Abstract
The efforts made in the last decade regarding the molecular landscape of acute myeloid leukemia (AML) have created the possibility of obtaining patients’ personalized treatment. Indeed, the improvement of accurate diagnosis and precise assessment of minimal residual disease (MRD) increased the number of new markers suitable for novel and targeted therapies. This progress was obtained thanks to the development of molecular techniques starting with real-time quantitative PCR (Rt-qPCR) passing through digital droplet PCR (ddPCR) and next-generation sequencing (NGS) up to the new attractive metabolomic approach. The objective of this surge in technological advances is a better delineation of AML clonal heterogeneity, monitoring patients without disease-specific mutation and designing customized post-remission strategies based on MRD assessment. In this context, metabolomics, which pertains to overall small molecules profiling, emerged as relevant access for risk stratification and targeted therapies improvement. In this review, we performed a detailed overview of the most popular modern methods used in hematological laboratories, pointing out their vital importance for MRD monitoring in order to improve overall survival, early detection of possible relapses and treatment efficacy.
1. Introduction
Acute myeloid leukemia (AML) is a clonal disorder that affects myeloid progenitor cells residing in the bone marrow (BM). This implies altered differentiation with subsequent abnormal proliferation and accumulation of inadequately matured myeloid cells [1,2]. AML arises mostly as a “de novo” neoplasm in healthy individuals; however, secondary forms of AML (sAML) derived from myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPNs) or therapies-related (i.e., topoisomerases II, radiation or chemotherapy) are probable [3]. From a molecular point of view, diagnosis is involved with a chromosomal translocation involving crucial genes such as t(8:21), resulting in the formation of RUNX1-RUNX1T1 fusion gene [4] t(15:17), which generates chimeric PML-RARA [5] and inv(16), involving the core binding factor β (CBF-β) and the smooth muscle myosin heavy chain (SMMHC), MYH11 [6].
A cytogenetic profile has allowed stratifying cases into groups that are favorable, intermediate and adverse-risk. Complete identification of genetic mutations has enhanced the previous classification, and it has been useful in determining personalized prognosis and risk stratification. Currently, NPM1, FLT-3, CEBPA, TP53, RUNX1 and ASXL1 are included to define the profile of prognostic-risk groups [6,7].
To date, diagnosis of AML according to the redesigned guidelines of the World Health Organization (WHO) must provide immunophenotypic, cytogenetic and molecular gene panel screening [8]. Moreover, research efforts in the last decade, carried out on large cohorts of AML patients highlighted the pivotal role of signal transduction alterations in promoting the onset of AML, including non-genomic loss of function of tumor suppressors [9,10]. With reference to this, epigenetic and splicing deregulations or mutations have arisen as third-class mutations due to their impact on cellular differentiation and proliferation. IDH-1, IDH-2, DNMT3A and TET2 emerged in this context [11]. These acquired driver alterations are expressed in more than 40% of AML cases and may be associated with leukemia progression when occurring with other mutations. Currently, they are evaluated in mostly relapsed cases, knowing that new drugs and specific inhibitors are available (i.e., IDH inhibitors and hypomethylating agents) [12,13].
Although ongoing studies have shown improvement in the outcome of AML patients, the real prognosis remains poor. Approximately 70% of patients aged ≥65 died within one year of diagnosis, despite the newly available target treatment options [14]. The molecular identity of AML, enhanced and revised several times from 1976 to 2016, proved its intrinsic genetic complexity. In this direction, diagnosis and prognosis profiles are currently achieved with more sensitive techniques and are used in tandem with canonical methods such as flow cytometry and cytogenetics in order to improve the monitoring of minimal residual disease (MRD), to optimize the treatment, increase overall survival (OS) and to prevent relapse [15,16,17]. In this review, we outlined molecular testing advised for AML diagnosis and measurable residual disease (MRD) assessment in order to translate molecular techniques into clinical practice. Our major focus will be RT-qPCR, digital PCR (ddPCR), next-generation sequencing (NGS) and the recent most attractive approach—metabolomic profiling (Figure 1).
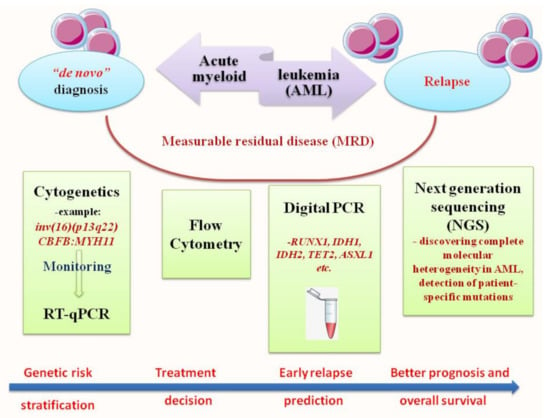
Figure 1.
Workflow of diagnostic methods used in “de novo” or relapsed form of AML, as well as its MRD assessment. This scheme highlights the network of methods used in hematology laboratories and the main targets tested, divided into four levels: cytogenetic and RT-qPCR, flow cytometry, digital PCR and next generation sequencing (NGS).
2. RT-qPCR: The Gold Standard for Diagnosis and Prognosis Stratification in AML
The detection of leukemic cells moved in the last two decades from immune-phenotyping to polymerase chain reaction (PCR) and real-time quantitative PCR (RT-qPCR) [18]. RT-qPCR has been consolidated not only for gene expression measurement and common fusion transcripts detection but also for genetic mutations in genes, such as NPM1, FLT3-ITD, CEBPA, IDH1/2, KIT, RAS, RUNX1 and TP53 or gene overexpression (WT1). It is commonly used as a standardized method in hematology laboratories to obtain a diagnosis and to monitor the kinetics of MRD without inter-laboratory mismatch [18,19]. This technique was shown to be reproducible, accurate and highly sensitive for MRD monitoring, with a significant capacity in predicting prognosis, treatment effectiveness and relapse risk [20] (Figure 2).
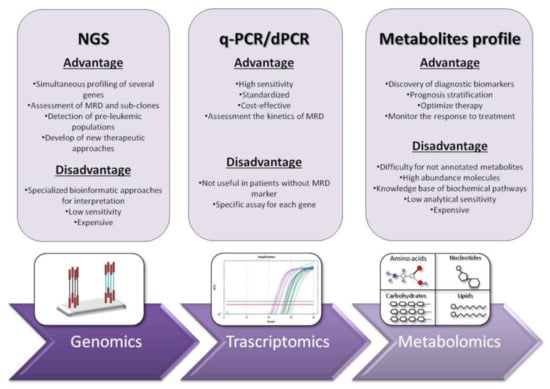
Figure 2.
