Freshwater Clam Extract Mitigates Neuroinflammation and Amplifies Neurotrophic Activity of Glia: Insights from In Vitro Model of Neurodegenerative Pathomechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Freshwater Clam Extract (FCE)
2.2. Preparation of Primary Glial Cell Cultures
2.3. In Vitro Models for Injury-Induced Neurodegeration
2.4. Cell Viability Analysis
2.5. Measurement of Intracellular ROS
2.6. Flow Cytometric Analysis of Cell Cycle Distribution
2.7. Flow Cytometry Analysis of Cell Apoptosis (Annexin V/Propidium Iodide Assay)
2.8. Nitric Oxide Assay
2.9. TNF-α Assay
2.10. Real-Time Quantitative RT-PCR Analysis (qRT-PCR) for Primary Glial Cells
2.11. Statistical Analysis
3. Results
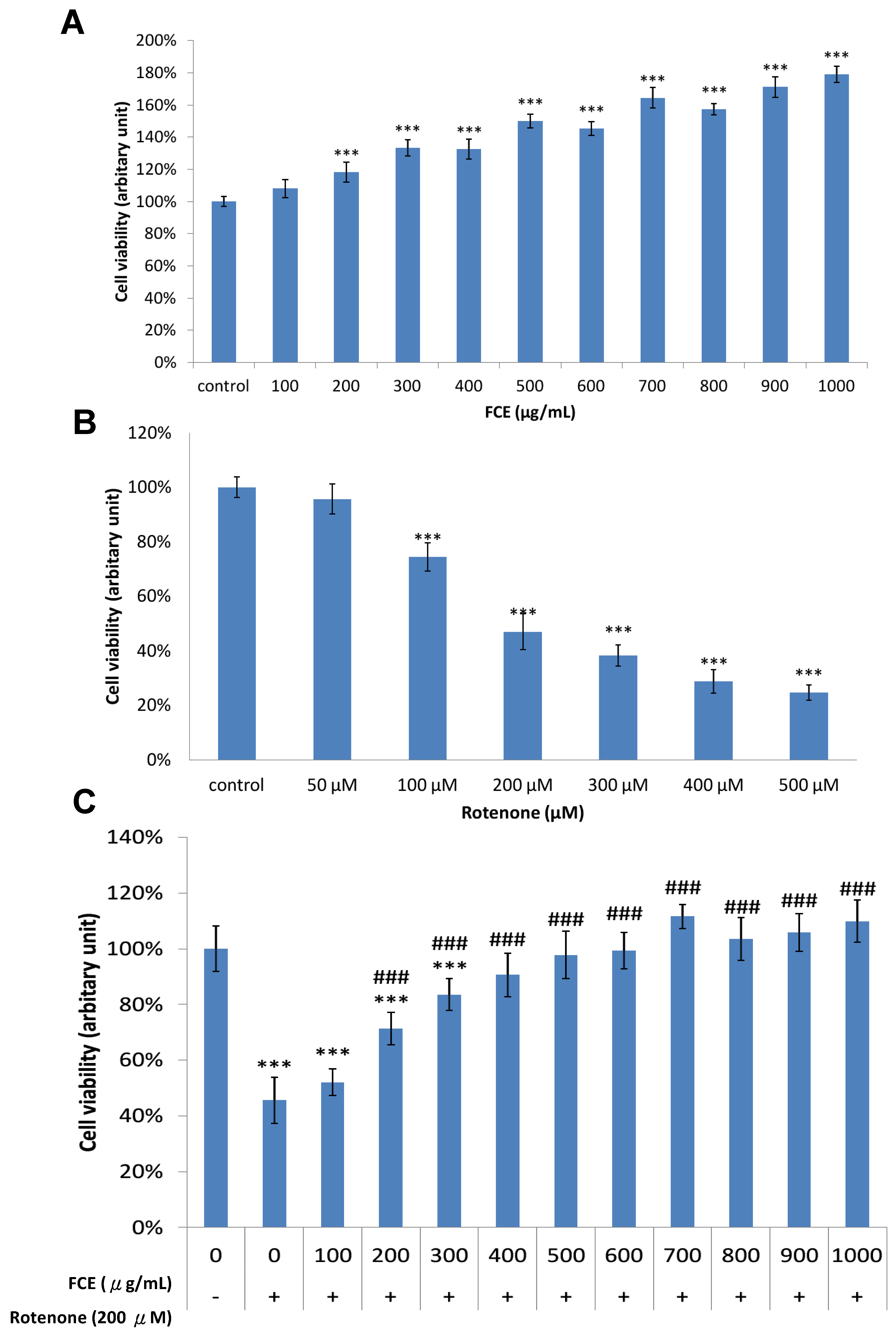
3.1. Pharmacological Effect of FCE and Rotenone on Cell Survival in Primary Glial Cells
3.2. FCE Protected against Rotenone-Induced Cytotoxicity in Primary Glial Cells
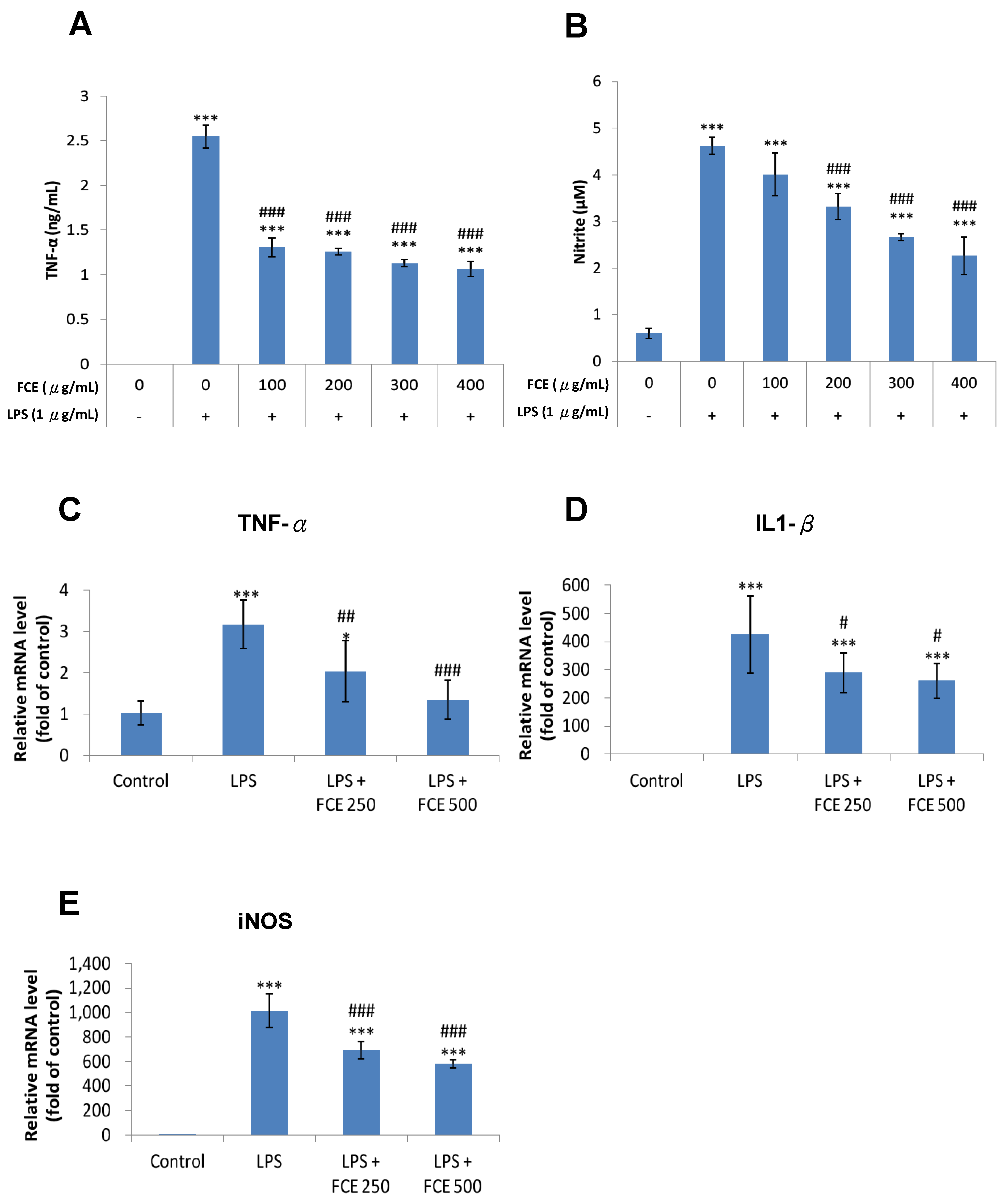
3.3. FCE Exerted Promising Anti-Inflammatory Effects against Injury-Induced Neuroinflammation in Primary Glial Cells
3.4. FCE Diminished Pro-Inflammatory mRNA Expression of TNF-α, iNOS and IL-1 Triggered by LPS in Primary Glial Cells
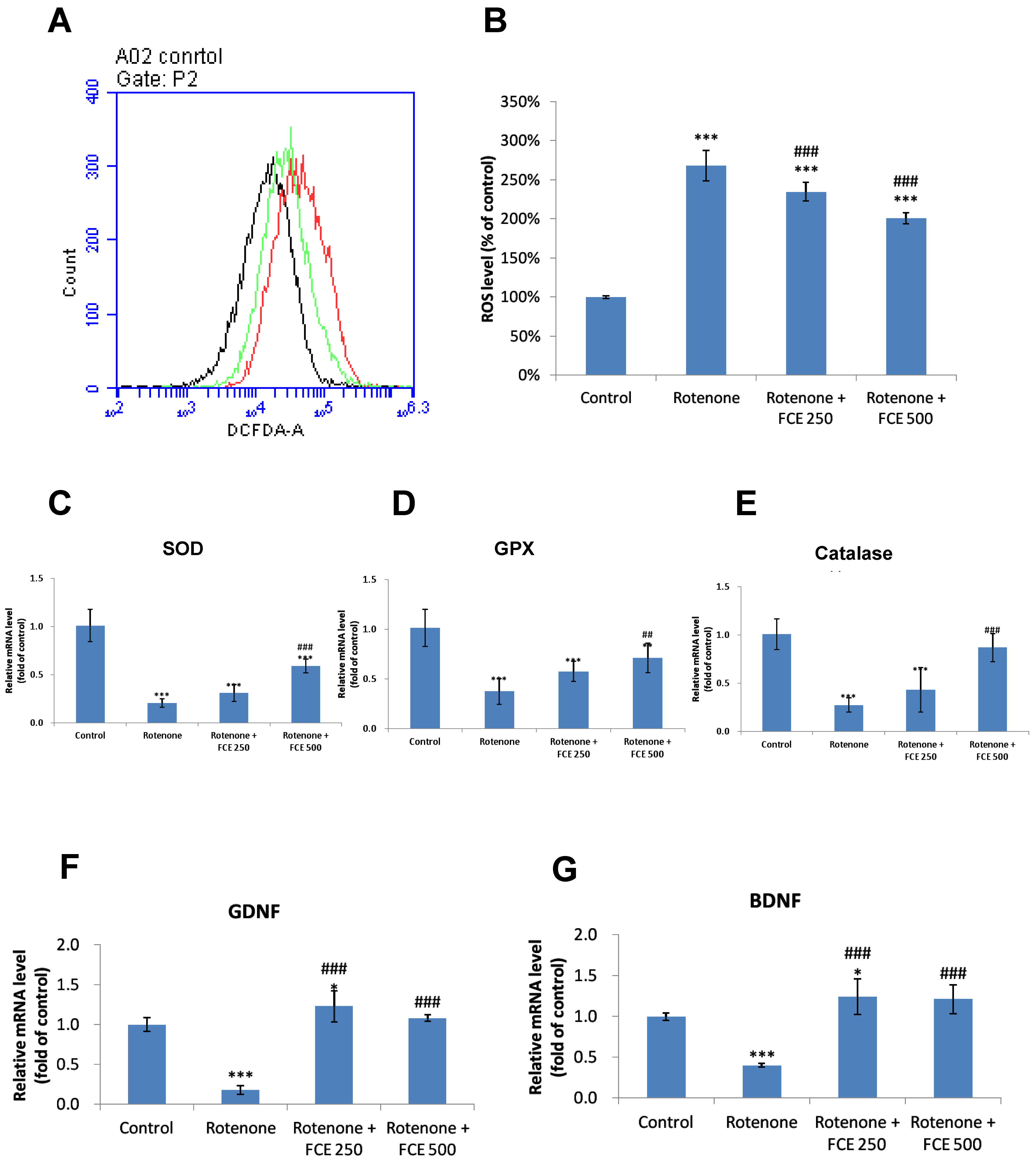
3.5. FCE Attenuated Rotenone-Induced Oxidative Stress in Primary Glial Cells
3.6. FCE Halted Rotenone-Induced Decrease in mRNA Expression of Antioxidant Enzymes in Primary Glial Cells
3.7. FCE Augmented mRNA Expression of Neurotrophic Factors in Primary Glial Cells
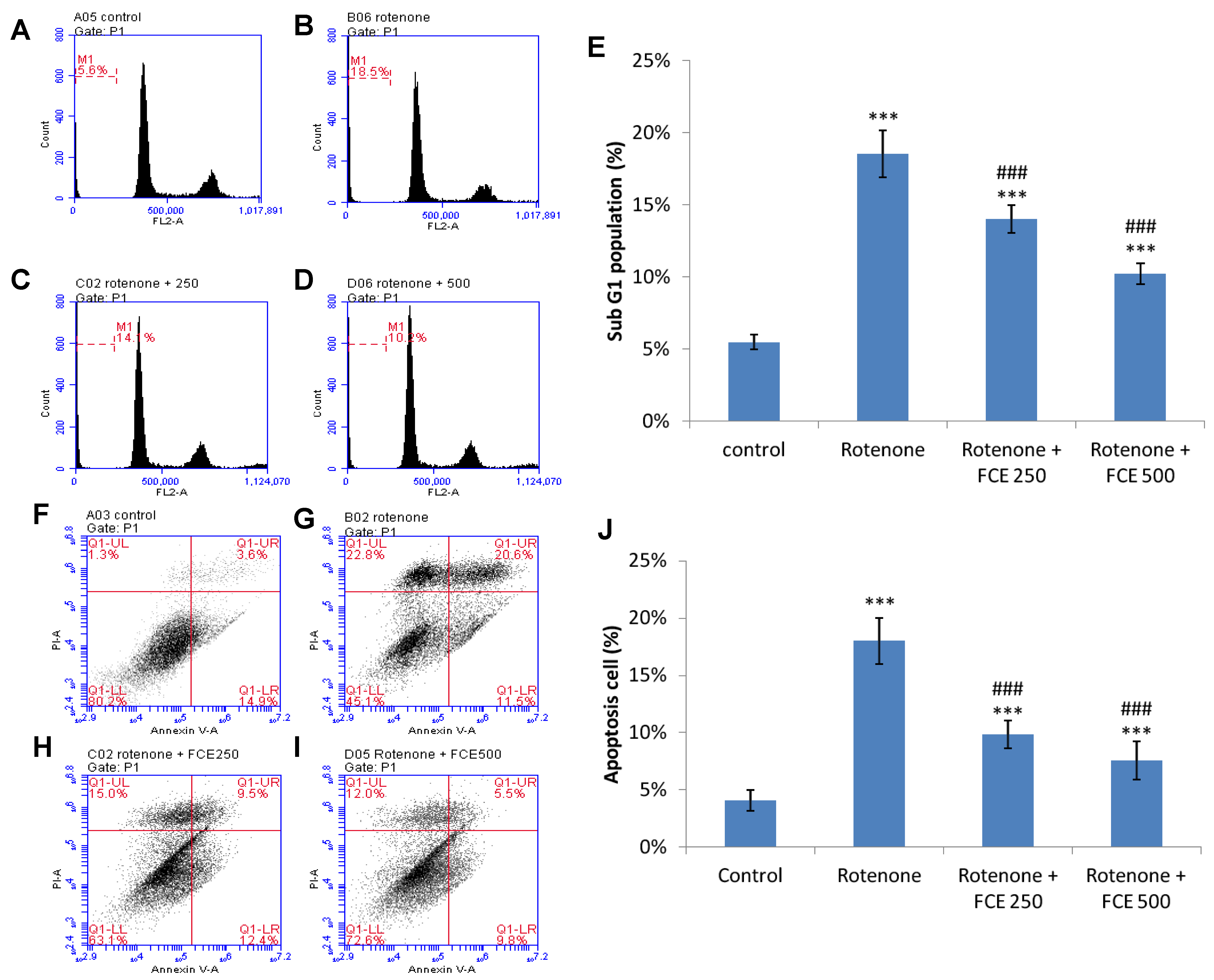
3.8. FCE Protected against Rotenone-Induced Apoptosis in Primary Glial Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Vartiainen, N.E.; Ying, W.; Chan, P.H.; Koistinaho, J.; Swanson, R.A. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J. Neurochem. 2001, 77, 1601–1610. [Google Scholar] [CrossRef]
