Colorectal Cancer Surveillance in Patients with Inflammatory Bowel Diseases: Chromoendoscopy or Non-Chromoendoscopy, That Is the Question
Abstract
1. Introduction
2. Virtual Chromoendoscopy: Technical Aspects
3. Methods
4. Results
4.1. DCE vs. HD-WLE
4.2. VCE
4.3. Random Biopsies in the Era of HD
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.C.; Colombel, J.F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Kaplan, G.G. The Global Burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Khor, B.; Gardet, A.; Xavier, R.J. Genetics and Pathogenesis of Inflammatory Bowel Disease. Nature 2011, 474, 307–317. [Google Scholar] [CrossRef]
- Cohen, L.J.; Cho, J.H.; Gevers, D.; Chu, H. Genetic Factors and the Intestinal Microbiome Guide Development of Microbe-Based Therapies for Inflammatory Bowel Diseases. Gastroenterology 2019, 156, 2174–2189. [Google Scholar] [CrossRef] [PubMed]
- Itzkowitz, S.H.; Yio, X. Inflammation and Cancer IV. Colorectal Cancer in Inflammatory Bowel Disease: The Role of Inflammation. Am. J. Physiol. Gastrointest. Liver. Physiol. 2004, 287, G7–G17. [Google Scholar] [CrossRef]
- Beaugerie, L.; Itzkowitz, S. Cancers Complicating Inflammatory Bowel Disease. N. Engl. J. Med. 2015, 372, 1441–1452. [Google Scholar] [CrossRef]
- Wheat, C.L.; Clark-Snustad, K.; Devine, B.; Grembowski, D.; Thornton, T.A.; Ko, C.W. Worldwide Incidence of Colorectal Cancer, Leukemia, and Lymphoma in Inflammatory Bowel Disease: An Updated Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2016, 2016, 1632439. [Google Scholar] [CrossRef]
- Bewtra, M.; Kaiser, L.M.; TenHave, T.; Lewis, J.D. Crohn’s Disease and Ulcerative Colitis Are Associated with Elevated Standardized Mortality Ratios: A Meta-Analysis. Inflamm. Bowel Dis. 2013, 19, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Bye, W.A.; Ma, C.; Nguyen, T.M.; Parker, C.E.; Jairath, V.; East, J.E. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2018, 113, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Rubin, D.T.; Ananthakrishnan, A.N.; Siegel, C.A.; Sauer, B.G.; Long, M.D. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am. J. Gastroenterol. 2019, 114, 384–413. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial Diagnosis, Monitoring of Known IBD, Detection of Complications. J. Crohns Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.R.; Scholefield, J.H.; Steele, R.J.; Dunlop, M.G.; Thomas, H.J.; Evans, G.D.; Eaden, J.A.; Rutter, M.D.; Atkin, W.P.; Saunders, B.P.; et al. Guidelines for Colorectal Cancer Screening and Surveillance in Moderate and High Risk Groups (Update from 2002). Gut 2010, 59, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Farraye, F.A.; Odze, R.D.; Eaden, J.; Itzkowitz, S.H.; McCabe, R.P.; Dassopoulos, T.; Lewis, J.D.; Ullman, T.A.; James, T., III; McLeod, R.; et al. AGA Medical Position Statement on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. Gastroenterology 2010, 138, 738–745. [Google Scholar] [CrossRef]
- Kucharzik, T.; Dignass, A.U.; Atreya, R.; Bokemeyer, B.; Esters, P.; Herrlinger, K.; Kannengiesser, K.; Kienle, P.; Langhorst, J.; Luegering, A. Updated S3-Guideline Ulcerative Colitis. German Society for Digestive and Metabolic Diseases (DGVS). Z. Gastroenterol. 2019, 57, 162–241. [Google Scholar]
- Winther, K.V.; Jess, T.; Langholz, E.; Munkholm, P.; Binder, V. Long-Term Risk of Cancer in Ulcerative Colitis: A Population-Based Cohort Study from Copenhagen County. Clin. Gastroenterol. Hepatol. 2004, 2, 1088–1095. [Google Scholar] [CrossRef]
- Lutgens, M.W.; Vleggaar, F.P.; Schipper, M.E.; Stokkers, P.C.; van der Woude, C.J.; Hommes, D.W.; de Jong, D.J.; Dijkstra, G.; van Bodegraven, A.A.; Oldenburg, B.; et al. High Frequency of Early Colorectal Cancer in Inflammatory Bowel Disease. Gut 2008, 57, 1246–1251. [Google Scholar] [CrossRef]
- Axelrad, J.E.; Shah, S.C. Diagnosis and Management of Inflammatory Bowel Disease-Associated Neoplasia: Considerations in the Modern Era. Therap. Adv. Gastroenterol. 2020, 6, 1756284820920779. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R.; SCENIC Guideline Development Panel. SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef] [PubMed]
