The Acute Effects of an Ultramarathon on Atrial Function and Supraventricular Arrhythmias in Master Athletes
Abstract
:1. Introduction
2. Methods
2.1. Study Group
2.2. Electrocardiography
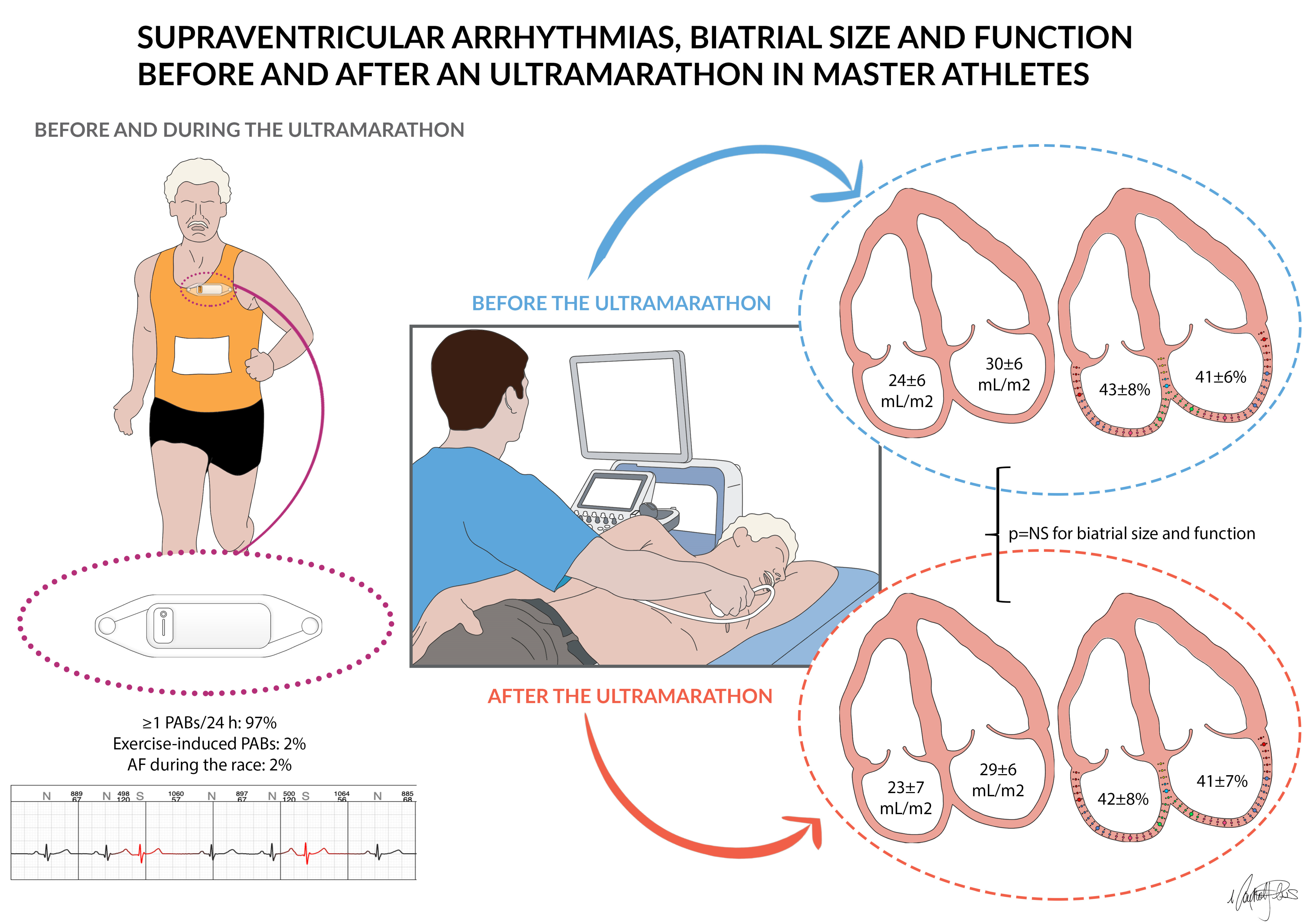
2.3. ECG Monitoring
2.4. Echocardiography
Two-Dimensional Speckle-Tracking Echocardiography (STE)
2.5. Statistical Analysis
3. Results
3.1. ECG Monitoring before and during the Race
3.2. Echocardiography
3.3. Correlation Analysis
4. Discussion
4.1. Atrial Volumes
4.2. Atrial Function
4.3. Electrical Function
4.4. Implications for AF Pathophysiology in the Athlete
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef] [Green Version]
- Tanasescu, M.; Leitzmann, M.F.; Rimm, E.B.; Willett, W.C.; Stampfer, M.J.; Hu, F.B. Exercise type and intensity in relation to coronary heart disease in men. JAMA 2002, 288, 1994–2000. [Google Scholar] [CrossRef]
- La Gerche, A.; Heidbuchel, H. Can intensive exercise harm the heart? You can get too much of a good thing. Circulation 2014, 130, 992–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, J.H.; Lavie, C.J. Run for your life... at a comfortable speed and not too far. Heart 2013, 99, 516–519. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Caso, P.; Scarafile, R.; Salerno, G.; De Corato, G.; Mita, C.; Di Salvo, G.; Allocca, F.; Colonna, D.; Caprile, M.; et al. Biventricular myocardial adaptation to different training protocols in competitive master athletes. Int. J. Cardiol. 2007, 115, 342–349. [Google Scholar] [CrossRef]
- Tso, J.; Kim, J.H. Master Endurance Athletes and Cardiovascular Controversies. Curr. Sports Med. Rep. 2020, 19, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Parry-Williams, G.; Sharma, S. The effects of endurance exercise on the heart: Panacea or poison? Nat. Rev. Cardiol. 2020, 17, 402–412. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Back, M.; Borjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2020, 42, 17–96. [Google Scholar] [CrossRef]
- Abdulla, J.; Nielsen, J.R. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace 2009, 11, 1156–1159. [Google Scholar] [CrossRef]
- Andersen, K.; Farahmand, B.; Ahlbom, A.; Held, C.; Ljunghall, S.; Michaelsson, K.; Sundstrom, J. Risk of arrhythmias in 52 755 long-distance cross-country skiers: A cohort study. Eur. Heart J. 2013, 34, 3624–3631. [Google Scholar] [CrossRef] [Green Version]
- D’Ascenzi, F.; Cameli, M.; Ciccone, M.M.; Maiello, M.; Modesti, P.A.; Mondillo, S.; Muiesan, M.L.; Scicchitano, P.; Novo, S.; Palmiero, P.; et al. The controversial relationship between exercise and atrial fibrillation: Clinical studies and pathophysiological mechanisms. J. Cardiovasc. Med. 2015, 16, 802–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzi, F.; Anselmi, F.; Focardi, M.; Mondillo, S. Atrial Enlargement in the Athlete’s Heart: Assessment of Atrial Function May Help Distinguish Adaptive from Pathologic Remodeling. J. Am. Soc. Echocardiogr. 