Schematic comparison of “omic” technologies with their main advantages and disadvantages.
In relation to this, the European LeukemiaNet (ELN), a group of 24 international experts in the clinical and translational knowledge in MRD in AML, has established the RT-qPCR approach as “highly sensitive” and as the “gold standard” [21].
ELN indicates that molecular evaluation of PML-RARA, RUNX1/RUNX1T1, CBFB-MYH11 and mutated NPM1 need to be considered as a standard of care for AML patients, and it recommends performing that assessment at diagnosis and every 3 months for 24 months after treatment editing [16].
PML-RARA is the fusion gene responsible for acute promyelocytic leukemia (APL). Three main alternative transcripts (bcr1, bcr2 and bcr3) may arise according to the location of breakpoints [5]. RT-qPCR has proved to be highly sensitive, readily standardized and to date represent the gold standard analysis for diagnosis and treatment monitoring of APL. MRD follow-up in patients treated with ATRA or ATO is considered important to achieve molecular remission [22]. Furthermore, given the small rates of relapses even in patients with intermediate-risk, the endpoint of treatment (previously associated with PCR negativity) has been substituted with a suggestion to maintain the treatment plan and MRD monitoring in BM until negativity, even if PML-RARA levels remain detectable [16]. Monitoring MRD for at least two years after the end of therapy remains mandatory for high-risk APL [16]. The molecular assessment involved the use of a single RT-qPCR protocol, meaning three reactions, one for each PML-RARA transcript [18]. This approach proved to be too laborious and costly. Consequently, a 3-plex RT-qPCR assay has been designed for fast molecular diagnosis and MRD monitoring of APL. Composed of multiple primers and probes, it allows seeing simultaneously all three main PML-RARA fusion transcripts [23].
Core-binding factor acute myeloid leukemia (CBF-AML) is a subtype of AML characterized by RUNX1/RUNX1T1 translocation or inv(16) and associated with a better prognosis compared to other types of AML [4,5]. RT-qPCR analysis of MRD has proved to be a sensitive and accurate method regarding peripheral blood (PB) levels of RUNX1/RUNX1T1 after consolidation therapy and during remission and in predicting relapse risk [24]. Jourdan et al. [25] illustrated that 3-log MRD of RUNX1/RUNX1T1 or inv(16) reduction is useful for discriminating high-risk patients from low-risk patients. However, a recent study investigating the real contribution of measuring transcript kinetics of CBF-AML showed that the majority of relapses were not predicted by molecular monitoring and occurred in a very short period of time, suggesting that MRD monitoring may be poorly informative in the follow-up of CBF-AML patients [26]. Moreover, the permanence of a low amount of positive transcripts in patients with long-term remission without effects on treatment outcome has encouraged the development of more accurate methods, such as ddPCR [23].
NPM1 has an incidence of mutation near to 30–40% of adult AML and is frequently associated with normal karyotype (50–60%). To date, more than 50 different mutations are described [27]. Types A, B and D are the most abundant (approximately 90% of NPM1 mutated AML patients) [28]. Being one of the most frequent molecular lesions observed in AML, it is an optimal leukemia-specific MRD target, with implications in clinical practice [29]. However, RT-qPCR is limited to patients carrying out types A, B and D since commercial plasmid standards are available for them.
ELN recommends the monitoring of NPM1 transcripts in BM and PB. The recurrence of NPM1 mutation in BM after treatment and the not attainment of a 4-log reduction in PB requires closer monitoring every 4 weeks for at least 3 months [16]. Indeed, even if NPM1 mutation is classified as a favorable risk, its persistence after the second cycle of chemotherapy was associated with a higher relapse risk and reduction in OS independently of other prognostic factors [30]. Finally, NPM1 qPCR analysis has proved to be essential as a marker of allogeneic stem cell transplantation in poor responders [31,32].
Nevertheless, RT-qPCR has some limitations: (1) more than 60% of AML patients cannot be analyzed by RT-qPCR because they lack common MRD molecular targets; (2) RT-qPCR needs a plasmid standard curve for each molecular target analysis, limiting its usage; (3) identification of the most clinically significant time-points and MRD thresholds; and (4) detection limit in monitoring AML patients with long term remission, where the level of fusion transcript is extremely low. These difficulties can interfere with risk assessment, and they have driven to development of droplet digital PCR (ddPCR), a recently designed technology that can reach high-precision absolute quantification (Figure 2).
3. Digital PCR: Emerging Approach for Diagnosis and Follow-Up in Myeloid Malignancies
Digital polymerase chain reaction (dPCR), or more precisely called droplet digital polymerase chain reaction (ddPCR), is a superior adaptation of real-time quantitative polymerase chain reaction (RT-qPCR). Since its first use in 1999 to detect ras mutations in colon cancer patients, it has been used primarily to detect mutations in genes linked to cancer genesis [33]. Although not a new method, dPCR has only recently entered the focus of scientific interest. Its field of application is increasing, not only in basic science but also in clinical diagnosis [34,35,36]. As with the classical PCR method, dPCR uses the same primer sets and fluorescent and enzymatic reagents. The difference is that the sample of interest is divided into thousands of individual PCRs-compartmentalisation, creating an environment with limited dilution. This eliminates the need to create a standard curve and achieves high precision and sensitivity [33]. Then, at the end of the PCR, fluorescence is measured (end-point PCR). Furthermore, Poisson statistics are used for data analysis. Likewise, ddPCR operates on the principle of fractionation of the sample of interest at about 20,000 droplets in an aqueous-oil emulsion, followed by a PCR reaction on each individual droplet [37].
The technical similarities and differences between dPCR and RT-qPCR are well defined, and the most important ones are listed as follows: (i) dPCR consists of thousands of individual PCR reactions divided during analysis while in RT-qPCR there is only one exponential amplification of the nucleic acids; (ii) dPCR measures the absolute number of the molecule of interest, while RT-qPCR determines the relative number using a standard curve; and (iii) dPCR has higher sensitivity and better accuracy compared to RT-qPCR [38,39]. The attractiveness of dPCR also increases with the fact that it does not require the use of standard reference curves. Given the existence of specific genetic aberrations in most hematological neoplasms, dPCR is increasingly widely used to make an accurate diagnosis of hematological diseases and to obtain pathological gene quantification [40,41,42].