- Iannone, A.; Ruospo, M.; Wong, G.; Principi, M.; Barone, M.; Strippoli, G.F.M.; Di Leo, A. Chromoendoscopy for Surveillance in Ulcerative Colitis and Crohn’s Disease: A Systematic Review of Randomized Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 1684–1697.e11. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Fritsch, J.; Holtmann, M.; Koehler, H.H.; Stolte, M.; Kanzler, S.; Nafe, B.; Jung, M.; Galle, P.R.; Neurath, M.F. Methylene Blue-Aided Chromoendoscopy for the Detection of Intraepithelial Neoplasia and Colon Cancer in Ulcerative Colitis. Gastroenterology 2003, 124, 880–888. [Google Scholar] [CrossRef]
- Subramanian, V.; Mannath, J.; Ragunath, K.; Hawkey, C.J. Meta-Analysis: The Diagnostic Yield of Chromoendoscopy for Detecting Dysplasia in Patients with Colonic Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2011, 33, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Furfaro, F.; Matsumoto, T.; Uraoka, T.; Smith, S.; Ghosh, S.; Kiesslich, R. Advanced Endoscopic Techniques in the Assessment of Inflammatory Bowel Disease: New Technology, New Era. Gut 2019, 68, 562–572. [Google Scholar] [CrossRef]
- Subramanian, V.; Ragunath, K. Advanced Endoscopic Imaging: A Review of Commercially Available Technologies. Clin. Gastroenterol. Hepatol. 2014, 12, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Ramappa, V.; Telakis, E.; Mannath, J.; Jawhari, A.U.; Hawkey, C.J.; Ragunath, K. Comparison of High Definition with Standard White Light Endoscopy for Detection of Dysplastic Lesions during Surveillance Colonoscopy in Patients with Colonic Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 350–355. [Google Scholar] [CrossRef] [PubMed]
- American Society for Gastrointestinal Endoscopy Standards of Practice Committee; Shergill, A.K.; Lightdale, J.R.; Bruining, D.H.; Acosta, R.D.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Evans, J.A.; et al. The Role of Endoscopy in Inflammatory Bowel Disease. Gastrointest. Endosc. 2015, 81, 1101–1121.e13. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Ono, A.; Sekiguchi, M.; Fujii, T.; Saito, Y. Advances in Image Enhancement in Colonoscopy for Detection of Adenomas. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 305–314. [Google Scholar] [CrossRef]
- Iacucci, M.; Cannatelli, R.; Tontini, G.E.; Panaccione, R.; Danese, S.; Fiorino, G.; Matsumoto, T.; Kochhar, G.S.; Shen, B.; Kiesslich, R.; et al. Improving the Quality of Surveillance Colonoscopy in Inflammatory Bowel Disease. Lancet Gastroenterol. Hepatol. 2019, 4, 971–983. [Google Scholar] [CrossRef]
- Clarke, W.T.; Feuerstein, J.D. Colorectal Cancer Surveillance in Inflammatory Bowel Disease: Practice Guidelines and Recent Developments. World J. Gastroenterol. 2019, 25, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; East, J.E.; Hassan, C.; Hazewinkel, Y.; Kamiński, M.F.; Neumann, H.; Pellisé, M.; Antonelli, G.; Bustamante Balen, M.; Coron, E.; et al. Advanced Imaging for Detection and Differentiation of Colorectal Neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2019. Endoscopy 2019, 51, 1155–1179. [Google Scholar]
- Atkinson, N.S.S.; Ket, S.; Bassett, P.; Aponte, D.; De Aguiar, S.; Gupta, N.; Horimatsu, T.; Ikematsu, H.; Inoue, T.; Kaltenbach, T.; et al. Narrow-Band Imaging for Detection of Neoplasia at Colonoscopy: A Meta-Analysis of Data From Individual Patients in Randomized Controlled Trials. Gastroenterology 2019, 157, 462–471. [Google Scholar] [CrossRef]
- East, J.E.; Vleugels, J.L.; Roelandt, P.; Bhandari, P.; Bisschops, R.; Dekker, E.; Hassan, C.; Horgan, G.; Kiesslich, R.; Longcroft-Wheaton, G.; et al. Advanced Endoscopic Imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy 2016, 48, 1029–1045. [Google Scholar] [CrossRef]
- ASGE Technology Committee; Manfredi, M.A.; Abu Dayyeh, B.K.; Bhat, Y.M.; Chauhan, S.S.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; et al. Electronic Chromoendoscopy. Gastrointest. Endosc. 2015, 81, 249–261. [Google Scholar] [CrossRef]
- Paggi, S.; Mogavero, G.; Amato, A.; Rondonotti, E.; Andrealli, A.; Imperiali, G.; Lenoci, N.; Mandelli, G.; Terreni, N.; Conforti, F.S.; et al. Linked Color Imaging Reduces the Miss Rate of Neoplastic Lesions in the Right Colon: A Randomized Tandem Colonoscopy Study. Endoscopy 2018, 50, 396–402. [Google Scholar]