- Desagher, S.; Glowinski, J.; Premont, J. Astrocytes protect neurons from hydrogen peroxide toxicity. J. Neurosci. 1996, 16, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Valette, J.; Lebon, V.; Bonvento, G. Neuron-astrocyte interactions in the regulation of brain energy metabolism: A focus on NMR spectroscopy. J. Neurochem. 2006, 99, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, A.; Dunnett, S.B.; Brundin, P.; Stoessl, A.J.; Freed, C.R.; Breeze, R.E.; Levivier, M.; Peschanski, M.; Studer, L.; Barker, R. Neural transplantation for the treatment of Parkinson’s disease. Lancet Neurol. 2003, 2, 437–445. [Google Scholar] [CrossRef]
- Choi-Lundberg, D.L.; Lin, Q.; Chang, Y.N.; Chiang, Y.L.; Hay, C.M.; Mohajeri, H.; Davidson, B.L.; Bohn, M.C. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science 1997, 275, 838–841. [Google Scholar] [CrossRef]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef]
- Emborg, M.E. Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. J. Neurosci. Methods 2004, 139, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s disease. Parkinsonism. Relat Disord. 2012, 18, S210–S212. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Brown, G.C. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 2007, 35, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Wood, W.G. Recent developments in understanding oxidative mechanisms and contributions of glial cell activation, mitochondrial dysfunction, and lipids and signaling pathways to neurodegenerative diseases. Preface. Mol. Neurobiol. 2010, 41, 53–54. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.E.; Iarkov, A.; Moran, V.E. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 340. [Google Scholar]
- Chijimatsu, T.; Umeki, M.; Okuda, Y.; Yamada, K.; Oda, H.; Mochizuki, S. The fat and protein fractions of freshwater clam ( Corbicula fluminea) extract reduce serum cholesterol and enhance bile acid biosynthesis and sterol excretion in hypercholesterolaemic rats fed a high-cholesterol diet. Br. J. Nutr. 2011, 105, 526–534. [Google Scholar] [CrossRef]
