Temporary Right-Ventricular Assist Devices: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
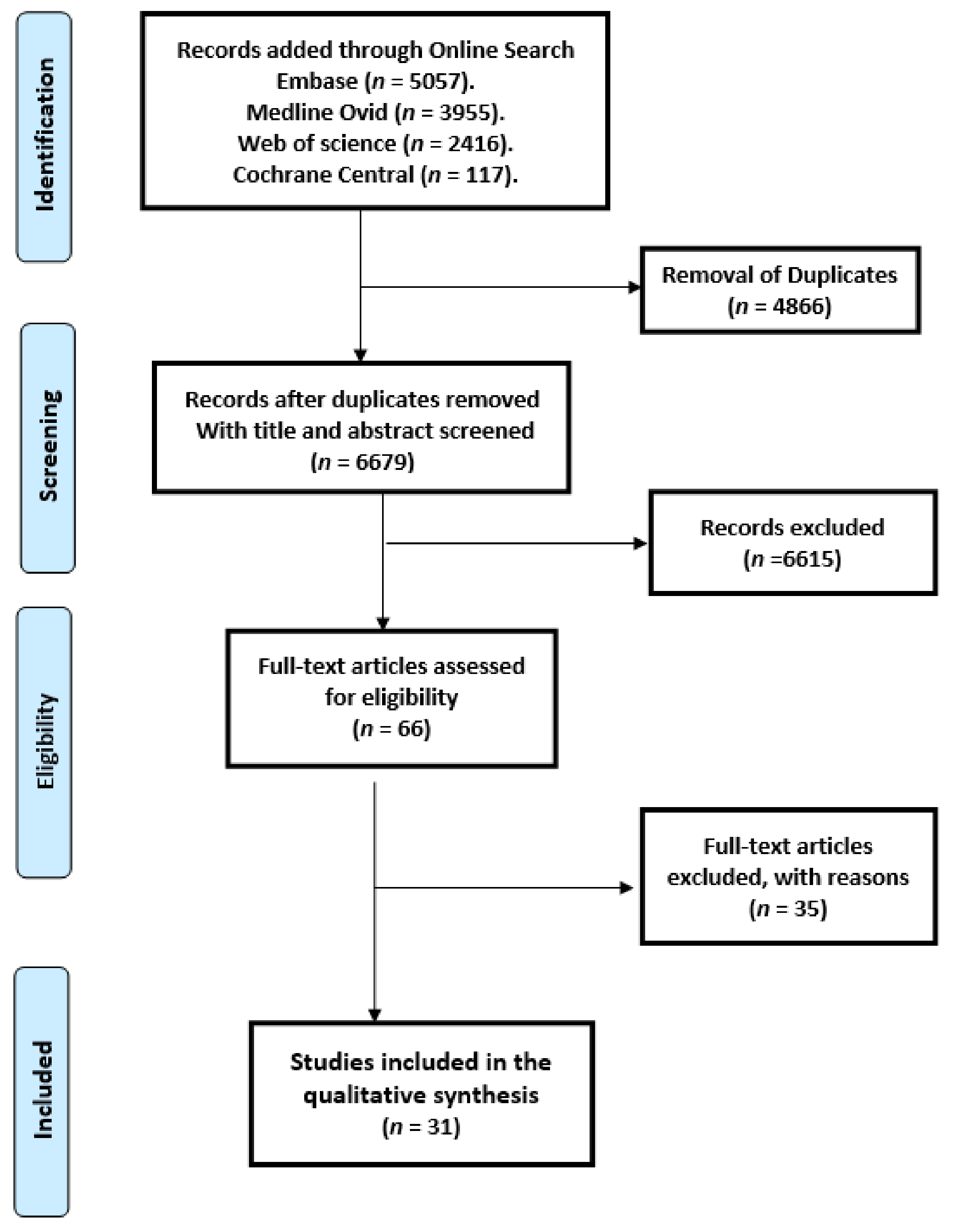
2.3. Study Selection
2.4. Data Extraction
3. Results
3.1. Patients’ Characteristics
3.2. Types of Temporary Right-Ventricular Assist Device (t-RVAD) and Approach to t-RVAD Implantation
3.2.1. ProtekDuo
3.2.2. Impella RP
3.2.3. Impella RD
3.2.4. TandemHeart RVAD (TH-RVAD)
3.2.5. Surgically Implanted t-RVADs
3.2.6. Timing of sRVAD Implantation
3.2.7. Oxy t-RVAD
3.3. Indication for t-RVAD Implantation (Patient’s Phenotypes)
3.4. Duration of t-RVAD Support
3.5. Survival Endpoints
3.6. Outcome Other Than Survival
3.7. Complications following t-RVAD Implantation
3.7.1. End Organ Dysfunction Post t-RVAD Implantation
3.7.2. Bleeding Post t-RVAD Implantation
3.7.3. Device Malfunction Post t-RVAD Implantation
3.7.4. Neurological Outcome Post t-RVAD Implantation
3.7.5. Sepsis Post-t-RAVD Implantation
3.7.6. Other Reported Complications
4. Discussion
- 1.
- We included 31 studies comprising 1598 patients in this systematic review
- 2.
- Successful t-RVAD weaning was reported in our review between 23% and 100%. Moreover, 30-day survival post temporary RAVD implantation ranged from 46% to 100%.
- 3.