- Yoshida, N.; Dohi, O.; Inoue, K.; Yasuda, R.; Murakami, T.; Hirose, R.; Inoue, K.; Naito, Y.; Inada, Y.; Ogiso, K.; et al. Blue Laser Imaging, Blue Light Imaging, and Linked Color Imaging for the Detection and Characterization of Colorectal Tumors. Gut Liver 2019, 13, 140–148. [Google Scholar] [CrossRef]
- Subramaniam, S.; Hayee, B.; Aepli, P.; Schoon, E.; Stefanovic, M.; Kandiah, K.; Thayalasekaran, S.; Alkandari, A.; Bassett, P.; Coron, E.; et al. Optical Diagnosis of Colorectal Polyps with Blue Light Imaging Using a New International Classification. United Eur. Gastroenterol. J. 2019, 7, 316–325. [Google Scholar] [CrossRef]
- Rondonotti, E.; Paggi, S.; Amato, A.; Mogavero, G.; Andrealli, A.; Conforti, F.S.; Conte, D.; Spinzi, G.; Radaelli, F. Blue-Light Imaging Compared with High-Definition White Light for Real-Time Histology Prediction of Colorectal Polyps Less than 1 Centimeter: A Prospective Randomized Study. Gastrointest. Endosc. 2019, 89, 554–564.e1. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, Q.; Liang, S.H.; Zhong, J.; Li, J.N.; Ran, Z.H.; Zhi, F.C.; Wang, X.D.; Zhang, X.L.; Wen, Z.H.; et al. Chromoendoscopy with Targeted Biopsies Is Superior to White-Light Endoscopy for the Long-Term Follow-up Detection of Dysplasia in Ulcerative Colitis Patients: A Multicenter Randomized-Controlled Trial. Gastroenterol. Rep. 2020, 9, 14–21. [Google Scholar] [CrossRef]
- Alexandersson, B.; Hamad, Y.; Andreasson, A.; Rubio, C.A.; Ando, Y.; Tanaka, K.; Ichiya, T.; Rezaie, R.; Schmidt, P. High-Definition Chromoendoscopy Superior to High-Definition White-Light Endoscopy in Surveillance of Inflammatory Bowel Diseases in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 2101–2107. [Google Scholar] [CrossRef]
- Kim, K.O.; Chiorean, M. Advanced Neoplasia Detection Using Chromoendoscopy and White Light Colonoscopy for Surveillance in Patients with Inflammatory Bowel Disease. Intest. Res. 2020, 18, 438–446. [Google Scholar] [CrossRef]
- Sekra, A.; Schauer, C.; Mills, L.; Vandal, A.C.; Rose, T.; Lal, D.; Ogra, R. Chromoendoscopy versus Standard Colonoscopy for Detection of Nonpolypoid Dysplasia in Patients with Inflammatory Bowel Disease. N. Z. Med. J. 2018, 131, 32–38. [Google Scholar]
- Deepak, P.; Hanson, G.J.; Fletcher, J.G.; Tremaine, W.J.; Pardi, D.S.; Kisiel, J.B.; Schroeder, K.W.; Wong Kee Song, L.M.; Harmsen, W.S.; Loftus, E.V., Jr.; et al. Incremental Diagnostic Yield of Chromoendoscopy and Outcomes in Inflammatory Bowel Disease Patients with a History of Colorectal Dysplasia on White-Light Endoscopy. Gastrointest. Endosc. 2016, 83, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Carballal, S.; Maisterra, S.; López-Serrano, A.; Gimeno-García, A.Z.; Vera, M.I.; Marín-Garbriel, J.C.; Díaz-Tasende, J.; Márquez, L.; Álvarez, M.A.; Hernández, L.; et al. Real-Life Chromoendoscopy for Neoplasia Detection and Characterisation in Long-Standing IBD. Gut 2018, 67, 70–78. [Google Scholar] [CrossRef]
- Coelho-Prabhu, N.; Bruining, D.H.; Faubion, W.A.; Kane, S.V.; Kisiel, J.B.; Papadakis, K.A.; Pardi, D.S.; Raffals, L.E.; Schroeder, K.W.; Tremaine, W.J.; et al. A 1-Year Cross-Sectional Inflammatory Bowel Disease Surveillance Colonoscopy Cohort Comparing High-Definition White Light Endoscopy and Chromoendoscopy. Inflamm. Bowel Dis. 2021, 27, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Mooiweer, E.; van der Meulen-de Jong, A.E.; Ponsioen, C.Y.; Fidder, H.H.; Siersema, P.D.; Dekker, E.; Oldenburg, B. Chromoendoscopy for Surveillance in Inflammatory Bowel Disease Does Not Increase Neoplasia Detection Compared With Conventional Colonoscopy With Random Biopsies: Results From a Large Retrospective Study. Am. J. Gastroenterol. 2015, 110, 1014–1021. [Google Scholar] [CrossRef]
- Clarke, K.; Kang, M.; Gorrepati, V.S.; Stine, J.G.; Tinsley, A.; Williams, E.; Moyer, M.; Coates, M. Dysplasia Detection Is Similar between Chromoendoscopy and High-Definition White-Light Colonoscopy in Inflammatory Bowel Disease Patients: A US-Matched Case-Control Study. Int. J. Color. Dis. 2020, 35, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; Kaplan, G.G.; Panaccione, R.; Akinola, O.; Lethebe, B.C.; Lowerison, M.; Leung, Y.; Novak, K.L.; Seow, C.H.; Urbanski, S.; et al. A Randomized Trial Comparing High Definition Colonoscopy Alone with High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy. Am. J. Gastroenterol. 2018, 113, 225–234. [Google Scholar] [CrossRef]