Nevertheless, the genetic complexity and molecular profile of AML complicates the decision to select a factor for determining MRD, which in turn is necessary for better risk stratification and therapy selection. In addition, the different approaches available for determining MRD have not been the subject of international standardization to date. Thus, monitoring patients with AML and early detection of possible relapse remains a major clinical challenge [10,16]. In this regard, the emerging dPCR approach is increasingly used in the detection of somatic mutations and the determination of MRD among AML patients [43]. The subclonal heterogeneity of AML requires the combined detection of multiple mutations simultaneously for better insight into disease progression and more recently for the implementation of the new target therapeutic agents.
In general, proving the presence of NPM1 mutations and the fusion genes (RUNX1-RUNX1T1, CBFB-MYH11 and PML-RARA) by using the dPCR technique and then evaluating them after induction and consolidation chemotherapy are suitable approaches for molecular MRD assessment [16]. There are several other somatic mutations that can be considered for MRD monitoring in AML, such as DNMT3A, NRAS, ASXL1, TET2, FLT3-ITD, FLT3-TKD, IDH1, IDH2, WT1, etc. A recent study evaluated the use of a newly developed double drop-off droplet dPCR (DDO-ddPCR) assay for mutation screening and AML disease monitoring using cell-free DNA [43]. In the study, assays were developed to determine the following mutations: NPM1 (a marker of residual disease) and IDH2 (therapeutic target for enasidenib). In conclusion, this new dPCR assay offers rapid diagnosis and detection of somatic mutations in AML patients, crucial for a prompt and targeted therapeutic decision, and then facilitates disease monitoring. The cost–benefit compared to other modern diagnostic methods such as NGS is on the dPCR side in terms of price, availability and sensitivity.
Several other studies have analyzed the IDH1/IDH2 mutation as an appropriate marker for molecular monitoring in AML using dPCR [44,45,46]. Given the frequent presence of these mutations in the molecular profile of AML as well as the development of new target therapies, IDH1/IDH2 will be the focus of future scientific research. dPCR can also be used to quantitatively assess the WT1 molecular marker in AML patients with high accuracy [47]. Its importance in disease follow-up has been recognized for years, and the RT-qPCR method has been standardized for its determination [48].
Finally, allogeneic hematopoietic stem cell transplantation (HSCT) as a curative method remains one of the greatest successful hopes over AML. Balsat et al. have shown that the presence of MRD prior to or after HSCT is associated with a negative outcome [32]. Currently, introducing a method into routine clinical practice with the capacity for early prediction of disease relapse is becoming an increasingly desirable goal for therapy. Indeed, eradication of MRD immediately preceding transplant increased the opportunity of long-term survival in AML [49]. dPCR as a method for assessing MRD has proven to be effective and highly trusted for the detection of pre-transplant NPM1 mutation burden and, thus, for the prediction of relapse in AML patients [18,50]. In another study, dPCR proved to be a more effective method of predicting disease relapse than RT-qPCR regarding the time frame [51].
The scientific data so far place dPCR at the zenith of interest when it comes to hematological diseases, especially where we need long-term monitoring. Its reliability as a method with high sensitivity and precision, most importantly in low levels of the disease, has been confirmed in numerous studies. The fact that, unlike RT-qPCR, there is no need for a standard curve and reference material makes it attractive, easier to access and fast to use. However, the main disadvantage of dPCR in monitoring AML patients is that it requires a specially designed assay for each specific gene aberration.
4. Next Generation Sequencing (NGS): Unveiling of the Molecular Landscape in Myeloid Neoplasms
NGS or massively parallel sequencing is a revolutionary method of DNA and RNA sequencing. It is called parallel because it sequences millions of DNA fragments simultaneously. Sequencing may be limited to selected segments of certain genes or to the entire exome [52]. The workflow that NGS runs is as follows: (i) library preparation, (ii) sequencing and (iii) data analysis. In the first stage, the DNA or RNA sample is prepared for sequencing, while fragmenting and adding special adapters to both ends of the fragments. In this manner, the fragments can be amplified. In the next step (ii), the fragments are placed in a flow cell and sequencer. The clusters of DNA or RNA fragments are then amplified in a process called cluster generation, creating millions of copies of single-stranded DNA or RNA. In this very step, chemically modified and fluorescently labeled nucleotides, through the principle of natural complementarity, bind to the DNA template and prevent the next base incorporation. The last step (iii) is data analysis, i.e., the determination of incorporated nucleotides [53].
In the early years of its appearance, NGS platforms were used primarily for cancer research purposes. Recently, they are increasingly emerging as irreplaceable diagnostic tools in clinical settings. So far, there are several commercially available NGS myeloid panels. They target about 30 genes directly or indirectly involved in the pathophysiology of myeloid neoplasms. Depending on their function, these genes may belong to the group of transcription factors, epigenetic modifiers, signal molecules, etc.
The clinical use of NGS proved to be important in demystifying myeloid neoplasms that lack classical chromosomal or gene aberrations. For example, in Ph-MPNs, in addition to the presence of “driver” mutations, the application of NGS in a clinical setting reveals new mutations that facilitate risk stratification and treatment decision [54]. NGS reveals an entirely new concept of disease understanding, pushing several layers deeper into the genetic profile and directly opening the possibility for new therapeutic approaches. The co-occurrence of newly discovered and “driver” mutations also offers a new concept regarding prognosis [55]. Of particular interest here is the detection of a mutation that will predict early progression (development of) in secondary AML. Thus, the co-existence of ASXL1, SRSF2, EZH2, IDH1 and IDH2 in PMF patients is associated with shorter leukemic-free survival and an increased risk of leukemic transformation [54,56]. Similarly, in ET and PV patients, mutations in the IDH2, U2AF1, EZH2, TP53, SH2B3 and SF3B1 genes are associated with a worse prognosis [57]. NGS enables more detailed detection of each patient’s molecular map and further efficient selection of HSCT candidates.
The idea of MRD assessment with NGS in AML patients has existed since the very beginning of NGS expansion in clinical settings. Scientific studies have shown that 96% of AML patients have at least one driver mutation, and 86% have at least two [58]. By improving clinical applicability and increasing sensitivity, NGS can be a valid tool for MRD assessment in AML patients, especially among those with rare gene mutations. One study, based on mutation detection in NPM1 and FLT3-ITD genes, showed that NGS had assured MRD assessment and 95% concordance with RT-qPCR for mutated NPM1 [59].