2018, 31, 148–157. [Google Scholar] [CrossRef] [PubMed]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Cameli, M.; Lisi, M.; Focardi, M.; Padeletti, M.; Palmitesta, P.; Corrado, D.; Bonifazi, M.; et al. Training-induced dynamic changes in left atrial reservoir, conduit, and active volumes in professional soccer players. Eur. J. Appl. Physiol. 2015, 115, 1715–1723. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Padeletti, M.; Lisi, M.; Zaca, V.; Natali, B.; Malandrino, A.; Alvino, F.; Morelli, M.; Vassallo, G.M.; et al. Characterization of right atrial function and dimension in top-level athletes: A speckle tracking study. Int. J. Cardiovasc. Imaging 2013, 29, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Brugger, N.; Krause, R.; Carlen, F.; Rimensberger, C.; Hille, R.; Steck, H.; Wilhelm, M.; Seiler, C. Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. Int. J. Cardiol. 2014, 170, 419–425. [Google Scholar] [CrossRef]
- Sanz-de la Garza, M.; Grazioli, G.; Bijnens, B.H.; Sarvari, S.I.; Guasch, E.; Pajuelo, C.; Brotons, D.; Subirats, E.; Brugada, R.; Roca, E.; et al. Acute, Exercise Dose-Dependent Impairment in Atrial Performance During an Endurance Race: 2D Ultrasound Speckle-Tracking Strain Analysis. JACC Cardiovasc. Imaging 2016, 9, 1380–1388. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zueger, T.; De Marchi, S.; Rimoldi, S.F.; Brugger, N.; Steiner, R.; Stettler, C.; Nuoffer, J.M.; Seiler, C.; Ith, M. Inflammation and atrial remodeling after a mountain marathon. Scand. J. Med. Sci. Sports 2014, 24, 519–525. [Google Scholar] [CrossRef]
- Passaglia, D.G.; Emed, L.G.; Barberato, S.H.; Guerios, S.T.; Moser, A.I.; Silva, M.M.; Ishie, E.; Guarita-Souza, L.C.; Costantini, C.R.; Faria-Neto, J.R. Acute effects of prolonged physical exercise: Evaluation after a twenty-four-hour ultramarathon. Arq. Bras. Cardiol. 2013, 100, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef]
- Cavigli, L.; Zorzi, A.; Spadotto, V.; Gismondi, A.; Sisti, N.; Valentini, F.; Anselmi, F.; Mandoli, G.E.; Spera, L.; Di Florio, A.; et al. The acute effects of an ultramarathon on biventricular function and ventricular arrhythmias in master athletes. Eur. Heart J. Cardiovasc. Imaging 2021. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Zaca, V.; Cameli, M.; Alvino, F.; Malandrino, A.; Palmitesta, P.; Zorzi, A.; Corrado, D.; et al. Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circ. Cardiovasc. Imaging 2014, 7, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713, quiz 786–688. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312–303. [Google Scholar]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Cameli, M.; Andrei, V.; Incampo, E.; Alvino, F.; Lisi, M.; Padeletti, M.; Focardi, M.; et al. Increased left atrial size is associated with reduced atrial stiffness and preserved reservoir function in athlete’s heart. Int. J. Cardiovasc. Imaging 2015, 31, 699–705. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Di Paolo, F.M.; Biffi, A.; Quattrini, F.M.; Pisicchio, C.; Roselli, A.; Caselli, S.; Culasso, F. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J. Am. Coll. Cardiol. 2005, 46, 690–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, A.; Riegler, L.; Cocchia, R.; Scarafile, R.; Salerno, G.; Gravino, R.; Golia, E.; Vriz, O.; Citro, R.; Limongelli, G.; et al. Left atrial volume index in highly trained athletes. Am. Heart J. 2010, 159, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete’s heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [CrossRef]
- D’Andrea, A.; Formisano, T.; Riegler, L.; Scarafile, R.; America, R.; Martone, F.; di Maio, M.; Russo, M.G.; Bossone, E.; Galderisi, M.; et al. Acute and Chronic Response to Exercise in Athletes: The “Supernormal Heart”. Adv. Exp. Med. Biol. 2017, 999, 21–41. [Google Scholar] [CrossRef]
- Trivax, J.E.; Franklin, B.A.; Goldstein, J.A.; Chinnaiyan, K.M.; Gallagher, M.J.; de Jong, A.T.; Colar, J.M.; Haines, D.E.; McCullough, P.A. Acute cardiac effects of marathon running. J. Appl. Physiol. 2010, 108, 1148–1153. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.; Sasson, Z.; Gray, T.; Chelvanathan, A.; Esfandiari, S.; Dimitry, J.; Armstrong, S.; Mak, S.; Goodman, J.M. Left atrial phasic function interacts to support left ventricular filling during exercise in healthy athletes. J. Appl. Physiol. 2015, 119, 328–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ascenzi, F.; Caselli, S.; Solari, M.; Pelliccia, A.; Cameli, M.; Focardi, M.; Padeletti, M.; Corrado, D.; Bonifazi, M.; Mondillo, S. Novel echocardiographic techniques for the evaluation of athletes’ heart: A focus on speckle-tracking echocardiography. Eur. J. Prev. Cardiol. 2016, 23, 437–446. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Zaca, V.; Lisi, M.; Santoro, A.; Causarano, A.; Mondillo, S. Supernormal diastolic function and role of left atrial myocardial deformation analysis by 2D speckle tracking echocardiography in elite soccer players. Echocardiography 2011, 28, 320–326. [Google Scholar] [CrossRef]
- Dawson, E.; George, K.; Shave, R.; Whyte, G.; Ball, D. Does the human heart fatigue subsequent to prolonged exercise? Sports Med. 2003, 33, 365–380. [Google Scholar] [CrossRef]
- Gabrielli, L.; Bijnens, B.H.; Brambila, C.; Duchateau, N.; Marin, J.; Sitges-Serra, I.; Mont, L.; Brugada, J.; Sitges, M. Differential atrial performance at rest and exercise in athletes: Potential trigger for developing atrial dysfunction? Scand. J. Med. Sci. Sports 2016, 26, 1444–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Warncke, M.L.; Muellerleile, K.; Saering, D.; Beitzen-Heineke, A.; Kisters, A.; Swiderska, M.; Cavus, E.; Jahnke, C.M.; Adam, G.; et al. Acute impact of an endurance race on biventricular and biatrial myocardial strain in competitive male and female triathletes evaluated by feature-tracking CMR. Eur. Radiol. 