- Yang, D.H.; Park, S.J.; Kim, H.S.; Park, Y.S.; Park, D.I.; Lee, K.M.; Jung, S.A.; Choi, C.H.; Koo, J.S.; Cheon, J.H.; et al. High-Definition Chromoendoscopy Versus High-Definition White Light Colonoscopy for Neoplasia Surveillance in Ulcerative Colitis: A Randomized Controlled Trial. Am. J. Gastroenterol. 2019, 114, 1642–1648. [Google Scholar] [CrossRef]
- Leifeld, L.; Rogler, G.; Stallmach, A.; Schmidt, C.; Zuber-Jerger, I.; Hartmann, F.; Plauth, M.; Drabik, A.; Hofstädter, F.; Dienes, H.P.; et al. White-Light or Narrow-Band Imaging Colonoscopy in Surveillance of Ulcerative Colitis: A Prospective Multicenter Study. Clin. Gastroenterol. Hepatol. 2015, 13, 1776–1781.e1. [Google Scholar] [CrossRef]
- Kandiah, K.; Subramaniam, S.; Thayalasekaran, S.; Chedgy, F.J.; Longcroft-Wheaton, G.; Fogg, C.; Brown, J.F.; Smith, S.C.; Iacucci, M.; Bhandari, P. Multicentre Randomised Controlled Trial on Virtual Chromoendoscopy in the Detection of Neoplasia during Colitis Surveillance High-Definition Colonoscopy (the VIRTUOSO Trial). Gut 2021, 70, 1684–1690. [Google Scholar] [CrossRef]
- Bisschops, R.; Bessissow, T.; Joseph, J.A.; Baert, F.; Ferrante, M.; Ballet, V.; Willekens, H.; Demedts, I.; Geboes, K.; De Hertogh, G.; et al. Chromoendoscopy versus Narrow Band Imaging in UC: A Prospective Randomised Controlled Trial. Gut 2018, 67, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Dubois, P.; Carter, B.; Cornelius, V.; Martyn, M.; Emmanuel, A.; Haji, A.; Hayee, B. A Randomized Crossover Trial of Conventional vs Virtual Chromoendoscopy for Colitis Surveillance: Dysplasia Detection, Feasibility, and Patient Acceptability (CONVINCE). Inflamm. Bowel Dis. 2019, 25, 1096–1106. [Google Scholar] [CrossRef]
- González-Bernardo, O.; Riestra, S.; Vivas, S.; de Francisco, R.; Pérez-Martínez, I.; Castaño-García, A.; Jiménez-Beltrán, V.; Rollé, V.; Suárez, P.; Suárez, A. Chromoendoscopy With Indigo Carmine vs Virtual Chromoendoscopy (ISCAN 1) for Neoplasia Screening in Patients With Inflammatory Bowel Disease: A Prospective Randomized Study. Inflamm. Bowel Dis. 2021, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- López-Serrano, A.; Suárez, M.J.; Besó, P.; Algarra, A.; Latorre, P.; Barrachina, M.M.; Paredes, J. Virtual Chromoendoscopy with ISCAN as an Alternative Method to Dye-Spray Chromoendoscopy for Dysplasia Detection in Long-Standing Colonic Inflammatory Bowel Disease: A Case-Control Study. Scand. J. Gastroenterol. 2021, 56, 820–828. [Google Scholar] [CrossRef]
- Bopanna, S.; Roy, M.; Das, P.; Dattagupta, S.; Sreenivas, V.; Mouli, V.P.; Kedia, S.; Dhingra, R.; Pradhan, R.; Kumar, N.S.; et al. Role of Random Biopsies in Surveillance of Dysplasia in Ulcerative Colitis Patients with High Risk of Colorectal Cancer. Intestig. Res. 2016, 14, 264–269. [Google Scholar] [CrossRef][Green Version]
- Gasia, M.F.; Ghosh, S.; Panaccione, R.; Ferraz, J.G.; Kaplan, G.G.; Leung, Y.; Novak, K.L.; Seow, C.H.; Iacucci, M. Targeted Biopsies Identify Larger Proportions of Patients With Colonic Neoplasia Undergoing High-Definition Colonoscopy, Dye Chromoendoscopy, or Electronic Virtual Chromoendoscopy. Clin. Gastroenterol. Hepatol. 2016, 14, 704–712.e4. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ajioka, Y.; Mitsuyama, K.; Watanabe, K.; Hanai, H.; Nakase, H.; Kunisaki, R.; Matsuda, K.; Iwakiri, R.; Hida, N.; et al. Comparison of Targeted vs Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer. Gastroenterology 2016, 151, 1122–1130. [Google Scholar] [CrossRef]
- Hata, K.; Ishihara, S.; Ajioka, Y.; Mitsuyama, K.; Watanabe, K.; Hanai, H.; Kunisaki, R.; Nakase, H.; Matsuda, K.; Iwakiri, R.; et al. Long-Term Follow-Up of Targeted Biopsy Yield (LOFTY Study) in Ulcerative Colitis Surveillance Colonoscopy. J. Clin. Med. 2020, 9, 2286. [Google Scholar] [CrossRef]
- Moussata, D.; Allez, M.; Cazals-Hatem, D.; Treton, X.; Laharie, D.; Reimund, J.M.; Bertheau, P.; Bourreille, A.; Lavergne-Slove, A.; Brixi, H.; et al. Are Random Biopsies Still Useful for the Detection of Neoplasia in Patients with IBD Undergoing Surveillance Colonoscopy with Chromoendoscopy? Gut 2018, 67, 616–624. [Google Scholar] [CrossRef]