In a study by Morita et al. using targeted sequencing of 295 genes in 131 AML patients, the lower cumulative incidence of relapse (CIR) and better overall survival (OS) were found among patients who had no residual mutations until 30 days after induction therapy [60]. RUNX1 gene evaluation with NGS is also a possible choice for MRD analysis in AML patients. In one study in this context, mutational burden <3.61% was associated with better event-free survival (EFS) and OS [61]. In a large study of 482 AML patients using a 54-gene NGS panel, samples were sequenced at the time of diagnosis and in the phase of clinical remission after induction chemotherapy. It was found that, in almost 90% of patients, at least one detectable mutation was present at the time of diagnosis. Using the same assay, the same analysis was performed after therapy, and a mutation was observed in 51% of the patients. A conclusion of great importance in this study is the fact that patients who had only DTA mutations (DNMT3A, TET2 and ASXL1) had a reduced risk of developing relapse, while patients with persistent mutations in other genes had an increased risk of developing relapse [62].
The fact that at least one leukemic mutation is present in a large number of AML patients permits us to believe that any of these mutations may be an appropriate marker for MRD, and NGS can provide an effective MRD assessment. Additionally, NGS can detect reciprocal gene rearrangements such as PML-RARA, RUNX1-RUNX1T1 and CBFB-MYH11. The NGS method is particularly superior in detecting intra-chromosomal rearrangements compared to FISH, which can detect these changes with great sensitivity only on larger chromosomes [63]. However, even if NGS can be used to detect MRD markers, association with cytogenetic and PCR-based approaches are essential to quantify correctly the presence of target [64]. At this time, detection of novel mutations or gene variants by NGS is not associated with change in treatment plan since their functional consequences are not yet fully understood. Furthermore, NGS is considered useful to define relapse, but it would be necessary to identify clinically the meaning of novel genetic mutation and their impact on disease patterns.
The future advent of genome-wide approaches in clinical practice could allow the identification of additional driver gene mutations and potential MRD markers suitable for prognosis and innovative therapeutic procedures [65].
NGS offers precise gene sequencing, but what is the true impact of the discovered mutations on leukemogenesis? These questions remain unclear to clinicians and scientists; therefore, one of the imperfections of NGS is the inability to determine the impact of a particular mutation. Hence, the challenge of introducing it into routine clinical practice [54]. Furthermore, a distinction must also be made between leukemia-related somatic mutations and clonal hematopoiesis of indeterminate potential (CHIP). CHIP by definition is a process associated with the aging of hematopoietic cells, in which they form clones that have acquired leukemia-related mutations with an allelic frequency of 2% or more. Thus, in AML patients, even in the period of clinical remission, certain mutations of genes such as TET2, ASXL1, RUNX1, IDH, DNMT3A and others may be present [66]. Along this line, another important aspect to consider when interpreting NGS assays includes germline mutations in certain genes that may be involved in leukemogenesis. The role of these germline mutations is not always clear; thus, their numbers are likely to grow in the future as NGS progresses. For example, one study reported germline p53 mutations in 6 of 107 AML patients after cancer treatment [67]. Thus, the role of this mutation in leukemogenesis is undoubtedly clear.
It will be of great importance in the future to create updated and extensive cancer databases that would include all mutations that can initiate the leukemogenic process. Moreover, NGS analysis in AML patients is of particular importance applied to the IDH1, IDH2 or FLT3-ITD/TKD genes, as they may represent a hot spot for target therapy. NGS as a newly introduced method will proceed through many more processes of intensive comparative analysis with existing methods of molecular diagnosis before being applied in clinical practice. A multidisciplinary approach must be taken to overcome technical, economic and organizational aspects.
5. Systemic Metabolomic Profiling: The New Era of Personalized Medicine
The qualitative and quantitative collection of cell metabolites including carbohydrates, vitamins and lipids featured in a specific condition or in a common metabolic reaction is defined as metabolome. It is a result of biochemical reactions catalyzed by the proteins of the proteome. Therefore, the metabolome can be considered as the interaction of genome and proteome information where microenvironmental factors can play an active role.
The understanding of metabolome functions in the past resulted in strong contributions in biological research. Roughly, from the 1920s to the 1960s, the “golden age of biochemistry” clarifies most metabolic networks involved in organisms and that are responsible for nutrient use and energy production. Thus, the main processes such as glycolysis, tricarboxylic acid (TCA), urea cycles respiration, glycogen catabolism, oxidative phosphorylation and the supremacy of ATP in energy transfer reactions have been characterized.
The latest scientific findings and the development of “omics” technologies open a modern concept of metabolism as a complex system operating in network with other biological systems. Metabolomics is the analytical and informatic technique used to study the metabolome and to define cell type, tissue, organ or organism metabolomic signatures in a specific condition [68]. Metabolomics studies allowed understanding disease mechanisms, identifying new diagnostic markers and figuring out drug effects and the individual variation in drug response (pharmacometabolomics). In addition, the metabolomic approach could be unlocking massive potentials for characterizing disease states in translational medicine [69]. The metabolomic approach has considerably impacted the pathophysiology of common complex diseases such as, e.g., Alzheimer [70], cardiovascular diseases [71], diabetes [71,72], asthma [73] and many cancers [74]. In this latest field, it offers a good method for the assessment of diagnosis, prognostication and disease monitoring [74].
In the last ten years, differential metabolomics approaches have been used to untangle AML and have paved the way to new information crucial for diagnosis, prognosis and target identification, although the complexity of the related technologies does make it easy to use in routine clinical practice.
Indeed, this approach provides several skills that many laboratories do not manage: the equipment is very expensive, the laboratory staff needs optimal knowledge on biochemical pathways and the samples must be prepared with the optimized and reproducible method in order to avoid bias associated to technical procedures (Figure 2).
By the Nuclear Magnetic Resonance (NMR)-based metabolism approach, significant serum metabolomic differences between different risk subgroups of AML were found. These studies enriched data concerning several metabolic pathways such as glycolysis, TCA cycle, proteins and lipoproteins biosynthesis, metabolism of fatty acids and cell membrane components [75]. All these results confirmed that the NMR-based metabolomics method was useful for rapid AML diagnosis and prognosis.
Being an early and noninvasive area of interest in AML, metabolomics can improve the investigation of a new biomarker. Combining gas chromatography coupled with triple quadrupole tandem mass spectrometry and statistical analysis, Musharaf et al. studied differentiated metabolic alterations in the serum of patients with acute lymphoblastic leukemia (ALL), and AML Fatty acid palmitic, stearic and oleic acid metabolism emerged as deregulated in patients with acute leukemia, thus representing an essential metabolic pathway associated with disease progression [76].
