquets-Pérez, N.; Blanco-Madrigal, J.M.; Díaz-Torne, C.; Sánchez-Alonso, F.; et al. Clinical Factors Associated with Discontinuation of Ts/BDMARDs in Rheumatic Patients from the BIOBADASER III Registry. Sci. Rep. 2021, 11, 11091. [Google Scholar] [CrossRef]
- Iannone, F.; Salaffi, F.; Fornaro, M.; Di Carlo, M.; Gentileschi, S.; Cantarini, L.; Lopalco, G. Influence of Baseline Modified Rheumatic Disease Comorbidity Index (MRDCI) on Drug Survival and Effectiveness of Biological Treatment in Patients Affected with Rheumatoid Arthritis, Spondyloarthritis and Psoriatic Arthritis in Real-World Settings. Eur. J. Clin. Investig. 2018, 48, e13013. [Google Scholar] [CrossRef]
- Lubrano, E.; Mesina, F.; Caporali, R. Clinical Remission in Rheumatoid Arthritis and Psoriatic Arthritis. Clin. Exp. Rheumatol. 2018, 36, 900–910. [Google Scholar]
- Parkinson, J.T.; Foley, É.M.; Jadon, D.R.; Khandaker, G.M. Depression in Patients with Spondyloarthritis: Prevalence, Incidence, Risk Factors, Mechanisms and Management. Ther. Adv. Musculoskelet. Dis. 2020, 12, 1759720X20970028. [Google Scholar] [CrossRef]
- Sakr, B.R.; Mohamed, H.E.; Effat, D.A. Relationship of Adherence to Treatment with Disease Activity, Physical Function, Quality of Life, Treatment Satisfaction and Beliefs in Axial Spondyloarthritis Patients. Egypt. Rheumatol. 2022, 44, 191–195. [Google Scholar] [CrossRef]
- Zhao, S.; Thong, D.; Miller, N.; Duffield, S.J.; Hughes, D.M.; Chadwick, L.; Goodson, N.J. The Prevalence of Depression in Axial Spondyloarthritis and Its Association with Disease Activity: A Systematic Review and Meta-Analysis. Arthritis Res. Ther. 2018, 20, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tweehuysen, L.; Huiskes, V.J.B.; van den Bemt, B.J.F.; Vriezekolk, J.E.; Teerenstra, S.; van den Hoogen, F.H.J.; van den Ende, C.H.; den Broeder, A.A. Open-Label, Non-Mandatory Transitioning from Originator Etanercept to Biosimilar SB4. Arthritis Rheumatol. 2018, 70, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Krüger, K.; Cantagrel, A.; Abad Hernández, M.A.; Freudensprung, U.; Farouk Rezk, M.; Addison, J. BENEFIT: Real-World Effectiveness of SB4 after Transition from Reference Etanercept in Rheumatoid Arthritis and Axial Spondyloarthritis. Clin. Exp. Rheumatol. 2021, 39, 365–371. [Google Scholar] [PubMed]
- Ebbers, H.C.; Pieper, B.; Issa, A.; Addison, J.; Freudensprung, U.; Rezk, M.F. Real-World Evidence on Etanercept Biosimilar SB4 in Etanercept-Naïve or Switching Patients: A Systematic Review. Rheumatol. Ther. 2019, 6, 317–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.; Flora, K.; Penn, H. Clinical Outcomes of a Multi-Disciplinary Switching Programme to Biosimilar Etanercept for Patients with Rheumatoid Arthritis. Rheumatology 2018, 57, 143–144. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Total Cohort n = 236 | RA n = 120 | PsA n = 80 | AS n = 36 |
|---|---|---|---|---|
| n (%) | 236 (100) | 120 (50.8) | 80 (33.9) | 36 (15.3) |
| Male gender (%) | 85 (36) | 23 (19.2) | 36 (45) | 26 (72.2) |