- Evidence stems from non-randomized heterogeneous trials which makes the comparison between the different studies or pooling the data in meta-analysis is not possible.
- 4.
- Acute kidney injury, post-operative bleeding, stroke, and device malfunction were the most commonly reported complications.
- 5.
- Subgroup analyses are obviously not adequately powered to investigate the determinants of device success.
4.1. Efficacy of t-RVAD in This Systematic Review
4.2. Duration of t-RVAD Dupport
4.3. Safety of t-RVAD in This Systematic Review
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Search Strategy (Terms) Database | Items |
| embase.com | 5057 |
| Medline Ovid | 3705 |
| Web of science | 2416 |
| Cochrane CENTRAL | 117 |
| Total | 11,295 |
References
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef] [PubMed]
- Soliman, O.; Muslem, R.; Caliskan, K. Right heart failure syndrome. Aging 2018, 11, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Esposito, M.L.; Bader, Y.; Morine, K.J.; Kiernan, M.S.; Pham, D.T.; Burkhoff, D. Mechanical Circulatory Support Devices for Acute Right Ventricular Failure. Circulation 2017, 136, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Temporary assist device support for the right ventricle: Pre-implant and post-implant challenges. Heart Fail. Rev. 2018, 23, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.M.; Turner, M.E.; O’Neill, W.; Denfield, S.W.; Aghili, N.; Badiye, A.; Gandhi, R.; Tehrani, B.; Chang, G.; Oyama, J.K.; et al. Percutaneous Impella RP use for refractory right heart failure in adolescents and young adults—A multicenter U.S. experience. Catheter. Cardiovasc. Interv. 2020, 96, 376–381. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Oliveros, E.; Collado, F.M.; Poulin, M.F.; Seder, C.W.; March, R.; Kavinsky, C.J. Percutaneous Right Ventricular Assist Device Using the TandemHeart ProtekDuo: Real-World Experience. J. Invasive Cardiol. 2021, 33, E407–E411. [Google Scholar]
- Shekiladze, N.; Condado, J.F.; Sandesara, P.B.; Kim, J.H.; Devireddy, C.; McDaniel, M.; Babaliaros, V.; Samady, H.; Kumar, G.; Jaber, W.A. A single healthcare experience with Impella RP. Catheter. Cardiovasc. Interv. 2020, 97, E161–E167. [Google Scholar] [CrossRef]
- Salna, M.; Garan, A.R.; Kirtane, A.J.; Karmpaliotis, D.; Green, P.; Takayama, H.; Sanchez, J.; Kurlansky, P.; Yuzefpolskaya, M.; Colombo, P.C.; et al. Novel percutaneous dual-lumen cannula-based right ventricular assist device provides effective support for refractory right ventricular failure after left ventricular assist device implantation. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 499–506. [Google Scholar] [CrossRef]
- Kremer, J.; Farag, M.; Brcic, A.; Zubarevich, A.; Schamroth, J.; Kreusser, M.M.; Karck, M.; Ruhparwar, A.; Schmack, B. Temporary right ventricular circulatory support following right ventricular infarction: Results of a groin-free approach. ESC Heart Fail. 2020, 7, 2853–2861. [Google Scholar] [CrossRef]
- Badu, B.; Cain, M.T.; Durham, L.A.; Joyce, L.D.; Sundararajan, S.; Gaglianello, N.; Ishizawar, D.; Saltzberg, M.; Mohammed, A.; Joyce, D.L. A Dual-Lumen Percutaneous Cannula for Managing Refractory Right Ventricular Failure. ASAIO J. 2020, 66, 915–921. [Google Scholar] [CrossRef]
- Vierecke, J.; Gahl, B.; De By, T.M.M.H.; Antretter, H.; Beyersdorf, F.; Caliskan, K.; Krachak, V.; Loforte, A.; Potapov, E.; Schoenrath, F.; et al. Results of primary biventricular support: An analysis of data from the EUROMACS registry. Eur. J. Cardio-Thorac. Surg. 2019, 56, 1037–1045. [Google Scholar] [CrossRef]
- Schmack, B.; Farag, M.; Kremer, J.; Grossekettler, L.; Brcic, A.; Raake, P.W.; Kreusser, M.M.; Goldwasser, R.; Popov, A.F.; Mansur, A.; et al. Results of concomitant groin-free percutaneous temporary RVAD support using a centrifugal pump with a double-lumen jugular venous cannula in LVAD patients. J. Thorac. Dis. 2019, 11, S913–S920. [Google Scholar] [CrossRef]