Metabolomic profiling by ultra-high performance liquid-chromatography mass spectrometry was used to study the metabolome of FLT3-ITD positive AML [77], which until now has been poorly understood. Using ultra-high performance liquid-chromatography-mass spectrometry, they identified a specific metabolomic signature in plasma and in leukemic cells according to FLT3 status, including lysophospholipid metabolism, cysteine/methionine metabolism, tryptophan metabolism, purine metabolism and biosynthesis and carnitine mediated fatty acid oxidation [77,78,79,80].
Recently, a global untargeted metabolomics approach was used to ascertain sorafenib resistance in FLT3/ITD-mutated leukemic cells [81]. Altered glycolytic activity and TCA cycle, increased antioxidant capacity of GSH and reduction in PPP flux rates result as major modifications between sensitive and resistant groups [81]. A combinatorial transcriptomics and metabolomics analysis provided evidence for additional therapeutic targets that are useful for enhancing the efficacy of gilteritinib, another FLT3 inhibitor. Metabolomic alterations are prevalently associated with decreased glutaminolysis and disruption of redox homeostasis, suggesting new therapeutical strategies for a subset of relapsed/refractory AML [82].
Combining different types of “omics” represents a promising future goal in order to offer personalized therapeutic approaches even for AML patients with identifiable and targetable genomic lesions [7,58,83]. Recent genomic-metabolic study on intracellular and biofluid metabolic AML samples allowed identifying a specific NPM1-mutated AML subgroup with high levels of serum choline, trimethylamine-N-oxide and leucine, characterized by common mutations of genes involved in DNA damage response and/or chromatid cohesion (NPM1/cohesion-mut) pathway. These data provided a pattern of the crosstalk between metabolic and genomicspathways in AML. These results, highlighted as an integrated genomic-metabolic study applied in pathology, could pave the way to personalized medicine [84].
6. Conclusions
Although there were enhancements in AML diagnosis, classification and remission achievement, more than 50% of patients relapsed due to the persistence of residual resistant clones. Considering this, the new era of the AML study approach passed through a precise and accurate evaluation of residual disease.
The improvement of new techniques such as ddPCR and NGS, attempting MRD detection, completely modified the relevance of this issue; currently, MRD monitoring has become an objective methodology for setting up remission statuses. In this context, the “minimal” residual disease (MRD) term has been substituted with “measurable” residual disease to emphasize the crucial role of modern techniques in determining the threshold of remission and in activating preventive treatment of molecular relapse. In addition to these technologies, metabolome analysis of AML serum highlighted the possibility to identify significant metabolic signatures suitable for the detection of relapsed patients and of highly responding patients with risk stratification. For this issue, standardization of sample selection and processing will be necessary to improve the use of metabolomics in AML stratification. In the near hematological future, characterizing novel drugs for metabolomics targeting and improving personalized treatment approaches will be challenging.
Author Contributions
Conceptualization: C.P., B.P. and A.J.; writing and original draft preparation: C.P., B.P., A.J. and M.S.A.; review and editing: C.P. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by AIRC (Italian Association for Cancer Research) investigator grant to GS (IG-23344).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- De Kouchkovsky, I.; Abdul-Hay, M. Acute myeloid leukemia: A comprehensive review and 2016 update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Grove, C.S.; Vassiliou, G.S. Acute myeloid leukaemia: A paradigm for the clonal evolution of cancer? Dis. Models Mech. 2014, 7, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Rahul, E.; Gupta, I.; Chopra, A.; Ranjan, A.; Gupta, A.K.; Meena, J.P.; Viswanathan, G.K.; Bakhshi, S.; Misra, A. Molecular and genomic landscapes in secondary & therapy related acute myeloid leukemia. Am. J. Blood Res. 2021, 11, 472. [Google Scholar] [PubMed]
- Höllein, A.; Nadarajah, N.; Meggendorfer, M.; Jeromin, S.; Kern, W.; Haferlach, C.; Haferlach, T. Molecular characterization of aml with runx1-runx1t1 at diagnosis and relapse reveals net loss of co-mutations. HemaSphere 2019, 3, e178. [Google Scholar] [CrossRef] [PubMed]
- Liquori, A.; Ibañez, M.; Sargas, C.; Sanz, M.Á.; Barragán, E.; Cervera, J. Acute promyelocytic leukemia: A constellation of molecular events around a single pml-rara fusion gene. Cancers 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Park, T.S.; Wan, T.S. Recurrent cytogenetic abnormalities in acute myeloid leukemia. Cancer Cytogenet. 2017, 1541, 223–245. [Google Scholar]
- Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Kansal, R. Toward integrated genomic diagnosis in routine diagnostic pathology by the world health organization classification of acute myeloid leukemia. J. Clin. Haematol. 2020, 1, 2. [Google Scholar]
- Carter, J.L.; Hege, K.; Yang, J.; Kalpage, H.A.; Su, Y.; Edwards, H.; Hüttemann, M.; Taub, J.W.; Ge, Y. Targeting multiple signaling pathways: The new approach to acute myeloid leukemia therapy. Signal Transduct. Target. Ther. 2020, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Panuzzo, C.; Stanga, S.; Andreani, G.; Ravera, S.; Maglione, A.; Pironi, L.; Petiti, J.; Shahzad Ali, M.; Scaravaglio, P. Deferasirox-dependent iron chelation enhances mitochondrial dysfunction and restores p53 signaling by stabilization of p53 family members in leukemic cells. Int. J. Mol. Sci. 2020, 21, 7674. [Google Scholar] [CrossRef] [PubMed]