- Chijimatsu, T.; Tatsuguchi, I.; Oda, H.; Mochizuki, S. A Freshwater clam (Corbicula fluminea) extract reduces cholesterol level and hepatic lipids in normal rats and xenobiotics-induced hypercholesterolemic rats. J. Agric. Food Chem. 2009, 57, 3108–3112. [Google Scholar] [CrossRef]
- Hsu, C.L.; Hsu, C.C.; Yen, G.C. Hepatoprotection by freshwater clam extract against CCl4-induced hepatic damage in rats. Am. J. Chin. Med. 2010, 38, 881–894. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Lin, M.S.; Hua, K.F.; Chen, W.J.; Lin, C.C. Neuroprotection by freshwater clam extract against the neurotoxin MPTP in C57BL/6 mice. Neurosci. Lett. 2017, 642, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.F.; Chen, G.M.; Ho, C.L.; Chen, M.C.; Chen, Y.L.; Chen, W.J.; Huang, J.F.; Perng, Y.S.; Lin, C.C. Freshwater clam extract inhibits inflammatory responses in LPS-activated macrophages by reducing the activation of mitogen-activated protein kinases and NF-kappaB. Nat. Prod. Commun. 2012, 7, 1435–1440. [Google Scholar] [CrossRef]
- Lin, M.S.; Sun, Y.Y.; Chiu, W.T.; Hung, C.C.; Chang, C.Y.; Shie, F.S.; Tsai, S.H.; Lin, J.W.; Hung, K.S.; Lee, Y.H. Curcumin Attenuates the Expression and Secretion of RANTES after Spinal Cord Injury In Vivo and Lipopolysaccharide-Induced Astrocyte Reactivation In Vitro. J. Neurotrauma 2011, 28, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chiang, T.H.; Chen, W.J.; Sun, Y.Y.; Lee, Y.H.; Lin, M.S. CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury 2015, 46, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Sherer, T.B.; Betarbet, R.; Stout, A.K.; Lund, S.; Baptista, M.; Panov, A.V.; Cookson, M.R.; Greenamyre, J.T. An in vitro model of Parkinson’s disease: Linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J. Neurosci. 2002, 22, 7006–7015. [Google Scholar] [CrossRef] [PubMed]
- Miriam, M.; Paula, M.I.; Leyre, M.; Miriam, H.; Jose, B.; Carmen, G. An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: A beginners approach. Protoc. Exch. 2011, 218. [Google Scholar]
- Radad, K.; Rausch, W.D.; Gille, G. Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem. Int. 2006, 49, 379–386. [Google Scholar] [CrossRef]