- Khorsandi, M.; Schroder, J.; Daneshmand, M.; Bishawi, M.; Bouamra, O.; Winterton, P.; Choi, A.Y.; Patel, C.; Rogers, J.; Del Rio, J.M.; et al. Outcomes After Extracorporeal Right Ventricular Assist Device Combined With Durable Left Ventricular Assist Device Support. Ann. Thorac. Surg. 2019, 107, 1768–1774. [Google Scholar] [CrossRef]
- Jaidka, A.; De, S.; Nagpal, A.D.; Chu, M.W.A. Prophylactic Right Ventricular Assist Device for High-Risk Patients Undergoing Valve Corrective Surgery. CJC Open 2019, 1, 19–27. [Google Scholar] [CrossRef]
- Coromilas, E.J.; Takeda, K.; Ando, M.; Cevasco, M.; Green, P.; Karmpaliotis, D.; Kirtane, A.; Topkara, V.K.; Yuzefpolskaya, M.; Takayama, H.; et al. Comparison of Percutaneous and Surgical Right Ventricular Assist Device Support After Durable Left Ventricular Assist Device Insertion. J. Card. Fail. 2019, 25, 105–113. [Google Scholar] [CrossRef]
- Ravichandran, A.K.; Baran, D.A.; Stelling, K.; Cowger, J.A.; Salerno, C.T. Outcomes with the tandem protek duo dual-lumen percutaneous right ventricular assist device. ASAIO J. 2018, 64, 570–572. [Google Scholar] [CrossRef]
- Bhama, J.K.; Bansal, U.; Winger, D.G.; Teuteberg, J.J.; Bermudez, C.; Kormos, R.L.; Bansal, A. Clinical experience with temporary right ventricular mechanical circulatory support. J. Thorac. Cardiovasc. Surg. 2018, 156, 1885–1891. [Google Scholar] [CrossRef]
- Anderson, M.; Morris, D.L.; Tang, D.; Batsides, G.; Kirtane, A.; Hanson, I.; Meraj, P.; Kapur, N.K.; O’Neill, W. Outcomes of patients with right ventricular failure requiring short-term hemodynamic support with the Impella RP device. J. Heart Lung Transplant. 2018, 37, 1448–1458. [Google Scholar] [CrossRef]
- Yoshioka, D.; Takayama, H.; Garan, R.A.; Topkara, V.K.; Han, J.; Kurlansky, P.; Yuzefpolskaya, M.; Colombo, P.C.; Naka, Y.; Takeda, K. Contemporary outcome of unplanned right ventricular assist device for severe right heart failure after continuous-flow left ventricular assist device insertion. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 828–834. [Google Scholar] [CrossRef]
- Schaefer, A.; Reichart, D.; Bernhardt, A.M.; Kubik, M.; Barten, M.J.; Wagner, F.M.; Reichenspurner, H.; Philipp, S.A.; Deuse, T. Outcomes of Minimally Invasive Temporary Right Ventricular Assist Device Support for Acute Right Ventricular Failure during Minimally Invasive Left Ventricular Assist Device Implantation. ASAIO J. 2017, 63, 546–550. [Google Scholar] [CrossRef]
- Kiernan, M.S.; Grandin, E.W.; Brinkley, M.; Kapur, N.K.; Pham, D.T.; Ruthazer, R.; Eduardo Rame, J.; Atluri, P.; Birati, E.Y.; Oliveira, G.H.; et al. Early Right Ventricular Assist Device Use in Patients Undergoing Continuous-Flow Left Ventricular Assist Device Implantation: Incidence and Risk Factors from the Interagency Registry for Mechanically Assisted Circulatory Support. Circ. Heart Fail. 2017, 10, e003863. [Google Scholar] [CrossRef]
- Leidenfrost, J.; Prasad, S.; Itoh, A.; Lawrance, C.P.; Bell, J.M.; Silvestry, S.C. Right ventricular assist device with membrane oxygenator support for right ventricular failure following implantable left ventricular assist device placement. Eur. J. Cardio-Thorac. Surg. 2016, 49, 73–77. [Google Scholar] [CrossRef]
- Deschka, H.; Holthaus, A.J.; Sindermann, J.R.; Welp, H.; Schlarb, D.; Monsefi, N.; Martens, S.; Scherer, M. Can Perioperative Right Ventricular Support Prevent Postoperative Right Heart Failure in Patients with Biventricular Dysfunction Undergoing Left Ventricular Assist Device Implantation? J. Cardiothorac. Vasc. Anesth. 2016, 30, 619–626. [Google Scholar] [CrossRef]
- Saeed, D.; Maxhera, B.; Kamiya, H.; Lichtenberg, A.; Albert, A. Alternative right ventricular assist device implantation technique for patients with perioperative right ventricular failure. J. Thorac. Cardiovasc. Surg. 2015, 149, 927–932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patil, N.P.; Mohite, P.N.; Sabashnikov, A.; Dhar, D.; Weymann, A.; Zeriouh, M.; Hards, R.; Hedger, M.; De Robertis, F.; Bahrami, T.; et al. Preoperative predictors and outcomes of right ventricular assist device implantation after continuous-flow left ventricular assist device implantation. J. Thorac. Cardiovasc. Surg. 2015, 150, 1651–1658. [Google Scholar] [CrossRef]
