Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery—A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Outcome Variables
2.2. Procedure, Data and Sample Collection
- After induction of anaesthesia-before skin incision
- Before start of CPB-after sternotomy/thoracotomy
- 30 min after start of CPB
- 120 min after start of CPB
- After end of CPB-before protamine administration
- 60 min after end of CPB
- 120 min after end of CPB
- 240 min after end of CPB
- 360 min after end of CPB
- –19. Postoperative Day (POD) 1–10
2.3. Bioelectrical Impedance Analysis
2.4. Statistics
3. Results
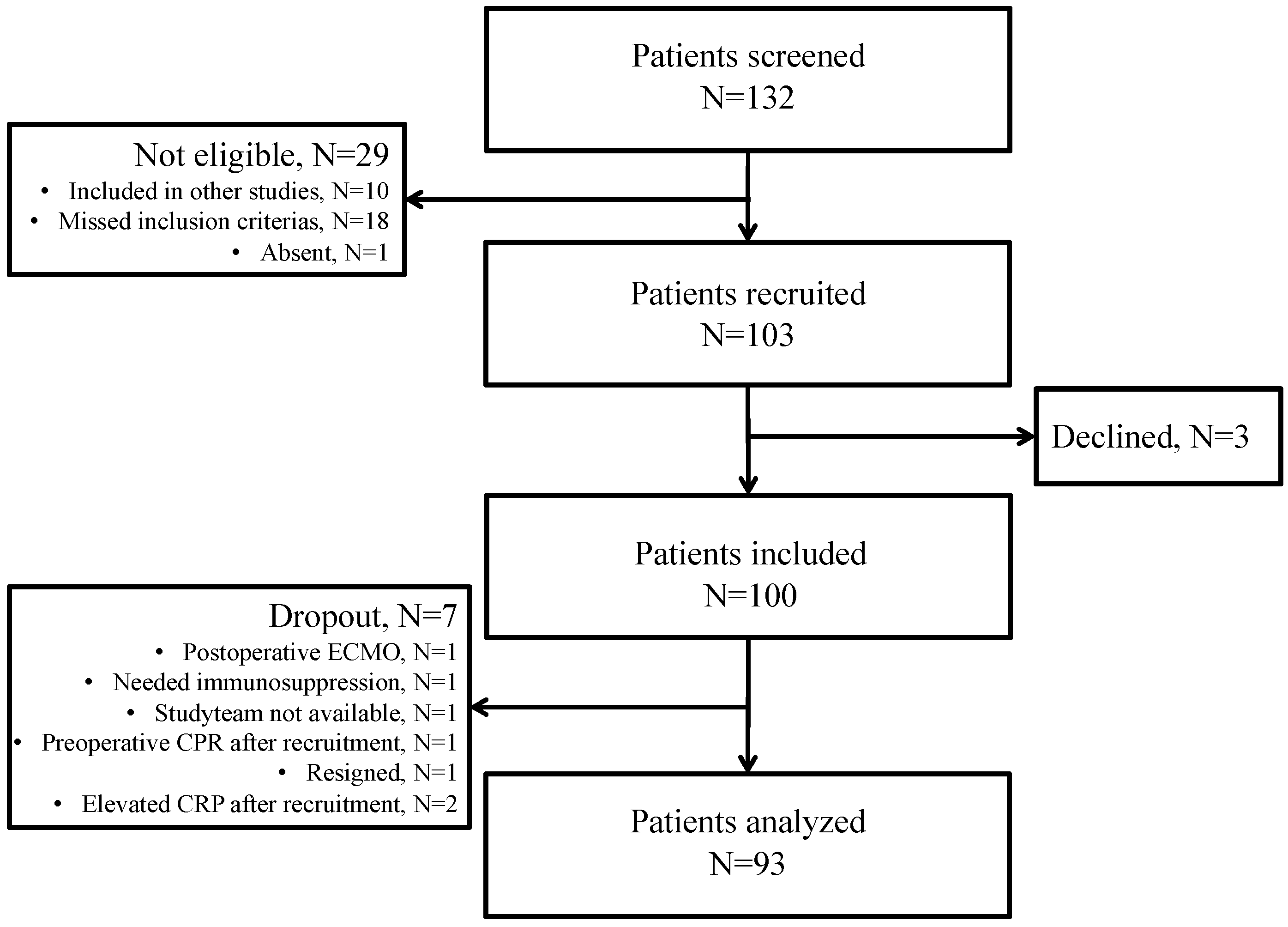
3.1. Patient Characteristics
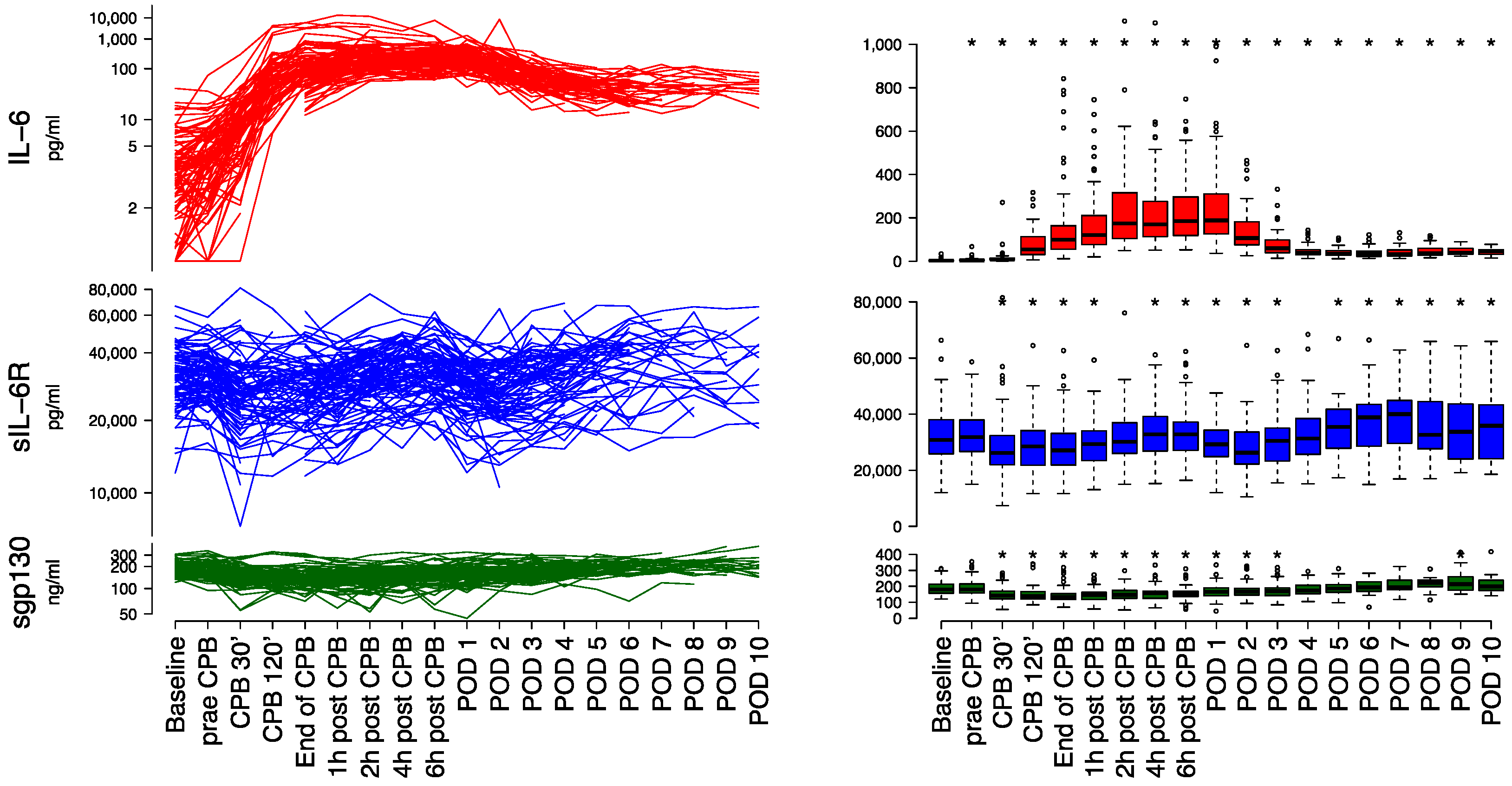
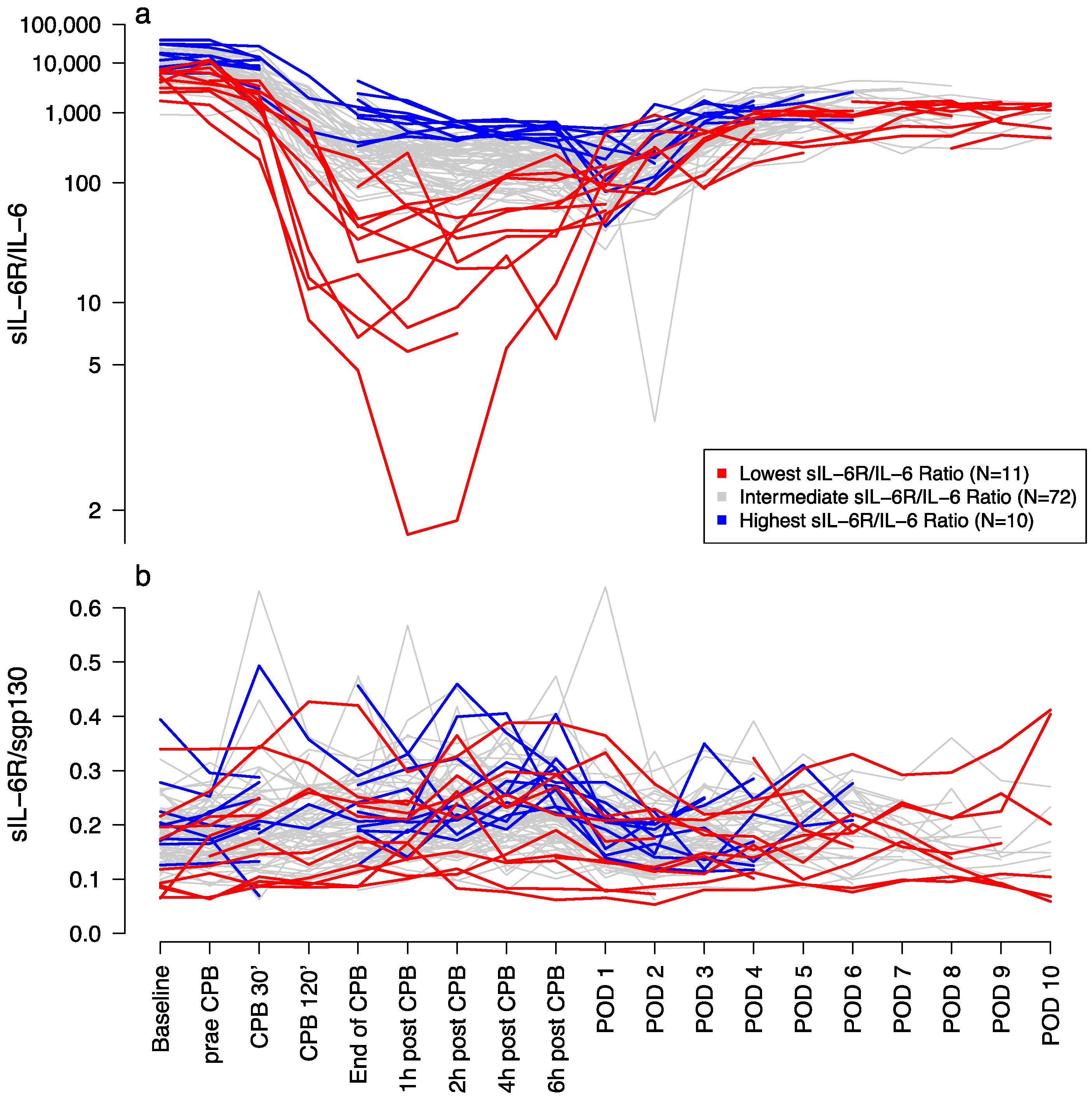
3.2. Laboratory Measurements
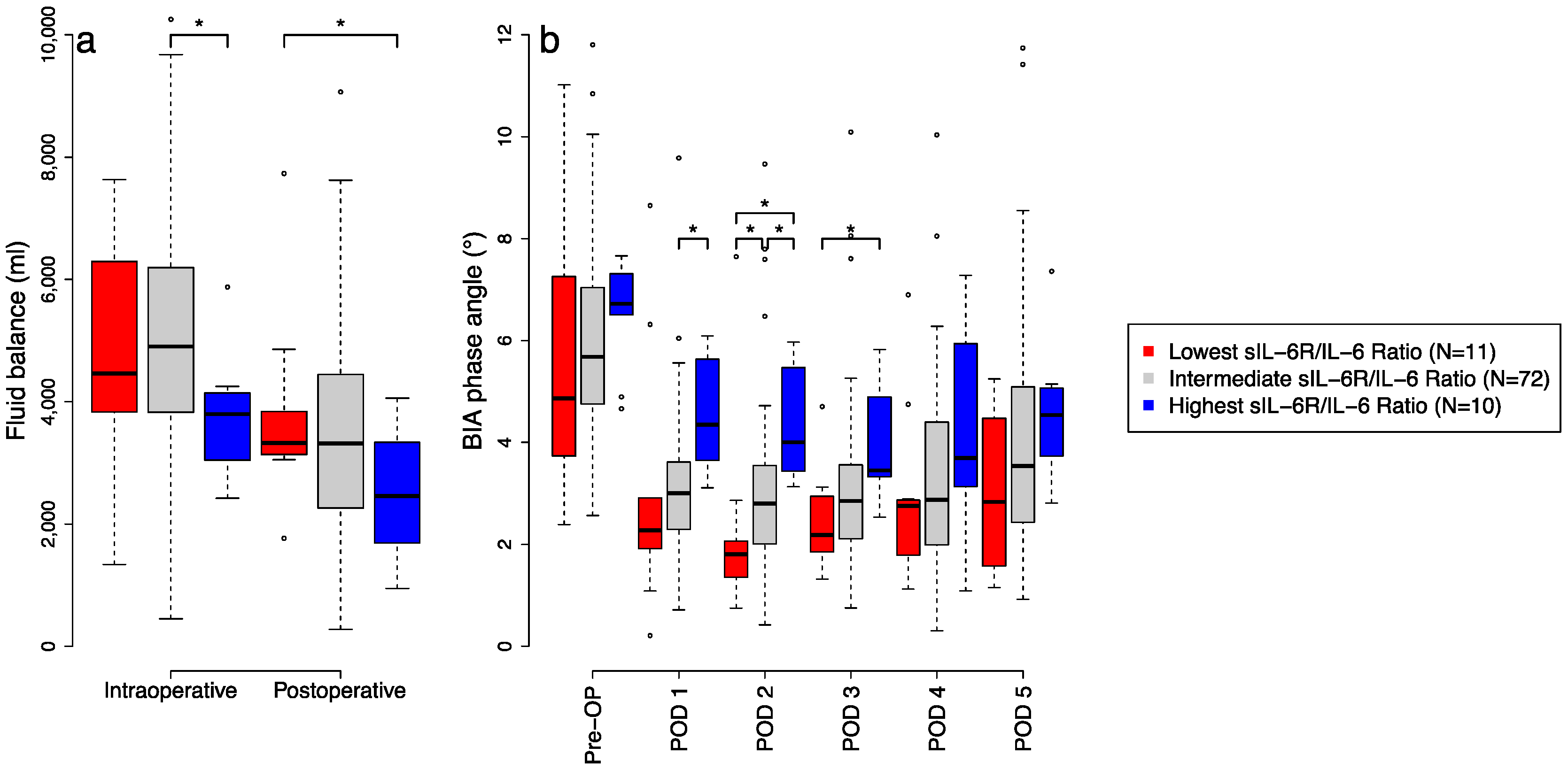
3.3. Pro-Inflammtory Effects on Fluid Balance and BIA
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, M.; Sun, T.; Zhao, Y.; Zhang, H. Detection of Serum Interleukin-6/10/18 Levels in Sepsis and Its Clinical Significance. J. Clin. Lab. Anal. 2016, 30, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Fink-Neuboeck, N.; Lindenmann, J.; Bajric, S.; Maier, A.; Riedl, R.; Weinberg, A.M.; Smolle-Juettner, F.M. Clinical impact of interleukin 6 as a predictive biomarker in the early diagnosis of postoperative systemic inflammatory response syndrome after major thoracic surgery: A prospective clinical trial. Surgery 2016, 160, 443–453. [Google Scholar] [CrossRef]
- Naffaa, M.; Makhoul, B.F.; Tobia, A.; Kaplan, M.; Aronson, D.; Azzam, Z.S.; Saliba, W. Procalcitonin and interleukin 6 for predicting blood culture positivity in sepsis. Am. J. Emerg Med. 2014, 32, 448–451. [Google Scholar] [CrossRef]