- Liang, Y.; Jing, X.; Zeng, Z.; Bi, W.; Chen, Y.; Wu, X.; Yang, L.; Liu, J.; Xiao, S.; Liu, S.; et al. Rifampicin attenuates rotenone-induced inflammation via suppressing NLRP3 inflammasome activation in microglia. Brain Res. 2015, 1622, 43–50. [Google Scholar] [CrossRef]
- Miller, R.L.; James-Kracke, M.; Sun, G.Y.; Sun, A.Y. Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem. Res. 2009, 34, 55–65. [Google Scholar] [CrossRef]
- Sherer, T.B.; Kim, J.H.; Betarbet, R.; Greenamyre, J.T. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp. Neurol. 2003, 179, 9–16. [Google Scholar] [CrossRef]
- Yuste, J.E.; Tarragon, E.; Campuzano, C.M.; Ros-Bernal, F. Implications of glial nitric oxide in neurodegenerative diseases. Front. Cell Neurosci. 2015, 9, 322. [Google Scholar] [CrossRef]
- Parga, J.A.; Rodriguez-Perez, A.I.; Garcia-Garrote, M.; Rodriguez-Pallares, J.; Labandeira-Garcia, J.L. NRF2 Activation and Downstream Effects: Focus on Parkinson’s Disease and Brain Angiotensin. Antioxidants 2021, 10, 1649. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Zhou, X.; Xie, A.; Cai, Z.; Fu, C.; Peng, Y.; Zhang, H.; Liu, L. Rotenone-Induced Neurodegeneration Is Enabled by a p38-Parkin-ROS Signaling Feedback Loop. J. Agric. Food Chem. 2021, 69, 13942–13952. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, L.; Jiao, Y.; Zhang, Y.; Wang, Y.; Zhu, K.; Sun, C. Thioredoxin-1 mediates neuroprotection of Schisanhenol against MPP(+)-induced apoptosis via suppression of ASK1-P38-NF-κB pathway in SH-SY5Y cells. Sci. Rep. 2021, 11, 21604. [Google Scholar] [CrossRef] [PubMed]
- Strycharz-Dudziak, M.; Kiełczykowska, M.; Drop, B.; Świątek, Ł.; Kliszczewska, E.; Musik, I.; Polz-Dacewicz, M. Total Antioxidant Status (TAS), Superoxide Dismutase (SOD), and Glutathione Peroxidase (GPx) in Oropharyngeal Cancer Associated with EBV Infection. Oxid. Med. Cell Longev. 2019, 2019, 5832410. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Venkatesh, P.; Ponnusankar, S. Ethnopharmacology and integrative medicine–Let the history tell the future. J. Ayurveda. Integr. Med. 2010, 1, 100–109. [Google Scholar] [CrossRef]