- Hu, A.B.; Burke, K.E.; Kochar, B.; Ananthakrishnan, A.N. Yield of Random Biopsies During Colonoscopies in Inflammatory Bowel Disease Patients Undergoing Dysplasia Surveillance. Inflamm. Bowel Dis. 2021, 27, 779–786. [Google Scholar] [CrossRef]
- El-Dallal, M.; Chen, Y.; Lin, Q.; Rakowsky, S.; Sattler, L.; Foromera, J.; Grossberg, L.; Cheifetz, A.S.; Feuerstein, J. Meta-Analysis of Virtual-Based Chromoendoscopy Compared With Dye-Spraying Chromoendoscopy Standard and High-Definition White Light Endoscopy in Patients With Inflammatory Bowel Disease at Increased Risk of Colon Cancer. Inflamm. Bowel Dis. 2020, 26, 1319–1329. [Google Scholar] [CrossRef]
- Bessissow, T.; Dulai, P.S.; Restellini, S.; Landry, T.; Bisschops, R.; Murad, M.H.; Singh, S. Comparison of Endoscopic Dysplasia Detection Techniques in Patients With Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. Inflamm. Bowel Dis. 2018, 24, 2518–2526. [Google Scholar] [CrossRef]
- Gondal, B.; Haider, H.; Komaki, Y.; Komaki, F.; Micic, D.; Rubin, D.T.; Sakuraba, A. Efficacy of Various Endoscopic Modalities in Detecting Dysplasia in Ulcerative Colitis: A Systematic Review and Network Meta-Analysis. World J. Gastrointest. Endosc. 2020, 12, 159–171. [Google Scholar] [CrossRef]
- Resende, R.H.; Ribeiro, I.B.; de Moura, D.T.H.; Galetti, F.; Rocha, R.S.P.; Bernardo, W.M.; Sakai, P.; de Moura, E.G.H. Surveillance in Inflammatory Bowel Disease: Is Chromoendoscopy the Only Way to Go? A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Endosc. Int. Open. 2020, 8, E578–E590. [Google Scholar] [CrossRef]
- Imperatore, N.; Castiglione, F.; Testa, A.; De Palma, G.D.; Caporaso, N.; Cassese, G.; Rispo, A. Augmented Endoscopy for Surveillance of Colonic Inflammatory Bowel Disease: Systematic Review With Network Meta-Analysis. J. Crohns Colitis 2019, 13, 714–724. [Google Scholar] [CrossRef]
- Har-Noy, O.; Katz, L.; Avni, T.; Battat, R.; Bessissow, T.; Yung, D.E.; Engel, T.; Koulaouzidis, A.; Eliakim, R.; Ben-Horin, S.; et al. Chromoendoscopy, Narrow-Band Imaging or White Light Endoscopy for Neoplasia Detection in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2017, 62, 2982–2990. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Rakowsky, S.; Sattler, L.; Yadav, A.; Foromera, J.; Grossberg, L.; Cheifetz, A. Meta-Analysis of Dye-Based Chromoendoscopy Compared with Standard-and High-Definition White-Light Endoscopy in Patients with Inflammatory Bowel Disease at Increased Risk of Colon Cancer. Gastrointest. Endosc. 2019, 90, 186–195. [Google Scholar] [CrossRef]
- Iannone, A.; Ruospo, M.; Palmer, S.C.; Principi, M.; Barone, M.; Di Leo, A.; Strippoli, G.F.M. Systematic Review with Network Meta-Analysis: Endoscopic Techniques for Dysplasia Surveillance in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2019, 50, 858–871. [Google Scholar] [CrossRef]
- Lv, X.H.; Wang, B.L.; Cao, G.W. Narrow Band Imaging for Surveillance in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 607–615. [Google Scholar] [CrossRef]
- Wan, J.; Wang, X.; Yang, Z.P.; Wu, K.C. Systematic Review with Meta-Analysis: Chromoendoscopy versus White Light Endoscopy in Detection of Dysplasia in Patients with Inflammatory Bowel Disease. J. Dig. Dis. 2019, 20, 206–214. [Google Scholar] [CrossRef]
- Azizi, S.; Al-Rubaye, H.; Turki, M.A.A.; Siddiqui, M.R.S.; Shanmuganandan, A.P.; Ehsanullah, B.; Brar, R.; Abulafi, A.M. Detecting Dysplasia Using White Light Endoscopy or Chromoendoscopy in Ulcerative Colitis Patients without Primary Sclerosing Cholangitis: A Systematic Review and Meta-Analysis. Int. J. Surg. 2018, 52, 180–188. [Google Scholar] [CrossRef]
- Wijnands, A.M.; Mahmoud, R.; Lutgens, M.W.M.D.; Oldenburg, B. Surveillance and Management of Colorectal Dysplasia and Cancer in Inflammatory Bowel Disease: Current Practice and Future Perspectives. Eur. J. Intern. Med. 2021, 93, 35–41. [Google Scholar] [CrossRef]
- Adamina, M.; Feakins, R.; Iacucci, M.; Spinelli, A.; Cannatelli, R.; D’Hoore, A.; Driessen, A.; Katsanos, K.; Mookhoek, A.; Myrelid, P.; et al. ECCO Topical Review Optimising Reporting in Surgery, Endoscopy, and Histopathology. J. Crohns Colitis 2021, 15, 1089–1105. [Google Scholar] [CrossRef]