- Rios-Toro, J.J.; Marquez-Coello, M.; Garcia-Alvarez, J.M.; Martin-Aspas, A.; Rivera-Fernandez, R.; Saez de Benito, A.; Giron-Gonzalez, J.A. Soluble membrane receptors, interleukin 6, procalcitonin and C reactive protein as prognostic markers in patients with severe sepsis and septic shock. PLoS ONE 2017, 12, e0175254. [Google Scholar] [CrossRef]
- Thao, P.T.N.; Tra, T.T.; Son, N.T.; Wada, K. Reduction in the IL-6 level at 24 h after admission to the intensive care unit is a survival predictor for Vietnamese patients with sepsis and septic shock: A prospective study. BMC Emerg Med. 2018, 18, 39. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.H.; Rinoesl, H.; Dragosits, K.; Ristl, R.; Hoffelner, F.; Opfermann, P.; Lamm, C.; Preissing, F.; Wiedemann, D.; Hiesmayr, M.J.; et al. Effect of hemoadsorption during cardiopulmonary bypass surgery—A blinded, randomized, controlled pilot study using a novel adsorbent. Crit. Care 2016, 20, 96. [Google Scholar] [CrossRef] [Green Version]
- Corbi, P.; Rahmati, M.; Delwail, A.; Potreau, D.; Menu, P.; Wijdenes, J.; Lecron, J.C. Circulating soluble gp130, soluble IL-6R, and IL-6 in patients undergoing cardiac surgery, with or without extracorporeal circulation. Eur. J. Cardio-thorac Surg. 2000, 18, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, T.; Hibi, M.; Murakami, M.; Narazaki, M.; Saito, M.; Taga, T. The molecular biology of interleukin 6 and its receptor. Ciba. Found. Symp. 1992, 167, 5–16, discussion 16–23. [Google Scholar] [CrossRef]
- Baran, P.; Hansen, S.; Waetzig, G.H.; Akbarzadeh, M.; Lamertz, L.; Huber, H.J.; Ahmadian, M.R.; Moll, J.M.; Scheller, J. The balance of interleukin (IL)-6, IL-6.soluble IL-6 receptor (sIL-6R), and IL-6.sIL-6R.sgp130 complexes allows simultaneous classic and trans-signaling. J. Biol. Chem. 2018, 293, 6762–6775. [Google Scholar] [CrossRef] [Green Version]