| Age (years) | 60.7 ± 13.8 | 61.8 ± 14.6 | 61.8 ± 12.8 | 54.9 ± 11.7 |
| BMI (Kg/M2) | 24.5 ± 4.2 | 24 ± 3.8 | 24.5 ± 3.9 | 24.7 ± 3.9 |
| Disease duration (years) | 15.9 ± 9.5 | 17.2 ± 10.6 | 13.8 ± 6.6 | 14.9 ± 9.5 |
| Comorbidities, n (%) | 33 (14) | 17 (14) | 15 (19) | 1 (3) |
| Diabetes | 13 (5.5) | 7 (5.8) | 6 (7.5) | - |
| Chronic bronchitis | 6 (2.5) | 3 (2.5) | 3 (3.8) | - |
| Cerebrovascular disease | 5 (2.1) | 1 (0.83) | 3 (3.8) | 1 (2.8) |
| Liver disease | 2 (0.85) | 2 (1.7) | - | - |
| Myocardial infarction | 2 (0.85) | 2 (1.7) | ||
| Heart failure | 1 (0.42) | 1 (0.83) | ||
| Renal failure | 1 (0.42) | 1 (0.83) | - | |
| Connectivitis | 2 (0.85) | - | 2 (2.5) | - |
| Peptic ulcer | 1 (0.42) | 1 (1.3) | ||
| ACPA/RF (+/+), n (%) | - | 76 (63.3) | - | - |
| RF+, n (%) | - | 75 (62.5) | - | - |
| HLAB27+, n (%) | - | - | 12 (15) | 18 (50) |
| TJC (median (IQR) | 0 (0–1) | 0 (0–2) | 0 (0–1) | 0 (0–0) |
| SJC (median (IQR) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0 (0–0) |
| CRP (mg/L) | 1.7 ± 2.3 | 2.2 ± 1.9 | 1.3 ± 2.8 | 1.3 ± 1.9 |
| DAS 28 | - | 2.5 ± 0.7 | - | - |
| DAPSA | - | - | 3.7 ± 2.7 | - |
| BASDAI | - | - | - | 1.5 ± 1.6 |
| HAQ | 0.7 ± 0.6 | 0.7 ± 0.5 | 0.7 ± 0.6 | 0.8 ± 0.7 |
| Medication | ||||
| Combination therapy, n (%) | 132 (55.9) | 77 (64.2) | 43 (53.8) | 12 (33.3) |
| Prednisone, n (%) | 85 (36) | 57 (47.5) | 23 (28.7) | 5 (13.9) |
| Etanercept duration (months) | 49.74 ± 40.75 | 56.43 ± 41.27 | 25.00 ± 13.67 | 40.08 ± 37.78 |
| SB4 duration (months) | 38.42 ± 11.41 | 37.77 ± 11.84 | 13.72 ± 13.49 | 41.39 ± 8.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisi, S.; Becciolini, A.; Ditto, M.C.; Rozza, D.; Zanetti, A.; Laganà, A.; Peroni, C.L.; Centanaro Di Vittorio, C.; Degiovanni, R.; Realmuto, C.; et al. Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study. J. Clin. Med. 2022, 11, 621. https://doi.org/10.3390/jcm11030621
Parisi S, Becciolini A, Ditto MC, Rozza D, Zanetti A, Laganà A, Peroni CL, Centanaro Di Vittorio C, Degiovanni R, Realmuto C, et al. Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study. Journal of Clinical Medicine. 2022; 11(3):621. https://doi.org/10.3390/jcm11030621
Chicago/Turabian StyleParisi, Simone, Andrea Becciolini, Maria Chiara Ditto, Davide Rozza, Anna Zanetti, Angela Laganà, Clara Lisa Peroni, Chiara Centanaro Di Vittorio, Rosanna Degiovanni, Cristina Realmuto, and et al. 2022. "Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study" Journal of Clinical Medicine 11, no. 3: 621. https://doi.org/10.3390/jcm11030621
APA StyleParisi, S., Becciolini, A., Ditto, M. C., Rozza, D., Zanetti, A., Laganà, A., Peroni, C. L., Centanaro Di Vittorio, C., Degiovanni, R., Realmuto, C., Scirè, C. A., Priora, M., Di Donato, E., Santilli, D., Mozzani, F., Lucchini, G., Ariani, A., Gardelli, L., Girelli, F., ... Fusaro, E. (2022). Efficacy and Drug Survival after Switching from Etanercept to the Biosimilar SB4: A Real-Life Long-Term Study. Journal of Clinical Medicine, 11(3), 621. https://doi.org/10.3390/jcm11030621