- Megna, B.; Weiss, J.; Ley, D.; Saha, S.; Pfau, P.; Grimes, I.; Li, Z.; Caldera, F. Clear Liquid Diet before Bowel Preparation Predicts Successful Chromoendoscopy in Patients with Inflammatory Bowel Disease. Gastrointest. Endosc. 2019, 89, 373–379.e2. [Google Scholar] [CrossRef]
- Ten Hove, J.R.; Bernstein, C.N.; Oldenburg, B. Putting Evidence into Practice: IBD Surveillance, Chromoendoscopy and Future Directions. Am. J. Gastroenterol. 2018, 113, 313–316. [Google Scholar] [CrossRef]
- Dekker, E.; Houwen, B.B.S.L.; Puig, I.; Bustamante-Balén, M.; Coron, E.; Dobru, D.E.; Kuvaev, R.; Neumann, H.; Johnson, G.; Pimentel-Nunes, P.; et al. Curriculum for Optical Diagnosis Training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, C10. [Google Scholar] [CrossRef]
- Zilli, A.; Capogreco, A.; Furfaro, F.; Allocca, M.; Roda, G.; Loy, L.; Fiorino, G.; Danese, S. Improving Quality of Care in Endoscopy of Inflammatory Bowel Disease: Can We Do Better? Expert Rev. Gastroenterol. Hepatol. 2020, 14, 819–828. [Google Scholar] [CrossRef]
- Parigi, T.L.; Peyrin-Biroulet, L.; Danese, S. Endoscopic Surveillance in IBD: The Virtue Is in High Definition. Gastroenterology 2021. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of Artificial Intelligence in Colonoscopy for Adenoma and Polyp Detection: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021, 93, 77–85.e6. [Google Scholar] [CrossRef]
- Konijeti, G.G.; Shrime, M.G.; Ananthakrishnan, A.N.; Chan, A.T. Cost-Effectiveness Analysis of Chromoendoscopy for Colorectal Cancer Surveillance in Patients with Ulcerative Colitis. Gastrointest. Endosc. 2014, 79, 455–465. [Google Scholar] [CrossRef]
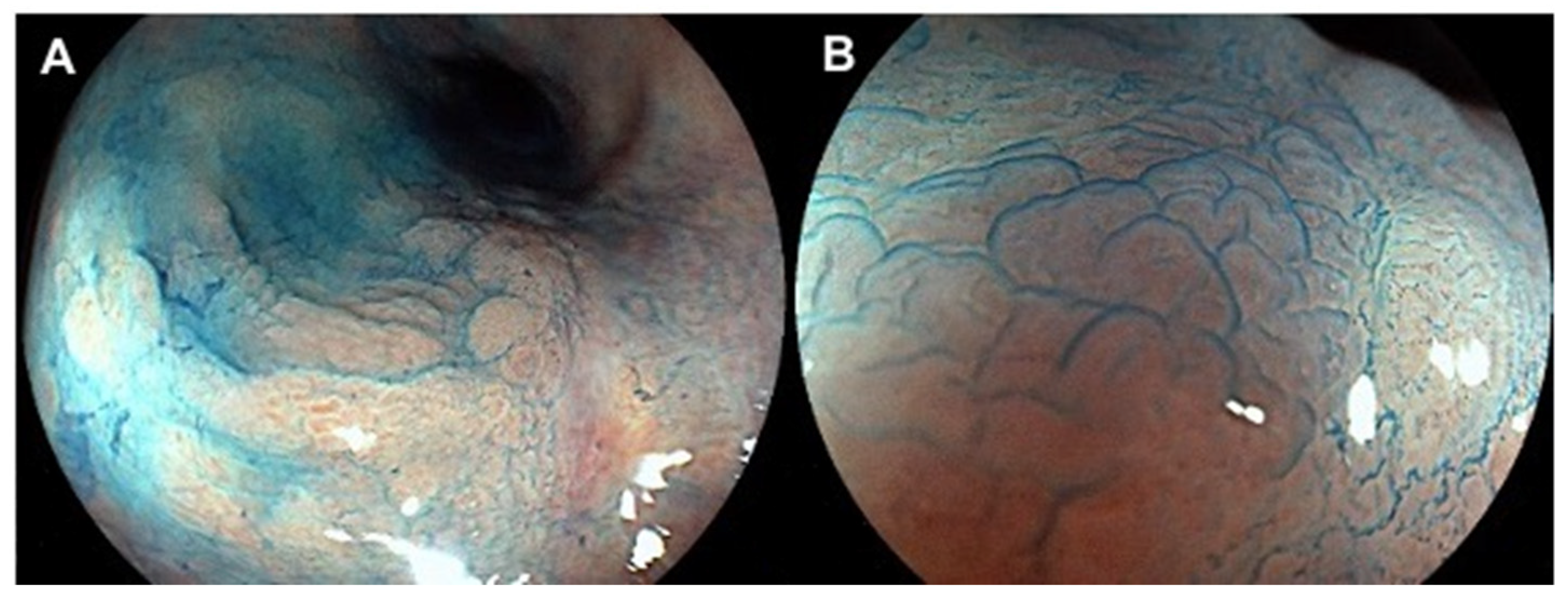
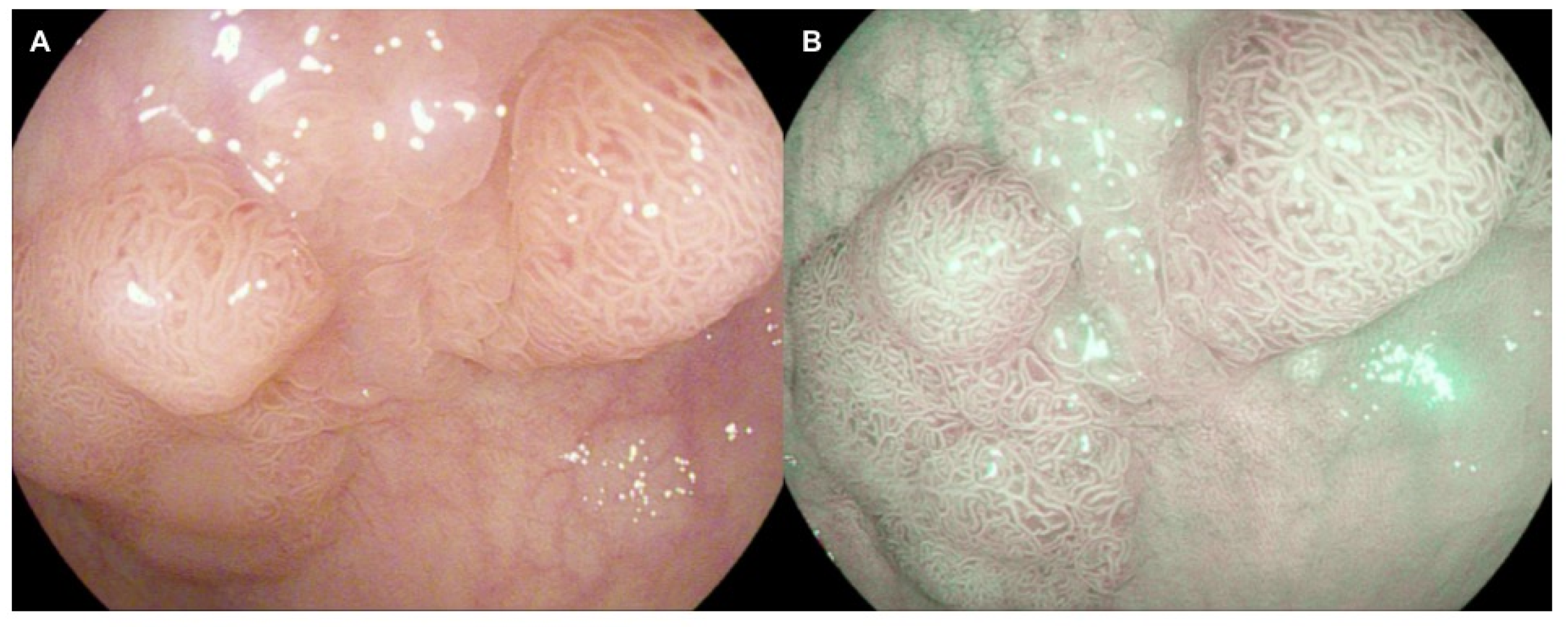
| Authors | Study Design | Methods | Colonoscope Technique | Results |
|---|---|---|---|---|
| Wan et al. [39] | Multi-center prospective randomized controlled trial | 122 UC with 447 colonoscopies. Randomization 1:1:1 to: HD-WLE with targeted biopsies (WLT) (n = 43) HD-WLE with random biopsies (WLR) (n = 40) HD-DCE with targeted biopsies (CET) (n = 39) | WLE vs. DCE with methylene blue | WLR and CET had more examinations that detected dysplasia than WLT (8.1%, 9.7% vs. 1.9%; p = 0.014 and 0.004). During a long-term follow-up (>36 months), CET exhibited more exams with dysplasia detection than WLT (13.3% vs. 1.6%, p = 0.015) |
| Alexandersson et al. [40] | Single-center prospective randomized controlled trial | 305 IBD. Randomization 1:1 to: HD-WLE with targeted plus random biopsies (n = 153) HD-DCE with targeted plus random biopsies (n = 152) | WLE vs. DCE with indigo carmine | DCE identified more colonic dysplasia than HD-WLE (17 vs. 7, p = 0.032) |
| Kim et al. [41] | Single-center retrospective study | 98 IBD with 290 colonoscopies. Comparison of HD-DCE (n = 159) vs. WLE (HD n = 124, SD n = 7) | WLE vs. DCE with methylene blue or indigo carmine | DCE achieved a higher dysplasia diagnostic yield compared to WLE (40.9% vs. 23.7%, p = 0.002). DCE identified a higher number of both polypoid and non-polypoid lesions than WLE. |
| Sekra et al. [42] | Single-center retrospective cohort study | 110 IBD. Comparison of HD-DCE with targeted biopsies (n = 34) vs. HD-WLE with targeted plus random biopsies (n = 76) | WLE vs. DCE with methylene blue or indigo carmine | DCE detected nonpolypoid dysplasia in 11.8% patients while HD-WLE did not identified any dysplastic lesion (risk difference 11.8, 95% CI 0.9–22.6, p = 0.008). No difference were observed in the polypoid dysplasia detection rate (p = 0.12) between the two techniques. |
| Deepak et al. [43] | Multi-center retrospective cohort study | 95 IBD. Subjects with dysplasia on index WLE who subsequently underwent CE | WLE vs. DCE with indigo carmine | 95 patients had an index WLE with dysplasia (55 found on targeted biopsies and 40 on random biopsies). DCE displayed a higher likelihood to identify flat dysplasia compared to WLE (OR 19.3, 95% CI 9.5–39.3). |
| Carballal et al. [44] | Multi-centre prospective cohort study | 350 IBD. Comparison of WLE (SD-WLE 41.5%, HD-WLE 58.5%) and DCE performed in the same procedure. | WLE vs. DCE with indigo carmine | 94 dysplastic lesions were identified. WLE missed 40/94 dysplastic lesions with a 57.4% incremental yield for DCE. The incremental diagnostic yield was similar in SD and HD-WLE (51.5% vs. 52.3%, p = 0.30). |