- Huang, K.C.; Wu, W.T.; Yang, F.L.; Chiu, Y.H.; Peng, T.C.; Hsu, B.G.; Liao, K.W.; Lee, R.P. Effects of freshwater clam extract supplementation on time to exhaustion, muscle damage, pro/anti-inflammatory cytokines, and liver injury in rats after exhaustive exercise. Molecules 2013, 18, 3825–3838. [Google Scholar] [CrossRef]
- Peng, T.C.; Subeq, Y.M.; Lee, C.J.; Lee, C.C.; Tsai, C.J.; Chang, F.M.; Lee, R.P. Freshwater clam extract ameliorates acute liver injury induced by hemorrhage in rats. Am. J. Chin. Med. 2008, 36, 1121–1133. [Google Scholar] [CrossRef]
- Soliman, A.M. Extract of Coelatura aegyptiaca, a freshwater clam, ameliorates hepatic oxidative stress induced by monosodium glutamate in rats. African. J. Pharmacy. Pharmacol. 2011, 5, 398–408. [Google Scholar] [CrossRef]
- Lee, R.P.; Subeq, Y.M.; Lee, C.J.; Hsu, B.G.; Peng, T.C. Freshwater clam extract decreased hemorrhagic shock-induced liver injury by attenuating TNF-alpha production. Biol. Res. Nurs. 2012, 14, 286–293. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Wu, D.C.; Jackson-Lewis, V.; Vila, M.; Tieu, K.; Teismann, P.; Vadseth, C.; Choi, D.K.; Ischiropoulos, H.; Przedborski, S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002, 22, 1763–1771. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.S.; et al. Aggregated alpha-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef]
- Doherty, G.H. Nitric oxide in neurodegeneration: Potential benefits of non-steroidal anti-inflammatories. Neurosci. Bull. 2011, 27, 366–382. [Google Scholar] [CrossRef]
- Liu, B.; Gao, H.M.; Wang, J.Y.; Jeohn, G.H.; Cooper, C.L.; Hong, J.S. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann. N. Y. Acad. Sci. 2002, 962, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Jezek, P.; Hlavata, L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Zuo, L.; Motherwell, M.S. The impact of reactive oxygen species and genetic mitochondrial mutations in Parkinson’s disease. Gene 2013, 532, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Schmidt, W.J. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002, 136, 317–324. [Google Scholar]