- Takeda, K.; Naka, Y.; Yang, J.A.; Uriel, N.; Colombo, P.C.; Jorde, U.P.; Takayama, H. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J. Heart Lung Transplant. 2014, 33, 141–148. [Google Scholar] [CrossRef]
- Cheung, A.W.; White, C.W.; Davis, M.K.; Freed, D.H. Short-term mechanical circulatory support for recovery from acute right ventricular failure: Clinical outcomes. J. Heart Lung Transplant. 2014, 33, 794–799. [Google Scholar] [CrossRef]
- Aissaoui, N.; Morshuis, M.; Paluszkiewicz, L.; Lauenroth, V.; Börgermann, J.; Gummert, J. Comparison of biventricular and left ventricular assist devices for the management of severe right ventricular dysfunction in patients with end-stage heart failure. ASAIO J. 2014, 60, 400–406. [Google Scholar] [CrossRef]
- Loforte, A.; Stepanenko, A.; Potapov, E.V.; Musumeci, F.; Dranishnikov, N.; Schweiger, M.; Montalto, A.; Pasic, M.; Weng, Y.; Dandel, M.; et al. Temporary right ventricular mechanical support in high-risk left ventricular assist device recipients versus permanent biventricular or total artificial heart support. Artif. Organs 2013, 37, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lazar, J.F.; Swartz, M.F.; Schiralli, M.P.; Schneider, M.; Pisula, B.; Hallinan, W.; Hicks, G.L., Jr.; Massey, H.T. Survival after left ventricular assist device with and without temporary right ventricular support. Ann. Thorac. Surg. 2013, 96, 2155–2159. [Google Scholar] [CrossRef] [PubMed]
- Khani-Hanjani, A.; Loor, G.; Chamogeorgakis, T.; Shafii, A.; Mountis, M.; Hanna, M.; Soltesz, E.; Gonzalez-Stawinski, G.V. Case series using the rotaflow system as a temporary right ventricular assist device after heartmate II implantation. ASAIO J. 2013, 59, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Paruchuri, V.; Jagannathan, A.; Steinberg, D.; Chakrabarti, A.K.; Pinto, D.; Aghili, N.; Najjar, S.; Finley, J.; Orr, N.M.; et al. Mechanical circulatory support for right ventricular failure. JACC Heart Fail. 2013, 1, 127–134. [Google Scholar] [CrossRef]
- Schopka, S.; Haneya, A.; Rupprecht, L.; Camboni, D.; Schmid, C.; Hilker, M. Temporary right heart assist: A minimally invasive approach. Artif. Organs 2012, 36, 700–704. [Google Scholar] [CrossRef]
- Saito, S.; Sakaguchi, T.; Miyagawa, S.; Nishi, H.; Yoshikawa, Y.; Fukushima, S.; Daimon, T.; Sawa, Y. Recovery of right heart function with temporary right ventricular assist using a centrifugal pump in patients with severe biventricular failure. J. Heart Lung Transpl. 2012, 31, 858–864. [Google Scholar] [CrossRef]
- Loforte, A.; Montalto, A.; Ranocchi, F.; Della Monica, P.L.; Casali, G.; Lappa, A.; Contento, C.; Musumeci, F. Levitronix CentriMag third-generation magnetically levitated continuous flow pump as bridge to solution. ASAIO J. 2011, 57, 247–253. [Google Scholar] [CrossRef]
- Lo Coco, V.; De Piero, M.E.; Massimi, G.; Chiarini, G.; Raffa, G.M.; Kowalewski, M.; Maessen, J.; Lorusso, R. Right ventricular failure after left ventricular assist device implantation: A review of the literature. J. Thorac. Dis. 2021, 13, 1256–1269. [Google Scholar] [CrossRef]
- Impella® RP with the Automated Impella® Controller Circulatory Support System. Instructions for use & Clinical Reference Manual. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf14/H140001d.pdf (accessed on 28 October 2021).
- TandemLife. Indication for Use Safety Inforamtion. Available online: http://www.tandemlife.com/indications-use-safety-information/ (accessed on 27 October 2021).
- CentriMag™ Blood Pump Instructions For Use (IFU). Available online: http://www.accessdata.fda.gov/cdrh_docs/pdf17/P170038C.pdf (accessed on 28 October 2021).