- Scheller, J.; Rose-John, S. Interleukin-6 and its receptor: From bench to bedside. Med. Microbiol. Immunol. 2006, 195, 173–183. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, S.A. Directing transition from innate to acquired immunity: Defining a role for IL-6. J. Immunol. 2005, 175, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Riethmueller, S.; Somasundaram, P.; Ehlers, J.C.; Hung, C.W.; Flynn, C.M.; Lokau, J.; Agthe, M.; Dusterhoft, S.; Zhu, Y.; Grotzinger, J.; et al. Proteolytic Origin of the Soluble Human IL-6R In Vivo and a Decisive Role of N-Glycosylation. PLoS Biol. 2017, 15, e2000080. [Google Scholar] [CrossRef]
- Garbers, C.; Thaiss, W.; Jones, G.W.; Waetzig, G.H.; Lorenzen, I.; Guilhot, F.; Lissilaa, R.; Ferlin, W.G.; Grotzinger, J.; Jones, S.A.; et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of interleukin 6 and soluble interleukin 6 receptor. J. Biol. Chem. 2011, 286, 42959–42970. [Google Scholar] [CrossRef] [Green Version]
- Narazaki, M.; Yasukawa, K.; Saito, T.; Ohsugi, Y.; Fukui, H.; Koishihara, Y.; Yancopoulos, G.D.; Taga, T.; Kishimoto, T. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood 1993, 82, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Kishimura, M.; Ozaki, S.; Osakada, F.; Hashimoto, H.; Okubo, M.; Murakami, M.; Nakao, K. Cloning of novel soluble gp130 and detection of its neutralizing autoantibodies in rheumatoid arthritis. J. Clin. Investig. 