- Panuzzo, C.; Signorino, E.; Calabrese, C.; Ali, M.S.; Petiti, J.; Bracco, E.; Cilloni, D. Landscape of tumor suppressor mutations in acute myeloid leukemia. J. Clin. Med. 2020, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.N.; John, A.J.; Kaufmann, S.H. Resistance to venetoclax and hypomethylating agents in acute myeloid leukemia. Cancer Drug Resist. 2021, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, Y. Isocitrate dehydrogenase inhibitors in acute myeloid leukemia. Biomark. Res. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A.; Guyatt, G.; Abel, G.; Alibhai, S.; Altman, J.K.; Buckstein, R.; Choe, H.; Desai, P.; Erba, H.; Hourigan, C.S. American society of hematology 2020 guidelines for treating newly diagnosed acute myeloid leukemia in older adults. Blood Adv. 2020, 4, 3528–3549. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Ottone, T.; Lavorgna, S.; Venditti, A.; Maurillo, L.; Lo-Coco, F.; Buccisano, F. Mrd in aml: The role of new techniques. Front. Oncol. 2019, 9, 655. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Béné, M.-C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S. Minimal/measurable residual disease in aml: A consensus document from the european leukemianet mrd working party. Blood J. Am. Soc. Hematol. 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Hauwel, M.; Matthes, T. Minimal residual disease monitoring: The new standard for treatment evaluation of haematological malignancies? Swiss Med. Wkly. 2014, 144, w13907. [Google Scholar] [CrossRef]
- Gabert, J.; Beillard, E.; van der Velden, V.H.J.; Bi, W.; Grimwade, D.; Pallisgaard, N.; Barbany, G.; Cazzaniga, G.; Cayuela, J.M.; Cavé, H.; et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia—A Europe against cancer program. Leukemia 2003, 17, 2318–2357. [Google Scholar] [CrossRef]
- Aitken, M.J.; Ravandi, F.; Patel, K.P.; Short, N.J. Prognostic and therapeutic implications of measurable residual disease in acute myeloid leukemia. J. Hematol. Oncol. 2021, 14, 137. [Google Scholar] [CrossRef]
- Ossenkoppele, G.; Schuurhuis, G.J. MRD in AML: Does it already guide therapy decision-making? Hematol. 2014 Am. Soc. Hematol. Educ. Program Book 2016, 2016, 356–365. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A. Diagnosis and management of aml in adults: 2017 eln recommendations from an international expert panel. Blood J. Am. Soc. Hematol. 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Chendamarai, E.; Balasubramanian, P.; George, B.; Viswabandya, A.; Abraham, A.; Ahmed, R.; Alex, A.A.; Ganesan, S.; Lakshmi, K.M.; Sitaram, U. Role of minimal residual disease monitoring in acute promyelocytic leukemia treated with arsenic trioxide in frontline therapy. Blood J. Am. Soc. Hematol. 2012, 119, 3413–3419. [Google Scholar] [CrossRef]
- Chen, Z.; Tong, Y.; Li, Y.; Gao, Q.; Wang, Q.; Fu, C.; Xia, Z. Development and validation of a 3-plex rt-qpcr assay for the simultaneous detection and quantitation of the three pml-rara fusion transcripts in acute promyelocytic leukemia. PLoS ONE 2015, 10, e0122530. [Google Scholar] [CrossRef]
- Willekens, C.; Blanchet, O.; Renneville, A.; Cornillet-Lefebvre, P.; Pautas, C.; Guieze, R.; Ifrah, N.; Dombret, H.; Jourdan, E.; Preudhomme, C. Prospective long-term minimal residual disease monitoring using rq-pcr in runx1-runx1t1-positive acute myeloid leukemia: Results of the french cbf-2006 trial. Haematologica 2016, 101, 328. [Google Scholar] [CrossRef]
- Jourdan, E.; Boissel, N.; Chevret, S.; Delabesse, E.; Renneville, A.; Cornillet, P.; Blanchet, O.; Cayuela, J.-M.; Recher, C.; Raffoux, E. Prospective evaluation of gene mutations and minimal residual disease in patients with core binding factor acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2013, 121, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Puckrin, R.; Atenafu, E.G.; Claudio, J.O.; Chan, S.; Gupta, V.; Maze, D.; McNamara, C.; Murphy, T.; Schuh, A.C.; Yee, K. Measurable residual disease monitoring provides insufficient lead-time to prevent morphological relapse in the majority of patients with core-binding factor acute myeloid leukemia. Haematologica 2021, 106, 56–63. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. Npm1-mutated acute myeloid leukemia: From bench to bedside. Blood 2020, 136, 1707–1721. [Google Scholar] [CrossRef] [PubMed]
- Gorello, P.; Cazzaniga, G.; Alberti, F.; Dell’Oro, M.; Gottardi, E.; Specchia, G.; Roti, G.; Rosati, R.; Martelli, M.; Diverio, D. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (npm1) gene mutations. Leukemia 2006, 20, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Forghieri, F.; Comoli, P.; Marasca, R.; Potenza, L.; Luppi, M. Minimal/measurable residual disease monitoring in npm1-mutated acute myeloid leukemia: A clinical viewpoint and perspectives. Int. J. Mol. Sci. 2018, 19, 3492. [Google Scholar] [CrossRef]
- Tiong, S.; Dillon, R.; Ivey, A.; Kok, C.H.; Kuzich, J.A.; Thiagarajah, N.; Bajel, A.; Potter, N.; Smith, M.; Hemmaway, C. The natural history of npm1mut measurable residual disease (MRD) positivity after completion of chemotherapy in acute myeloid leukemia (AML). Blood 2020, 136, 25–27. [Google Scholar] [CrossRef]
- Lussana, F.; Caprioli, C.; Stefanoni, P.; Pavoni, C.; Spinelli, O.; Buklijas, K.; Michelato, A.; Borleri, G.; Algarotti, A.; Micò, C. Molecular detection of minimal residual disease before allogeneic stem cell transplantation predicts a high incidence of early relapse in adult patients with npm1 positive acute myeloid leukemia. Cancers 2019, 11, 1455. [Google Scholar] [CrossRef] [PubMed]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.-P.; Nibourel, O.; Berthon, C. Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with npm1 mutation: A study by the acute leukemia french association group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Digital pcr. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef] [PubMed]