- Bjorklund, A.; Kirik, D.; Rosenblad, C.; Georgievska, B.; Lundberg, C.; Mandel, R.J. Towards a neuroprotective gene therapy for Parkinson’s disease: Use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000, 886, 82–98. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Kordower, J.H. In vivo gene delivery of glial cell line--derived neurotrophic factor for Parkinson’s disease. Ann. Neurol. 2003, 53 (Suppl. 3), S120–S132. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Kirik, D.; Rosenblad, C.; Bjorklund, A.; Mandel, R.J. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: Intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 2000, 20, 4686–4700. [Google Scholar] [CrossRef] [PubMed]
- Hyman, C.; Hofer, M.; Barde, Y.A.; Juhasz, M.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef]
- Altar, C.A.; Boylan, C.B.; Jackson, C.; Hershenson, S.; Miller, J.; Wiegand, S.J.; Lindsay, R.M.; Hyman, C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc. Natl. Acad. Sci. USA 1992, 89, 11347–11351. [Google Scholar] [CrossRef]
- Klein, R.L.; Lewis, M.H.; Muzyczka, N.; Meyer, E.M. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999, 847, 314–320. [Google Scholar] [CrossRef]
- Gash, D.M.; Zhang, Z.; Cass, W.A.; Ovadia, A.; Simmerman, L.; Martin, D.; Russell, D.; Collins, F.; Hoffer, B.J.; Gerhardt, G.A. Morphological and functional effects of intranigrally administered GDNF in normal rhesus monkeys. J. Comp. Neurol. 1995, 363, 345–358. [Google Scholar] [CrossRef]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature 1996, 380, 252–255. [Google Scholar] [CrossRef]