2000, 106, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Jostock, T.; Mullberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Aparicio-Siegmund, S.; Garbers, Y.; Flynn, C.M.; Waetzig, G.H.; Gouni-Berthold, I.; Krone, W.; Berthold, H.K.; Laudes, M.; Rose-John, S.; Garbers, C. The IL-6-neutralizing sIL-6R-sgp130 buffer system is disturbed in patients with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E411–E420. [Google Scholar] [CrossRef]
- Hirota, H.; Izumi, M.; Hamaguchi, T.; Sugiyama, S.; Murakami, E.; Kunisada, K.; Fujio, Y.; Oshima, Y.; Nakaoka, Y.; Yamauchi-Takihara, K. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart Vessels 2004, 19, 237–241. [Google Scholar] [CrossRef]
- Kaminski, K.A.; Kozieradzka, A.; Bonda, T.; Banach, M.; Kozuch, M.; Wojtkowska, I.; Dobrzycki, S.; Kralisz, P.; Nowak, K.; Prokopczuk, P.; et al. Percutaneous coronary interventions affect concentrations of interleukin 6 and its soluble receptors in coronary sinus blood in patients with stable angina. Angiology 2009, 60, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Bonda, T.; Kaminski, K.A.; Kozuch, M.; Kozieradzka, A.; Wojtkowska, I.; Dobrzycki, S.; Kralisz, P.; Nowak, K.; Prokopczuk, P.; Musial, W.J. Circadian variations of interleukin 6 in coronary circulations of patients with myocardial infarction. Cytokine 2010, 50, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Day, J.R.; Taylor, K.M. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int. J. Surg. 2005, 3, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mat-Nor, M.B.; Md Ralib, A.; Abdulah, N.Z.; Pickering, J.W. The diagnostic ability of procalcitonin and interleukin-6 to differentiate infectious from noninfectious systemic inflammatory response syndrome and to predict mortality. J. Crit. Care 2016, 33, 245–251. [Google Scholar] [CrossRef]