2021. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Anselmi, F.; Ceccon, C.; Baccani, B.; Sisti, N.; Gismondi, A.; Sciaccaluga, C.; Aprile, F.; Fiorentini, C.; Graziano, F.; et al. The acute impact of an ultramarathon on right heart: A 12-lead ECG study. Scand. J. Med. Sci. Sports 2020, 30, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.; George, K.; Somauroo, J.; Jain, N.; Reese, K.; Hoffman, M.D.; Haddad, F.; Ashley, E.; Jones, H.; Oxborough, D. Exploratory insights from the right-sided electrocardiogram following prolonged endurance exercise. Eur. J. Sport Sci. 2016, 16, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M. Atrial fibrillation in endurance athletes. Eur. J. Prev. Cardiol. 2014, 21, 1040–1048. [Google Scholar] [CrossRef]
- Wilhelm, M.; Roten, L.; Tanner, H.; Wilhelm, I.; Schmid, J.P.; Saner, H. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am. J. Cardiol. 2011, 108, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Nuoffer, J.M.; Schmid, J.P.; Wilhelm, I.; Saner, H. Comparison of pro-atrial natriuretic peptide and atrial remodeling in marathon versus non-marathon runners. Am. J. Cardiol. 2012, 109, 1060–1065. [Google Scholar] [CrossRef]
- Baldesberger, S.; Bauersfeld, U.; Candinas, R.; Seifert, B.; Zuber, M.; Ritter, M.; Jenni, R.; Oechslin, E.; Luthi, P.; Scharf, C.; et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur. Heart J. 2008, 29, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, A.; Vio, R.; Mastella, G.; Ciarmatori, N.; Del Monte, A.; Trovato, D.; Iliceto, S.; Schiavon, M.; Bertaglia, E.; Corrado, D.; et al. Burden of premature atrial beats in middle-aged endurance athletes with and without lone atrial fibrillation versus sedentary controls. Eur. J. Prev. Cardiol. 2020, 27, 1555–1563. [Google Scholar] [CrossRef]
- Molina, L.; Mont, L.; Marrugat, J.; Berruezo, A.; Brugada, J.; Bruguera, J.; Rebato, C.; Elosua, R. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: A follow-up study. Europace 2008, 10, 618–623. [Google Scholar] [CrossRef]
- Pagourelias, E.D.; Kouidi, E.; Efthimiadis, G.K.; Deligiannis, A.; Geleris, P.; Vassilikos, V. Right atrial and ventricular adaptations to training in male Caucasian athletes: An echocardiographic study. J. Am. Soc. Echocardiogr. 2013, 26, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
| Variables | |
|---|---|
| Age, years | 47.9 ± 7.8 |
| Males, n (%) | 47 (69) |
| Hours of Training per Week | 6.7 ± 4.4 |
| Years of Training | 11.9 ± 8.2 |
| Duration of Marathon Race, Hours | 5.3 ± 7.6 |
| Family History for CAD, n (%) | 10 (15) |
| Family History for SCD, n (%) | 0 (0) |
| Height, cm | 173 ± 8 |
| Weight, kg | 71 ± 11 |
| BSA, m2 | 1.8 ± 0.17 |
| Variables | Pre-Race | Post-Race | p Value |
|---|---|---|---|