- Eslamboli, A.; Georgievska, B.; Ridley, R.M.; Baker, H.F.; Muzyczka, N.; Burger, C.; Mandel, R.J.; Annett, L.; Kirik, D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J. Neurosci. 2005, 25, 769–777. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Lin, Q.; Collier, T.J.; Frim, D.M.; Breakefield, X.O.; Bohn, M.C. Astrocytes retrovirally transduced with BDNF elicit behavioral improvement in a rat model of Parkinson’s disease. Brain Res. 1995, 691, 25–36. [Google Scholar] [CrossRef]
- Levivier, M.; Przedborski, S.; Bencsics, C.; Kang, U.J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 1995, 15, 7810–7820. [Google Scholar] [CrossRef]
- Oh, Y.T.; Lee, J.Y.; Lee, J.; Kim, H.; Yoon, K.S.; Choe, W.; Kang, I. Oleic acid reduces lipopolysaccharide-induced expression of iNOS and COX-2 in BV2 murine microglial cells: Possible involvement of reactive oxygen species, p38 MAPK, and IKK/NF-kappaB signaling pathways. Neurosci. Lett. 2009, 464, 93–97. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Zhang, W.; Wang, H.; Ong, K.T.; Woo, S.L.; Walzem, R.L.; Mashek, D.G.; et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012, 7, e39286. [Google Scholar] [CrossRef]
- Patel, N.K.; Khan, M.S.; Bhutani, K.K. Investigations on Leucas cephalotes (Roth.) Spreng. for inhibition of LPS-induced pro-inflammatory mediators in murine macrophages and in rat model. EXCLI. J 2015, 14, 508–516. [Google Scholar]
- Montserrat-de la, P.S.; Fernandez-Arche, Á.; Ángel-Martin, M.; Garcia-Gimenez, M.D. The sterols isolated from Evening Primrose oil modulate the release of proinflammatory mediators. Phytomedicine 2012, 19, 1072–1076. [Google Scholar] [CrossRef]
- Kung, W.M.; Lin, M.S. The NFkB Antagonist CDGSH Iron-Sulfur Domain 2 Is a Promising Target for the Treatment of Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 934. [Google Scholar] [CrossRef]
| cDNA Target | Sequence (5’ -> 3’) | Product Size (bp) | Sequence Reference | |
|---|---|---|---|---|
| Actin | forward | GCTACAGCTTCACCACCACA | 123 | NM_007393.5 |
| reverse | TCTCCAGGGAGGAAGAGGAT | |||
| BDNF | forward | TGGCTGACACTTTTGAGCAC | 131 | NM_001316310.1 |
| reverse | CAAAGGCACTTGACTGCTGA | |||
| GDNF | forward | TGGGCTATGAAACCAAGGAG | 142 | NM_001301357.1 |