- Reinhart, K.; Meisner, M.; Brunkhorst, F.M. Markers for sepsis diagnosis: What is useful? Crit. Care Clin. 2006, 22, 503–519. [Google Scholar] [CrossRef]
- Wei, M.; Kuukasjarvi, P.; Laurikka, J.; Pehkonen, E.; Kaukinen, S.; Laine, S.; Tarkka, M. Inflammatory cytokines and soluble receptors after coronary artery bypass grafting. Cytokine 2001, 15, 223–228. [Google Scholar] [CrossRef]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Stapel, S.N.; Looijaard, W.; Dekker, I.M.; Girbes, A.R.J.; Weijs, P.J.M.; Oudemans-van Straaten, H.M. Bioelectrical impedance analysis-derived phase angle at admission as a predictor of 90-day mortality in intensive care patients. Eur. J. Clin. Nutr. 2018, 72, 1019–1025. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, J.D.; Kang, D.R.; Hong, J.; Lee, J.M. Bioelectrical impedance analysis values as markers to predict severity in critically ill patients. J. Crit. Care 2017, 40, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Galicia, J.C.; Tai, H.; Komatsu, Y.; Shimada, Y.; Akazawa, K.; Yoshie, H. Polymorphisms in the IL-6 receptor (IL-6R) gene: Strong evidence that serum levels of soluble IL-6R are genetically influenced. Genes Immun. 2004, 5, 513–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbers, C.; Monhasery, N.; Aparicio-Siegmund, S.; Lokau, J.; Baran, P.; Nowell, M.A.; Jones, S.A.; Rose-John, S.; Scheller, J. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim. Biophys. Acta 2014, 1842, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Hamid, Y.H.; Urhammer, S.A.; Jensen, D.P.; Glumer, C.; Borch-Johnsen, K.; Jorgensen, T.; Hansen, T.; Pedersen, O. Variation in the interleukin-6 receptor gene associates with type 2 diabetes in Danish