- Anderson, M.B.; Goldstein, J.; Milano, C.; Morris, L.D.; Kormos, R.L.; Bhama, J.; Kapur, N.K.; Bansal, A.; Garcia, J.; Baker, J.N.; et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J. Heart Lung Transplant. 2015, 34, 1549–1560. [Google Scholar] [CrossRef]
- Takeda, K.; Naka, Y.; Yang, J.A.; Uriel, N.; Colombo, P.C.; Jorde, U.P.; Takayama, H. Timing of temporary right ventricular assist device insertion for severe right heart failure after left ventricular assist device implantation. ASAIO J. 2013, 59, 564–569. [Google Scholar] [CrossRef]
- Aissaoui, N.; Morshuis, M.; Schoenbrodt, M.; Hakim Meibodi, K.; Kizner, L.; Börgermann, J.; Gummert, J. Temporary right ventricular mechanical circulatory support for the management of right ventricular failure in critically ill patients. J. Thorac. Cardiovasc. Surg. 2013, 146, 186–191. [Google Scholar] [CrossRef]
- Kapur, N.K.; Paruchuri, V.; Korabathina, R.; Al-Mohammdi, R.; Mudd, J.O.; Prutkin, J.; Esposito, M.; Shah, A.; Kiernan, M.S.; Sech, C.; et al. Effects of a percutaneous mechanical circulatory support device for medically refractory right ventricular failure. J. Heart Lung Transplant. 2011, 30, 1360–1367. [Google Scholar] [CrossRef]
- Bhama, J.K.; Kormos, R.L.; Toyoda, Y.; Teuteberg, J.J.; McCurry, K.R.; Siegenthaler, M.P. Clinical Experience Using the Levitronix CentriMag System for Temporary Right Ventricular Mechanical Circulatory Support. J. Heart Lung Transplant. 2009, 28, 971–976. [Google Scholar] [CrossRef]
| Author | Indication for t-RVAD; [Underlying Disease for LVAD (%)]/Subgroups (No.) | Patients No. | Male (%) | Age * | Follow Up * |
|---|---|---|---|---|---|
| Aissaoui et al., 2014 [30] | Post-LVAD | 57 | nr | 54 ± 14 | nr |
| Anderson et al., 2018 [20] | Post-LVAD (31); PCCS (13); HTX (7); post-RV infarction (9) | 60 | 68 | 59 ± 15 | nr |
| Badu et al., 2020 [12] | PCCS (18); CM (12); respiratory failure (10) | 40 | 73 | 55 ± 16 | nr |
| Bhama et al., 2018 [19] | Post-LVAD (42) [ICM (52%), DCM (43%), other (5%)]; PCCS (13); HTX (25) | 80 | nr | nr | nr |
| Cheung et al., 2014 [29] | Post-LVAD (2); PCCS (4); myocarditis (2); post-RV infarction (7); HTx (3) | 18 | 67 | 57 ± 10 | 365 |
| Coromilas et al., 2019 [17] | Post-LVAD/pRVAD (19); sRVAD (21) | 40 | 85 | 59 ± 12 | nr |
| Deschka et al., 2016 [25] | Post-LVAD [ICM (56%), DCM (40%), chronic rejection (4%)] | 25 | 80 | 55 ± 12 | 575 ± 541 |
| Jaidka et al., 2019 [16] | Pre-valvular surgery | 10 | 40 | 66 ± 15 | nr |
| Kapur et al., 2013 [34] | Different indications/pRVAD (22); sRVAD (24) | 46 | nr | nr | nr |
| Khani-Hanjani et al., 2013 [33] | Post-LVAD [ICM (33%), DCM (67%)] | 12 | 84 | 51 (24–69) | 371 |
| Khorsandi et al., 2019 [15] | Post-LVAD/concurrent t-RVAD (29); staged t-RVAD (14) | 43 | 86 | 51 (19–76) | 453 (2–3560) |
| Kiernan et al., 2017 [23] | Post-LVAD | 386 | 79 | nr | nr |
| Kremer et al., 2020 [11] | Post-MI | 10 | 90 | nr | 96 ± 108 |
| Lazar et al., 2013 [32] | Post-LVAD | 34 | 68 | 52 ± 12 | nr |
| Leidenfrost et al., 2016 [24] | Post-LVAD/t-RVAD only (27) [ICM (80%]; t-RVAD-MO (12) | 27 | nr | 56 ± 15 | nr |
| Loforte et al., 2011 [37] | Post-LVAD (10); PCCS (9) | 19 | nr | nr | nr |
| Loforte et al., 2013 [31] | Post-LVAD [ICM (30%), DCM (55%), myocarditis (4%), others (11%)] | 46 | nr | 55 (25–70) | nr |
| Oliveros et al., 2021 [8] | Different indications, post LVAD (1) | 11 | 54 | 59 ± 16 | nr |
| Patil et al., 2015 [27] | Post-LVAD [ICM (11%), DCM (80%), myocarditis (3%), CHD (3%), PPCM (3%)] | 35 | 66 | 40 ± 15 | nr |
| Qureshi et al., 2020 [6] | Different indications | 12 | 67 | 18 | nr |
| Ravichandran et al., 2018 [18] | Different indication, post LVAD (12) | 17 | 76 | 56 ± 8 | nr |
| Saeed et al., 2015 [26] | Post-LVAD (17) [ICM (57%), DCM (24%), post MI (19%)]; PCCS (4) | 21 | 71 | 58 ± 14 | nr |
| Saito et al., 2012 [36] | Post LVAD | 26 | 62 | 33 ± 15 | nr |
| Salna et al., 2020 [10] | Post LVAD | 27 | 78 | 63 | 408 |
| Schaefer et al., 2017 [22] | Post-LVAD [ICM (20%), DCM (50%), myocarditis (10%), post-MI (20%)]; minimally invasive (10); sternotomy (11) | 21 | 10 | 50 ± 15 | 274 ± 179 |