| reverse | CAACATGCCTGGCCTACTTT | |||
| TNF-α | forward | CAGGGGCCACCACGCTCTTC | 371 | NM_001278601.1 |
| reverse | CTTGGGGCAGGGGCTCTTGAC | |||
| IL-1β | forward | CAGGCTCCGAGATGAACAACAAAA | 332 | NM_008361.4 |
| reverse | TGGGGAACTCTGCAGACTCAAACT | |||
| iNOS | forward | TCACTGGGACAGCACAGAAT | 510 | NM_001313922.1 |
| reverse | TGTGTCTGCAGATGTGCTGA | |||
| GPx | forward | CCTCAAGTACGTCCGGCCTG | 197 | NM_008160.6 |
| reverse | CAACATCGTTGCGACACACC | |||
| SOD | forward | TGGGTTCCACGTCCATCAGTA | 151 | NM_011434.1 |
| reverse | ACCGTCCTTTCCAGCAGTCA | |||
| Catalase | forward | TTCAGAAGAAAGCGGTCAAGAAT | 59 | NM_009804.2 |
| reverse | GATGCGGGCCCCATAGTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-S.; Chen, S.-M.; Hua, K.-F.; Chen, W.-J.; Hsieh, C.-C.; Lin, C.-C. Freshwater Clam Extract Mitigates Neuroinflammation and Amplifies Neurotrophic Activity of Glia: Insights from In Vitro Model of Neurodegenerative Pathomechanism. J. Clin. Med. 2022, 11, 553. https://doi.org/10.3390/jcm11030553
Lin M-S, Chen S-M, Hua K-F, Chen W-J, Hsieh C-C, Lin C-C. Freshwater Clam Extract Mitigates Neuroinflammation and Amplifies Neurotrophic Activity of Glia: Insights from In Vitro Model of Neurodegenerative Pathomechanism. Journal of Clinical Medicine. 2022; 11(3):553. https://doi.org/10.3390/jcm11030553
Chicago/Turabian StyleLin, Muh-Shi, Shu-Mei Chen, Kuo-Feng Hua, Wei-Jung Chen, Cho-Chen Hsieh, and Chai-Ching Lin. 2022. "Freshwater Clam Extract Mitigates Neuroinflammation and Amplifies Neurotrophic Activity of Glia: Insights from In Vitro Model of Neurodegenerative Pathomechanism" Journal of Clinical Medicine 11, no. 3: 553. https://doi.org/10.3390/jcm11030553
APA StyleLin, M.-S., Chen, S.-M., Hua, K.-F., Chen, W.-J., Hsieh, C.-C., & Lin, C.-C. (2022). Freshwater Clam Extract Mitigates Neuroinflammation and Amplifies Neurotrophic Activity of Glia: Insights from In Vitro Model of Neurodegenerative Pathomechanism. Journal of Clinical Medicine, 11(3), 553. https://doi.org/10.3390/jcm11030553