whites. Diabetes 2004, 53, 3342–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IL6R Genetics Consortium Emerging Risk Factors Collaboration; Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, H.R., Jr.; Koyama, T.; Koehler, E.A.; Siew, E.; Curtis, B.R.; Fremont, R.D.; May, A.K.; Bernard, G.R.; Ware, L.B. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit. Care Med. 2011, 39, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Landis, R.C.; Brown, J.R.; Fitzgerald, D.; Likosky, D.S.; Shore-Lesserson, L.; Baker, R.A.; Hammon, J.W. Attenuating the Systemic Inflammatory Response to Adult Cardiopulmonary Bypass: A Critical Review of the Evidence Base. J. Extra Corpor. Technol. 2014, 46, 197–211. [Google Scholar]
| Preoperative Risk Indicators | |
|---|---|
| Male | 59 (63.4) |
| Female | 34 (36.6) |
| Age (y) | 69.0 [60 to 76] |
| Height (cm) | 172 [165 to 178] |
| Weight (kg) | 81.0 [69 to 88] |
| Resistance | 386.5 [345 to 463] |
| Reactance | 43.0 [34.0 to 50.8] |
| Phase angle | 5.7 [4.7 to 7.2] |
| Frailty scale | 2.0 [2 to 3] |
| Comorbidities | |
| Asthma | 4 (4.3) |
| COPD | 15 (16.1) |
| NIDDM | 14 (15.1) |
| IDDM | 4 (4.3) |
| Chronic kidney disease | 6 (6.5) |
| Cardiac decompensation | 1 (1.1) |
| PAOD | 6 (6.5) |
| Atrial fibrillation | 26 (28) |
| Angina pectoris | |
| Absent | 68 (73.1) |
| Stable | 23 (24.7) |
| Unstable | 2 (2.2) |
| LVEF | |
| >50% | 65 (69.9) |
| 30–50% | 23 (24.7) |
| <30% | 5 (5.4) |
| Surgical characteristics | |
| Procedure | |
| CABG | 14 (15.1) |
| Combined | 26 (28) |
| Valve | 53 (57) |
| Reoperation | 16 (17.2) |
| Anaesthesia duration (min) | 395 [339 to 457] |
| Surgery (min) | 307 [255 to 542] |
| CPB (min) | 148 [111 to 192] |