| Schmack et al., 2019 [14] | Post-LVAD [ICM (55%), DCM (45%)] | 11 | 91 | 52 ± 13 | 215 ± 283 |
| Schopka et al., 2012 [35] | Different indications | 12 | 83 | nr | nr |
| Shekiladze et al., 2020 [9] | Post-LVAD (6); acute PE (9); PCCS (8); post-MI (11); non ischemic CM (5) | 39 | nr | 57 ± 16 | nr |
| Takeda et al., 2014 [28] | Post-LVAD [ICM (41%), DCM (41%), myocarditis (6.8%), others (11%)/weaning group (21); failure group (23) | 44 | nr | nr | nr |
| Vierecke et al., 2019 [13] | Post-LVAD [ICM (24%), DCM (26%), myocarditis (6%), CHD (1%)] | 342 | nr | 55 (46–62) | nr |
| Yoshioka et al., 2017 [21] | Post-LVAD [ICM (44%), DCM (56%)] | 27 | nr | 50 ± 15 | nr |
| Device | Features (Description) | Approach for Implantation and Configuration | Duration of Support | Advantages | Disadvantages (Limitations) |
|---|---|---|---|---|---|
| ProtekDuo | Dual lumen cannula and must connected to extracorporeal pump usually TandemHeart or less frequent CentriMag. | Percutaneously through IJV, inflow in the RA and outflow in the PA. | 30 days | Single venous access, IJV access allowing patient to remain ambulatory, oxygenator can be added. | May cause SVC syndrome with larger cannula size. |
| Impella RP | Intra-corporeal dual lumen cannula with microaxial pump with flow rate up to 4.5 L/min. | Percutaneously through Fem. V, inflow in the IVC and the outflow in the PA. | 14 days | Single venous access, small dimension of the machine. | No oxygenation capacity, femoral access limit the patient’s mobility. |
| CentriMag | Extracorporeal centrifugal pump up to 10 L/min. | 1—Surgically (sternotomy) via direct cannulation of RA (inflow) and PA (outflow). 2—Minimally invasive surgical approach via left-sided mini-thoracotomy to suture the outflow cannula of t-RVAD with PA and t-RAVD inflow via Fem. V. 3—By peripheral cannulation of the Fem. V and direct cannulation of the PA. 4—Percutaneously via ProtekDuo. 5—Percutaneously peripheral cannulation of the Fem. V and a percutaneous PA cannula (Fem. V or IJV). | 30 days | Variety of connection methods, oxygenator can be added. | Usually surgically implanted. |
| TandemHeart | Extracorporeal centrifugal pump. flow rate up to 4.5 L/min. | 1—Percutaneously via ProtekDuo cannula. 2—Percutaneously peripheral cannulation of the Fem. V and a percutaneous PA cannula (Fem. V or IJV). | 30 days | Single venous access, IJV access allowing patient to remain ambulatory, oxygenator can be added. | May cause SVC syndrome with larger cannula size. femoral access limit the patients mobility. |
| Author | Type of t-RVAD Device | Approach of Implantation | t-RVAD Duration * |
|---|---|---|---|
| Aissaoui et al., 2014 [30] | CentriMag (40), Thoratec PVAD (17) | Surgical | 32 (3–400) |
| Anderson et al., 2018 [20] | Impella RP | Fem. V | 4 ± 2 |
| Badu et al., 2020 [12] | ProtekDuo(weaned)/(for died) | IJV | 14 ± 7/10 ± 12 |
| Bhama et al., 2018 [19] | CentriMag | Surgical | 6 |
| Cheung et al., 2014 [29] | Impella RP (3)/Impella RD (15) | Fem. V(RP)/surgical (RD) | 7 (2–19) |
| Coromilas et al., 2019 [17] | ProtekDuo (15)/Impella RP (4)/CentriMag (21) | IJV/Fem. V/surgical | 9–18 |
| Deschka et al., 2016 [25] | Biomedicus Bio-Pump or Rotaflow RF32+ Oxygenator | Surgical | 11 ± 7 |
| Jaidka et al., 2019 [16] | CentriMag | Surgical | 4 ± 1 |
| Kapur et al., 2013 [34] | TandemHeart | Percutaneous (22), surgical (24) | 5 ± 5 |
| Khani-Hanjani et al., 2013 [33] | Rotaflow | Surgical | 8 (3–18) |
| Khorsandi et al., 2019 [15] | CentriMag (34), Rotaflow (8), AB5000(1) | Surgical | nr |
| Kiernan et al., 2017 [23] | nr | nr | nr |
| Kremer et al., 2020 [11] | ProtekDuo | IJV | 10 ± 7 |
| Lazar et al., 2013 [32] | CentriMag | Surgical | nr |
| Leidenfrost et al., 2016 [24] | CentriMag (25), Impella LD (1), AB5000(1)/+ Oxygenator (12) | Surgical | 10 ± 9/5 ± 3 |
| Loforte et al., 2011 [37] | CentriMag (PCCS/post LVAD) | Surgical | (9 ± 3)/(19 ± 9) |