- Badbaran, A.; Mailer, R.; Dahlke, C.; Woens, J.; Fathi, A.; Mellinghoff, S.C.; Renne, T.; Addo, M.M.; Riecken, K.; Fehse, B. Digital pcr to quantify chadox1 ncov-19 copies in blood and tissues. bioRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Della Starza, I.; Nunes, V.; Lovisa, F.; Silvestri, D.; Cavalli, M.; Garofalo, A.; Campeggio, M.; De Novi, L.A.; Soscia, R.; Oggioni, C. Droplet digital pcr improves ig-/tr-based mrd risk definition in childhood b-cell precursor acute lymphoblastic leukemia. HemaSphere 2021, 5, e543. [Google Scholar] [CrossRef]
- Hiemcke-Jiwa, L.S.; Minnema, M.C.; Radersma-van Loon, J.H.; Jiwa, N.M.; de Boer, M.; Leguit, R.J.; de Weger, R.A.; Huibers, M.M. The use of droplet digital pcr in liquid biopsies: A highly sensitive technique for myd88 p.(l265p) detection in cerebrospinal fluid. Hematol. Oncol. 2018, 36, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Duewer, D.L.; Kline, M.C.; Romsos, E.L.; Toman, B. Evaluating droplet digital pcr for the quantification of human genomic DNA: Converting copies per nanoliter to nanograms nuclear DNA per microliter. Anal. Bioanal. Chem. 2018, 410, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, A.; Petiti, J.; Giugliano, E.; Gottardi, E.M.; Saglio, G.; Cilloni, D.; Fava, C. Standardization of bcr-abl1 p210 monitoring: From nested to digital pcr. Cancers 2020, 12, 3287. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Petiti, J.; Rosso, V.; Andreani, G.; Dragani, M.; Fava, C.; Saglio, G. Digital pcr in myeloid malignancies: Ready to replace quantitative pcr? Int. J. Mol. Sci. 2019, 20, 2249. [Google Scholar] [CrossRef]
- Fava, C.; Varotto, M.; Berchialla, P.; Gottardi, E.; Daraio, F.; Lorenzatti, R.; Giugliano, E.; Barberio, D.; Iurlo, A.; Orlandi, E. Dropled digital pcr may have a prognostic value for predicting relapse after imatinib discontinuation. Clin. Lymphoma Myeloma Leuk. 2016, 16, S62–S63. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Heinrich, M.C.; Dakhil, S.R.; Goldberg, S.L.; Wadleigh, M.; Kuriakose, P.; Cortes, J.; Radich, J.; Helton, B.; Rizzieri, D. Rates of deep molecular response by digital and conventional pcr with frontline nilotinib in newly diagnosed chronic myeloid leukemia: A landmark analysis. Leuk. Lymphoma 2019, 60, 2384–2393. [Google Scholar] [CrossRef]
- Bochicchio, M.T.; Petiti, J.; Berchialla, P.; Izzo, B.; Giugliano, E.; Ottaviani, E.; Errichiello, S.; Rege-Cambrin, G.; Venturi, C.; Luciano, L. Droplet digital pcr for bcr–abl1 monitoring in diagnostic routine: Ready to start? Cancers 2021, 13, 5470. [Google Scholar] [CrossRef] [PubMed]
- Rausch, C.; Rothenberg-Thurley, M.; Buerger, S.A.; Tschuri, S.; Dufour, A.; Neusser, M.; Schneider, S.; Spiekermann, K.; Metzeler, K.H.; Ziemann, F. Double drop-off droplet digital pcr: A novel, versatile tool for mutation screening and residual disease monitoring in acute myeloid leukemia using cellular or cell-free DNA. J. Mol. Diagn. 2021, 23, 975–985. [Google Scholar] [CrossRef]
- Petrova, L.; Vrbacky, F.; Lanska, M.; Zavrelova, A.; Zak, P.; Hrochova, K. Idh1 and idh2 mutations in patients with acute myeloid leukemia: Suitable targets for minimal residual disease monitoring? Clin. Biochem. 2018, 61, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Brambati, C.; Galbiati, S.; Xue, E.; Toffalori, C.; Crucitti, L.; Greco, R.; Sala, E.; Crippa, A.; Chiesa, L.; Soriani, N. Droplet digital polymerase chain reaction for dnmt3a and idh1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica 2016, 101, e157. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Guerrini, F.; Ciabatti, E.; Puccetti, R.; Salehzadeh, S.; Metelli, M.R.; Di Vita, A.; Domenichini, C.; Caracciolo, F.; Orciuolo, E. Digital droplet pcr is a specific and sensitive tool for detecting idh2 mutations in acute myeloid leukemia patients. Cancers 2020, 12, 1738. [Google Scholar] [CrossRef]
- Koizumi, Y.; Furuya, D.; Endo, T.; Asanuma, K.; Yanagihara, N.; Takahashi, S. Quantification of wilms’ tumor 1 mrna by digital polymerase chain reaction. Int. J. Hematol. 2018, 107, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V.; Gottardi, E.; Fava, M.; Schnittger, S.; Weiss, T. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized wt1 assay to enhance risk stratification in acute myeloid leukemia: A european leukemianet study. J. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef]
- Ball, B.; Stein, E.M. Which are the most promising targets for minimal residual disease-directed therapy in acute myeloid leukemia prior to allogeneic stem cell transplant? Haematologica 2019, 104, 1521. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Grimm, J.; Jentzsch, M.; Kloss, L.; Goldmann, K.; Schulz, J.; Beinicke, S.; Häntschel, J.; Cross, M.; Vucinic, V. Digital droplet pcr-based absolute quantification of pre-transplant npm1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann. Hematol. 2018, 97, 1757–1765. [Google Scholar] [CrossRef]
- Valero-Garcia, J.; González-Espinosa, M.d.C.; Barrios, M.; Carmona-Antoñanzas, G.; García-Planells, J.; Ruiz-Lafora, C.; Fuentes-Gálvez, A.; Jiménez-Velasco, A. Earlier relapse detection after allogeneic haematopoietic stem cell transplantation by chimerism assays: Digital pcr versus quantitative real-time pcr of insertion/deletion polymorphisms. PLoS ONE 2019, 14, e0212708. [Google Scholar]
- Behjati, S.; Tarpey, P.S. What is next generation sequencing? Arch. Dis. Child. Educ. Pract. 2013, 98, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.W.; Schrijver, I. Next generation DNA sequencing and the future of genomic medicine. Genes 2010, 1, 38–69. [Google Scholar] [CrossRef]
- Bacher, U.; Shumilov, E.; Flach, J.; Porret, N.; Joncourt, R.; Wiedemann, G.; Fiedler, M.; Novak, U.; Amstutz, U.; Pabst, T. Challenges in the introduction of next-generation sequencing (ngs) for diagnostics of myeloid malignancies into clinical routine use. Blood Cancer J. 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood J. Am. Soc. Hematol. 2017, 129, 667–679. [Google Scholar] [CrossRef]