| Resting heart rate, bpm | 61 ± 8 | 85 ± 14 | <0.0001 |
| PR interval, ms | 157 ± 24 | 156 ± 20 | 0.49 |
| P wave voltage, mV | 0.16 ± 0.05 | 0.20 ± 0.0.6 | <0.0001 |
| P wave duration, ms | 98 ± 17 | 103 ± 16 | 0.094 |
| RA enlargement, n (%) | 5 (7.4) | 15 (22) | <0.0001 |
| LA enlargement, n (%) | 22 (32.4) | 23 (33.8) | 0.66 |
| Variables | |
|---|---|
| Min HR, bpm | 41 (38–45) |
| Max HR, bpm | 172 (161–182) |
| Max Theoretical HR, % | 98 (91–105) |
| Number of PABs/24 h, n ^ | 7 (3–19) |
| ≥1 PAB(s)/24 h, n (%) | 57 (97%) |
| ≥100 PABs/24 h, n (%) | 5 (8%) |
| ≥1 PAB(s) during the Race, n (%) | 13 (22%) |
| Exercise-Induced PABs *, n (%) | 1 (2%) |
| ≥1 Couplet(s), n (%) | 12 (21%) |
| ≥1 Couplet(s) during the Race, n (%) | 2 (4%) |
| ≥1 Triplet(s), n (%) | 10 (17%) |
| ≥1 Triplet(s) during the Race, n (%) | 1 (2%) |
| ≥1 Non-Sustained SVT(s), n (%) | 11 (19%) |
| ≥1 Non-Sustained SVT(s) during the Race, n (%) | 0 |
| Echocardiographic Variables | Pre-Race | Post-Race | p Values |
|---|---|---|---|
| Left atrium | |||
| LA volume, mL | 54.3 ± 11.5 | 52.2 ± 12.1 | 0.15 |
| LA volume index, mL/m2 | 30.0 ± 5.5 | 28.6 ± 6.2 | 0.16 |
| LA PALS, % | 40.6 ± 6.1 | 41.1 ± 7.1 | 0.46 |
| LA PACS, % | 18.3 ± 4.4 | 20.7 ± 5.5 | 0.001 |
| Right atrium | |||
| RA volume, mL | 43.6 ± 11.3 | 42.1 ± 12.3 | 0.25 |
| RA volume index, mL/m2 | 23.8 ± 6.0 | 23.1 ± 6.9 | 0.31 |
| RA PALS, % | 43.4 ± 7.8 | 41.7 ± 7.8 | 0.057 |
| RA PACS, % | 19.0 ± 5.0 | 20.9 ± 7.5 | 0.076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavigli, L.; Zorzi, A.; Spadotto, V.; Mandoli, G.E.; Melani, A.; Fusi, C.; D’Andrea, A.; Focardi, M.; Valente, S.; Cameli, M.; et al. The Acute Effects of an Ultramarathon on Atrial Function and Supraventricular Arrhythmias in Master Athletes. J. Clin. Med. 2022, 11, 528. https://doi.org/10.3390/jcm11030528
Cavigli L, Zorzi A, Spadotto V, Mandoli GE, Melani A, Fusi C, D’Andrea A, Focardi M, Valente S, Cameli M, et al. The Acute Effects of an Ultramarathon on Atrial Function and Supraventricular Arrhythmias in Master Athletes. Journal of Clinical Medicine. 2022; 11(3):528. https://doi.org/10.3390/jcm11030528
Chicago/Turabian StyleCavigli, Luna, Alessandro Zorzi, Veronica Spadotto, Giulia Elena Mandoli, Andrea Melani, Chiara Fusi, Antonello D’Andrea, Marta Focardi, Serafina Valente, Matteo Cameli, and et al. 2022. "The Acute Effects of an Ultramarathon on Atrial Function and Supraventricular Arrhythmias in Master Athletes" Journal of Clinical Medicine 11, no. 3: 528. https://doi.org/10.3390/jcm11030528
APA StyleCavigli, L., Zorzi, A., Spadotto, V., Mandoli, G. E., Melani, A., Fusi, C., D’Andrea, A., Focardi, M., Valente, S., Cameli, M., Bonifazi, M., & D’Ascenzi, F. (2022). The Acute Effects of an Ultramarathon on Atrial Function and Supraventricular Arrhythmias in Master Athletes. Journal of Clinical Medicine, 11(3), 528. https://doi.org/10.3390/jcm11030528