| Loforte et al., 2013 [31] | CentriMag | Surgical | 16 (2–50) |
| Oliveros et al., 2021 [8] | ProtekDuo | IJV | 58 ± 47 |
| Patil et al., 2015 [27] | CentriMag | Surgical | nr |
| Qureshi et al., 2020 [6] | Impella RP | Fem. V | 7 (0.2–18) |
| Ravichandran et al., 2018 [18] | ProtekDuo | IJV | 11 ± 7 |
| Saeed et al., 2015 [26] | CentriMag ± oxygenator (12) | Surgical | 9 (2–88) |
| Saito et al., 2012 [36] | Capiox or Gyropump | Surgical | 5 ± 3 |
| Salna et al., 2020 [10] | ProtekDuo | IJV | 11 |
| Schaefer et al., 2017 [22] | CentriMag/Deltastream pump | Surgical (minimally invasive) | 16 ± 12 |
| Schmack et al., 2019 [14] | ProtekDuo | IJV | 17 ± 10 |
| Schopka et al., 2012 [35] | Rotaflow ± oxygenator | Surgical (minimal invasive) | 11 (2–43) |
| Shekiladze et al., 2020 [9] | Impella RP | Fem. V | 3 |
| Takeda et al., 2014 [28] | CentriMag (17), AB5000(25), Thoratec PVAD (1) | Surgical | nr |
| Vierecke et al., 2019 [13] | CentriMag (128), others (214) | NR | nr |
| Yoshioka et al., 2017 [21] | CentriMag | Surgical | 14 (10–18) |
| Author | No. | To Weaning (%) | To Discharge (%) | 30 Day (%) | 180 Day (%) | Died (%) # |
|---|---|---|---|---|---|---|
| Aissaoui et al., 2014 [30] | 57 | nr | nr | nr | 47 | nr |
| Anderson et al., 2018 [20] | 60 | nr | nr | 72 ** | 62 | 27 |
| Badu et al., 2020 [12] | 40 | 73 | 68 | nr | nr | nr |
| Bhama et al., 2018 [19] | 80 | nr | nr | 64 | nr | 58 |
| Cheung et al., 2014 [29] | 18 | 78 | nr | 72 | 50 * | nr |
| Coromilas et al., 2019 (pRVAD/sRVAD) [17] | 19/21 | nr | nr | 84/67 | nr | nr |
| Deschka et al., 2016 [25] | 25 | nr | 68 | nr | 56 * | 52 |
| Jaidka et al., 2019 [16] | 10 | 100 | 100 | 100 | 80 | 20 |
| Kapur et al., 2013 (pRVAD/sRVAD) [34] | 22/24 | nr | 50/38 | nr | nr | nr |
| Khani-Hanjani et al., 2013 [33] | 12 | nr | 92 | nr | 92 * | 8 |
| Khorsandi et al., 2019 (concurrent/staged) [15] | 29/14 | nr | 90/36 | 93/71 | nr | 51 |
| Kiernan et al., 2017 [23] | 386 | nr | nr | 78 | 64 | nr |
| Kremer et al., 2020 [11] | 10 | 40 | nr | 60 | nr | 40 |
| Lazar et al., 2013 [32] | 34 | nr | 88 | nr | 76 * | 24 |
| Leidenfrost et al., 2016 (t-RVAD only/t-RVAD-MO) [24] | 15/12 | nr | nr | 53/92 | 63 *** | nr |
| Loforte et al., 2011 (PCCS/post LVAD) [37] | 9/10 | 56/80 | nr | nr | nr | nr |
| Loforte et al., 2013 [31] | 46 | nr | 57 | 74 | 54 | nr |
| Oliveros et al., 2021 [8] | 11 | nr | nr | 82 | 72 | 36 |
| Patil et al., 2015 [27] | 35 | nr | nr | 94 | 73 | nr |
| Qureshi et al., 2020 [6] | 12 | nr | 83 | nr | nr | 33 |
| Ravichandran et al., 2018 [18] | 17 | nr | nr | nr | nr | 41 |
| Saeed et al., 2015 [26] | 21 | nr | 62 | nr | 52 * | 38 |
| Saito et al., 2012 (all/weaned) [36] | 26/11 | nr | nr | nr | nr/82 | nr |
| Salna et al., 2020 [10] | 27 | nr | nr | nr | 81 * | 19 |
| Schaefer et al., 2017 (minimally invasive/sternotomy) [22] | 10/11 | 100/nr | nr | 80/46 | nr | 20/nr |
| Schmack et al., 2019 [14] | 11 | 91 | nr | 73 | nr | 36 |
| Schopka et al., 2012 [35] | 12 | nr | 50 | nr | nr | nr |
| Shekiladze et al., 2020 [9] | 39 | nr | nr | 49 | nr | nr |
| Takeda et al., 2014 (weaning group/failure group) [28] | 21/23 | nr | 86/36 | nr | 75/13 | nr |
| Vierecke et al., 2019 ## [13] | 342 | nr | nr | 73 | 60 | nr |
| Yoshioka et al., 2017 [21] | 27 | nr | 59 | nr | 59 | 41 |
| Author | Weaned (%) | Switch to Permanent RVAD (%) (Switch to HTx, %) | ICU Stay (Days) * | AKI/RRT (%) |
|---|---|---|---|---|
| Aissaoui et al., 2014 [30] | nr | nr—(18) | nr | 33 |
| Badu et al., 2020 [12] | 73 | nr | nr | nr |
| Bhama et al., 2018 [19] | 78 | nr | nr | nr |
| Cheung et al., 2014 [29] | 78 | nr | nr | nr |
| Coromilas et al., 2019 (pRVAD) [17] | nr | nr | 21 (10–27) | 33 |
| Coromilas et al., 2019 (sRVAD) [17] | nr | nr | 27 (15–44) | 43 |
| Deschka et al., 2016 [25] | 92 | nr | 37 ± 32 | 36 |
| Jaidka et al., 2019 [16] | 100 | nr | 8 | 0 |
| Khani-Hanjani et al., 2013 [33] | nr | nr | 19 (15–22) | 18 |
| Khorsandi et al., 2019 (concurrent) [15] | 73 | 34—(69) | nr | 40 |