| Coelho-Prabhu et al. [45] | Single-center retrospective cohort study | 808 IBD. Comparison of HD-WLE with targeted plus random biopsies (n = 658) vs. HD-DCE with targeted plus random biopsies (n = 150). | WLE vs. DCE with indigo carmine | Polypoid dysplasia and dysplasia on random biopsies were both higher in DCE than HD-WLE (Polypoid: 33.0% vs. 12.0% respectively, p < 0.01. Random: 10% vs. 3.6% respectively, p < 0.001). Adjustment for dysplasia risk factors revealed a similar diagnostic yield between the two techniques. |
| Mooiweer et al. [46] | Multi-center retrospective study | 2242 IBD. Comparison of DCE with targeted biopsies (n = 440) vs. WLE with targeted plus random biopsies (n = 1802). | WLE vs. DCE with methylene blue or indigo carmine | Dysplasia detection rate was similar in each group (DCE 11% vs. WLE 10%, p = 0.80). Targeted biopsies displayed a comparable dysplasia diagnostic yield in both techniques (p = 0.30). |
| Clarke et al. [47] | Single-center retrospective case-control study | 187 IBD. Comparison of HD-DCE (n = 98) vs. HD-WLE (n = 89). | WLE vs. DCE with methylene blue or indigo carmine | Dysplastic lesions detection rate was not significantly different between DCE and HD-WLE (10.2% vs. 6.7% respectively, p = 0.39). |
| Iacucci et al. [48] | Single-center randomized prospective trial | 270 IBD. Randomization 1:1:1 to: HD-DCE (n = 90) HD-VCE (n = 90) HD-WLE (n = 90) | WLE vs. DCE with methylene blue or indigo carmine vs. VCE (i-SCAN 2–3) | The diagnostic yield for neoplastic lesions (polypoid, non-polypoid, and CRC) was similar in the three groups (WLE 18.9%, DCE 17.8%, VCE 11.1%; p = 0.91). |
| Yang et al. [49] | Multicenter prospective randomized controlled trial | 210 UC. Randomization 1:1 to: HD-DCE with targeted biopsies (n = 108) HD-WLE with targeted plus random biopsies (n = 102) | WLE vs. DCE with methylene blue or indigo carmine | HD-WLE and DCE achieved similar colitis-associated dysplasia detection rate (5.6% vs. 3.9% respectively, p = 0.749). |
| Authors | Study Design | Methods | Colonoscope Technique | Results |
|---|---|---|---|---|
| Leifeld et al. [50] | Multi-center prospective randomized study | 159 UC. Subjects underwent two colonoscopies (HD-WLE and HD-VCE) in a randomized sequence. | WLE vs. VCE (NBI) | NBI detected a comparable number of intraepithelial neoplasia to HD-WLE (31 vs. 26, p = 0.888). Considering only targeted biopsies in both groups, NBI showed a trend of more detection of dysplasia (1.6 times) than HD-WLE (24 vs. 15, p = 0.175). |
| Kandiah et al. [51] | Multi-center randomized controlled trial | 188 IBD. Randomization 1:1 to HD-VCE (n = 94) or HD-WLE (n = 94) with targeted plus random biopsies in both arms | WLE vs. VCE (i-SCAN OE mode 2) | No difference was observed in the neoplasia detection between the two techniques (VCE 14.9% vs. WLE 24.2%; p = 0.14). |
| Bisschops et al. [52] | Multi-center randomized controlled trial | 131 UC. Randomization 1:1 to HD-VCE (n = 65) or HD-DCE (n = 66) with targeted biopsies in both arms | VCE (NBI) vs. DCE with methylene blue | No difference was found in the detection of colitis-associated neoplasia between DCE and NBI [21.2% vs. 21.5%; OR 1.02 (95% CI 0.44–2.35, p = 0.964)]. |
| Gulati et al. [53] | Single-center randomized crossover trial | 48 IBD. Subjects underwent two colonoscopies (HD-DCE and HD-VCE) in a randomized sequence (1:1). | VCE (FICE) vs. DCE with indigo carmine | The diagnostic accuracy for the diagnosis of dysplasia applying DCE or VCE was respectively 76.9% vs. 93.7%; DCE missed 2 dysplastic lesions (18.2%) while VCE 1 dysplastic lesion (9.1%) [OR 2.0 (95% CI 0.10 to 118.0)]. |
| González-Bernardo et al. [54] | Single-center prospective randomized study | 129 IBD. Randomization 1:1 to HD-VCE (n = 62) or HD-DCE (n = 67) with targeted biopsies in both arms. | VCE (i-SCAN 1) vs. DCE with indigo carmine | No difference in the rate of detection of neoplastic lesions was observed between the two techniques (DCE 17.9% vs. VCE 11.3%; p = 0.2). |