| AoCC (min) | 98 ± 44 |
| Balanceintraoperative (mL) | 4460 [3767 to 6096] |
| PRBC (units) | 0 [0 to 1] |
| Platelets (units) | 0 [0 to 0] |
| Fresh frozen plasma (units) | 0 [0 to 0] |
| Fibrinogen (g) | 0 [0 to 2] |
| Coagulation factors (I.U.) | 0 [0 to 0] |
| Postoperative risk indicators | |
| SAPS 3 | 41.1 ± 11.6 |
| SOFA on ICU admission | 7 [6 to 9] |
| Length of ICU stay (d) | 2 [1 to 4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puchinger, J.; Ryz, S.; Nixdorf, L.; Edlinger-Stanger, M.; Lassnigg, A.; Wiedemann, D.; Hiesmayr, M.; Spittler, A.; Bernardi, M.H. Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery—A Prospective Cohort Study. J. Clin. Med. 2022, 11, 590. https://doi.org/10.3390/jcm11030590
Puchinger J, Ryz S, Nixdorf L, Edlinger-Stanger M, Lassnigg A, Wiedemann D, Hiesmayr M, Spittler A, Bernardi MH. Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery—A Prospective Cohort Study. Journal of Clinical Medicine. 2022; 11(3):590. https://doi.org/10.3390/jcm11030590
Chicago/Turabian StylePuchinger, Jürgen, Sylvia Ryz, Larissa Nixdorf, Maximilian Edlinger-Stanger, Andrea Lassnigg, Dominik Wiedemann, Michael Hiesmayr, Andreas Spittler, and Martin H. Bernardi. 2022. "Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery—A Prospective Cohort Study" Journal of Clinical Medicine 11, no. 3: 590. https://doi.org/10.3390/jcm11030590
APA StylePuchinger, J., Ryz, S., Nixdorf, L., Edlinger-Stanger, M., Lassnigg, A., Wiedemann, D., Hiesmayr, M., Spittler, A., & Bernardi, M. H. (2022). Characteristics of Interleukin-6 Signaling in Elective Cardiac Surgery—A Prospective Cohort Study. Journal of Clinical Medicine, 11(3), 590. https://doi.org/10.3390/jcm11030590