| Khorsandi et al., 2019 (staged) [15] | 71 | 29—(21) | nr | nr |
| Kremer et al., 2020 [11] | 40 | 20 | 16 ± 12 | 80 |
| Lazar et al., 2013 [32] | 92 | nr | nr | nr |
| Leidenfrost et al., 2016 (t-RVAD only/t-RVAD-MO) [24] | 66/83 | nr | nr | nr |
| Loforte et al., 2011 (PCCS/post LVAD) [37] | 56/80 | nr | nr | nr |
| Loforte et al., 2013 [31] | 65 | 7—(9) | 22 (15–50) | 15 |
| Oliveros et al., 2021 [8] | 55 | nr | nr | 46 |
| Patil et al., 2015 [27] | nr | nr | 23 (5–35) | nr |
| Qureshi et al., 2020 [6] | 75 | nr | nr | nr |
| Ravichandran et al., 2018 [18] | 23 | 35 | nr | nr |
| Saeed et al., 2015 [26] | nr | nr—(10) | nr | 52 |
| Saito et al., 2012 [36] | 42 | nr | nr | nr |
| Salna et al., 2020 [10] | 86 | 11 | 36 (22–48) | 0 |
| Schaefer et al., 2017 (minimally invasive) [22] | 100 | nr | nr | 40 |
| Schmack et al., 2019 [14] | 91 | nr | 24 ± 17 | nr |
| Schopka et al., 2012 [35] | 58 | nr | nr | nr |
| Takeda et al., 2014 [28] | 49 | nr | nr | nr |
| Yoshioka et al., 2017 [21] | 63 | nr | 28 (15–35) | 41 |
| Author | Major Hge | GI Hge | Reoperation for Hge | Thrombosis | Stroke | ICH | Sepsis | Pulmonary Hge | Hemolysis |
|---|---|---|---|---|---|---|---|---|---|
| Anderson et al., 2018 [20] | 48 | nr | nr | nr | nr | nr | nr | 0 | 22 |
| Badu et al., 2020 [12] | 0 | nr | nr | 3 | nr | nr | nr | nr | nr |
| Bhama et al., 2018 [19] | nr | nr | 28 | nr | nr | nr | 55 | nr | nr |
| Cheung et al., 2014 [29] | nr | nr | nr | nr | nr | nr | nr | nr | 22 |
| Deschka et al., 2016 [25] | 4 | 12 | 40 | nr | 8 | 8 | 20 | 20 | nr |
| Jaidka et al., 2019 [16] | nr | 0 | 0 | nr | nr | nr | nr | nr | nr |
| Kapur et al., 2013 [34] | 44 | nr | nr | nr | nr | nr | nr | nr | nr |
| Khani-Hanjani et al., 2013 [33] | nr | nr | 36 | nr | 0 | nr | 0 | nr | nr |
| Khorsandi et al., 2019 [15] | 33 | nr | nr | 16 | 23 | nr | 51 | 7 | nr |
| Kremer et al., 2020 [11] | 40 | nr | 20 | nr | nr | 10 | nr | nr | nr |
| Loforte et al., 2013 [31] | 43 | nr | nr | nr | nr | 2 | 15 | 9 | nr |
| Oliveros et al., 2021 [8] | nr | 46 | nr | nr | 18 | nr | 64 | nr | nr |
| Qureshi et al., 2020 [6] | nr | nr | nr | 8 | nr | nr | nr | nr | 42 |
| Ravichandran et al., 2018 [18] | nr | 6 | nr | nr | nr | 12 | nr | nr | nr |
| Saeed et al., 2015 [26] | 29 | nr | nr | nr | 0 | nr | 19 | 0 | nr |
| Salna et al., 2020 [10] | nr | nr | nr | 4 | nr | nr | nr | nr | 15 |
| Schaefer et al., 2017 [22] | 0 | 0 | nr | 0 | nr | 10 | nr | nr | nr |
| Schmack et al., 2019 [14] | nr | nr | nr | nr | nr | 9 | nr | nr | nr |
| Schopka et al., 2012 [35] | 0 | nr | nr | nr | 17 | nr | 8 | nr | nr |
| Shekiladze et al., 2020 [9] | nr | nr | nr | nr | nr | nr | nr | nr | 26 |
| Vierecke et al., 2019 [13] | 12 | nr | nr | 3 | 3 | nr | 8 | nr | nr |
| Yoshioka et al., 2017 [21] | nr | nr | nr | nr | 19 | nr | 30 | nr | nr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelshafy, M.; Caliskan, K.; Guven, G.; Elkoumy, A.; Elsherbini, H.; Elzomor, H.; Tenekecioglu, E.; Akin, S.; Soliman, O. Temporary Right-Ventricular Assist Devices: A Systematic Review. J. Clin. Med. 2022, 11, 613. https://doi.org/10.3390/jcm11030613
Abdelshafy M, Caliskan K, Guven G, Elkoumy A, Elsherbini H, Elzomor H, Tenekecioglu E, Akin S, Soliman O. Temporary Right-Ventricular Assist Devices: A Systematic Review. Journal of Clinical Medicine. 2022; 11(3):613. https://doi.org/10.3390/jcm11030613
Chicago/Turabian StyleAbdelshafy, Mahmoud, Kadir Caliskan, Goksel Guven, Ahmed Elkoumy, Hagar Elsherbini, Hesham Elzomor, Erhan Tenekecioglu, Sakir Akin, and Osama Soliman. 2022. "Temporary Right-Ventricular Assist Devices: A Systematic Review" Journal of Clinical Medicine 11, no. 3: 613. https://doi.org/10.3390/jcm11030613
APA StyleAbdelshafy, M., Caliskan, K., Guven, G., Elkoumy, A., Elsherbini, H., Elzomor, H., Tenekecioglu, E., Akin, S., & Soliman, O. (2022). Temporary Right-Ventricular Assist Devices: A Systematic Review. Journal of Clinical Medicine, 11(3), 613. https://doi.org/10.3390/jcm11030613