| López-Serrano et al. [55] | Single-center retrospective case-control study | 191 IBD. Comparison of HD-DCE (n = 98) vs. HD-VCE (n = 93) with targeted biopsies in both groups. | VCE (i-SCAN twin-mode 1–3) vs. DCE with indigo carmine | No significant difference in dysplasia detection was observed in the per lesion (DCE 14.6% vs. VCE 15.6%, p = 0.526) and per patient analysis (DCE 12.2% vs. VCE 9.7%, p = 0.647). |
| Authors | Study Design | Methods | Colonoscope Technique | Results |
|---|---|---|---|---|
| Bopanna et al. [56] | Single-center prospective randomized study | 28 UC. HD-WLE with 4 quadrantic random biopsies every 10 cm. | HD-WLE | No dysplasia was found in the 924 biopsy samples. Indefinite for dysplasia was observed in only seven biopsies (0.7%). |
| Gasia et al. [57] | Single-center retrospective audit | 454 IBD. Assessing the role of surveillance strategies: SD-WLE, HD-WLE, VCE, DCE, random plus targeted biopsies, targeted biopsies only. | SD-WLE, HD-WLE, VCE (iSCAN) DCE with methylene blue or indigo carmine | Targeted biopsies with HD systems achieved a higher neoplasia diagnostic yield than random plus targeted biopsies with HD systems (respectively 19.1% vs. 10.4%, p = 0.02). |
| Watanabe et al. [58] | Multi-center randomized controlled trial | 221 UC. Randomization 1:1 to HD-WLE with targeted plus random biopsies (n = 107) and HD-WLE with only targeted biopsies (n = 114). | HD-WLE in the majority of cases | Targeted biopsies group detected a similar amount of neoplasia compared to random plus targeted biopsies group (respectively 11.4% vs. 9.3%; p = 0.617). |
| Hata et al. [59] | Multi-center retrospective cohort study | 195 UC. Comparing long-term efficacy of targeted vs. targeted plus random biopsies using follow-up data of the Watanabe et al. trial. | HD-WLE in the majority of cases | The likelihood to develop CRC in subjects with a negative examination was low (Invasive CRC: 0.77 per 1000 patient-years. Advanced neoplasia HGD/CRC-TIS: 2.3 per 1000 patient-years). |
| Moussata et al. [60] | Multi-center prospective cohort study | 1000 IBD. Evaluation of the role of additional random biopsies in HD-DCE. | HD-DCE with indigo carmine | Random biopsies exhibited a low dysplasia diagnostic yield (0.2% per biopsy, 68/31865). Personal history of neoplasia, tubular colon, and PSC were independently associated with the detection of dysplasia by random biopsies. |
| Hu et al. [61] | Multi-center retrospective study | 300 IBD contributing 442 colonoscopes with detection of dysplasia. Determination of the additional dysplasia diagnostic yield by random biopsies in HD-WLE and HD-DC. | HD-WLE and HD-DCE | Dysplasia discovered by random biopsies was linked to longer disease duration (OR 1.04, 95% CI 1.01–1.07), active inflammation (OR 2.89, 95% CI 1.26–6.67), PSC (OR 3.66, 95% CI 1.21–11.08). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbiadini, R.; D’Amico, F.; De Marco, A.; Terrin, M.; Zilli, A.; Furfaro, F.; Allocca, M.; Fiorino, G.; Danese, S. Colorectal Cancer Surveillance in Patients with Inflammatory Bowel Diseases: Chromoendoscopy or Non-Chromoendoscopy, That Is the Question. J. Clin. Med. 2022, 11, 509. https://doi.org/10.3390/jcm11030509
Gabbiadini R, D’Amico F, De Marco A, Terrin M, Zilli A, Furfaro F, Allocca M, Fiorino G, Danese S. Colorectal Cancer Surveillance in Patients with Inflammatory Bowel Diseases: Chromoendoscopy or Non-Chromoendoscopy, That Is the Question. Journal of Clinical Medicine. 2022; 11(3):509. https://doi.org/10.3390/jcm11030509
Chicago/Turabian StyleGabbiadini, Roberto, Ferdinando D’Amico, Alessandro De Marco, Maria Terrin, Alessandra Zilli, Federica Furfaro, Mariangela Allocca, Gionata Fiorino, and Silvio Danese. 2022. "Colorectal Cancer Surveillance in Patients with Inflammatory Bowel Diseases: Chromoendoscopy or Non-Chromoendoscopy, That Is the Question" Journal of Clinical Medicine 11, no. 3: 509. https://doi.org/10.3390/jcm11030509
APA StyleGabbiadini, R., D’Amico, F., De Marco, A., Terrin, M., Zilli, A., Furfaro, F., Allocca, M., Fiorino, G., & Danese, S. (2022). Colorectal Cancer Surveillance in Patients with Inflammatory Bowel Diseases: Chromoendoscopy or Non-Chromoendoscopy, That Is the Question. Journal of Clinical Medicine, 11(3), 509. https://doi.org/10.3390/jcm11030509