- Vannucchi, A.M.; Lasho, T.; Guglielmelli, P.; Biamonte, F.; Pardanani, A.; Pereira, A.; Finke, C.; Score, J.; Gangat, N.; Mannarelli, C. Mutations and prognosis in primary myelofibrosis. Leukemia 2013, 27, 1861–1869. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Guglielmelli, P.; Finke, C.M.; Rotunno, G.; Elala, Y.; Pacilli, A.; Hanson, C.A.; Pancrazzi, A.; Ketterling, R.P. Targeted deep sequencing in polycythemia vera and essential thrombocythemia. Blood Adv. 2016, 1, 21–30. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Kölking, B.; Damm, F.; Reinhardt, K.; Klusmann, J.H.; Reinhardt, D.; von Neuhoff, N.; Brugman, M.H.; Schlegelberger, B.; Suerbaum, S. Next-generation sequencing for minimal residual disease monitoring in acute myeloid leukemia patients with flt3-itd or npm1 mutations. Genes Chromosomes Cancer 2012, 51, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Kantarjian, H.M.; Wang, F.; Yan, Y.; Bueso-Ramos, C.; Sasaki, K.; Issa, G.C.; Wang, S.; Jorgensen, J.; Song, X. Clearance of somatic mutations at remission and the risk of relapse in acute myeloid leukemia. J. Clin. Oncol. 2018, 36, 1788. [Google Scholar] [CrossRef]
- Kohlmann, A.; Nadarajah, N.; Alpermann, T.; Grossmann, V.; Schindela, S.; Dicker, F.; Roller, A.; Kern, W.; Haferlach, C.; Schnittger, S. Monitoring of residual disease by next-generation deep-sequencing of runx1 mutations can identify acute myeloid leukemia patients with resistant disease. Leukemia 2014, 28, 129–137. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; Al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.; Gradowska, P.L.; Meijer, R.; Cloos, J. Molecular minimal residual disease in acute myeloid leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef]
- Mertens, F.; Johansson, B.; Fioretos, T.; Mitelman, F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer 2015, 15, 371–381. [Google Scholar] [CrossRef]
- Leisch, M.; Jansko, B.; Zaborsky, N.; Greil, R.; Pleyer, L. Next generation sequencing in aml—On the way to becoming a new standard for treatment initiation and/or modulation? Cancers 2019, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Metzeler, K.H.; Herold, T.; Rothenberg-Thurley, M.; Amler, S.; Sauerland, M.C.; Görlich, D.; Schneider, S.; Konstandin, N.P.; Dufour, A.; Bräundl, K. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2016, 128, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, B.; Eisfeld, A.K.; Nicolet, D.; Mrózek, K.; Blachly, J.S.; Orwick, S.; Lucas, D.M.; Kohlschmidt, J.; Blum, W.; Kolitz, J.E. Persistence of dnmt 3a r882 mutations during remission does not adversely affect outcomes of patients with acute myeloid leukaemia. Br. J. Haematol. 2016, 175, 226–236. [Google Scholar] [CrossRef]
- Zebisch, A.; Lal, R.; Müller, M.; Lind, K.; Kashofer, K.; Girschikofsky, M.; Fuchs, D.; Wölfler, A.; Geigl, J.B.; Sill, H. Acute myeloid leukemia with tp53 germ line mutations. Blood J. Am. Soc. Hematol. 2016, 128, 2270–2272. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Andrjaj, L.; Dudzik, D.; Barbas, C.; Milkovi, L.; Grune, T.; Zarkovj, N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018, 14, 47–58. [Google Scholar]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Metabolite profiling for the identification of altered metabolic pathways in alzheimer’s disease. J. Pharm. Biomed. Anal. 2015, 107, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Laborde, C.M.; Mourino-Alvarez, L.; Posada-Ayala, M.; Alvarez-Llamas, G.; Serranillos-Reus, M.G.; Moreu, J.; Vivanco, F.; Padial, L.R.; Barderas, M.G. Plasma metabolomics reveals a potential panel of biomarkers for early diagnosis in acute coronary syndrome. Metabolomics 2014, 10, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Reinke, S.N.; Gallart-Ayala, H.; Gómez, C.; Checa, A.; Fauland, A.; Naz, S.; Kamleh, M.A.; Djukanović, R.; Hinks, T.S.C.; Wheelock, C.E. Metabolomics analysis identifies different metabotypes of asthma severity. Eur. Respir. J. 2017, 49, 1601740. [Google Scholar] [CrossRef] [PubMed]
- Puchades-Carrasco, L.; Pineda-Lucena, A. Metabolomics applications in precision medicine: An oncological perspective. Curr. Top. Med. Chem. 2017, 17, 2740–2751. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Chen, W.-L.; Wang, J.-H.; Li, N.; Li, J.-M.; Mi, J.-Q.; Zhang, W.-N.; Li, Y.; Wu, S.-F. Rapid diagnosis and prognosis of de novo acute myeloid leukemia by serum metabonomic analysis. J. Proteome Res. 2013, 12, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Siddiqui, A.J.; Shamsi, T.; Choudhary, M.I.; Rahman, A.-u. Serum metabonomics of acute leukemia using nuclear magnetic resonance spectroscopy. Sci. Rep. 2016, 6, 30693. [Google Scholar] [CrossRef] [PubMed]
- Stockard, B.; Garrett, T.; Guingab-Cagmat, J.; Meshinchi, S.; Lamba, J. Distinct metabolic features differentiating flt3-itd aml from flt3-wt childhood acute myeloid leukemia. Sci. Rep. 2018, 8, 5534. [Google Scholar] [CrossRef]
- Bhanot, H.; Reddy, M.M.; Nonami, A.; Weisberg, E.L.; Bonal, D.; Kirschmeier, P.T.; Salgia, S.; Podar, K.; Galinsky, I.; Chowdary, T.K. Pathological glycogenesis through glycogen synthase 1 and suppression of excessive amp kinase activity in myeloid leukemia cells. Leukemia 2015, 29, 1555–1563. [Google Scholar] [CrossRef]
- Tan, G.; Zhao, B.; Li, Y.; Liu, X.; Zou, Z.; Wan, J.; Yao, Y.; Xiong, H.; Wang, Y. Pharmacometabolomics identifies dodecanamide and leukotriene b4 dimethylamide as a predictor of chemosensitivity for patients with acute myeloid leukemia treated with cytarabine and anthracycline. Oncotarget 2017, 8, 88697. [Google Scholar] [CrossRef][Green Version]
- Tiziani, S.; Lodi, A.; Khanim, F.L.; Viant, M.R.; Bunce, C.M.; Günther, U.L. Metabolomic profiling of drug responses in acute myeloid leukaemia cell lines. PLoS ONE 2009, 4, e4251. [Google Scholar] [CrossRef]
- You, X.; Jiang, W.; Lu, W.; Zhang, H.; Yu, T.; Tian, J.; Wen, S.; Garcia-Manero, G.; Huang, P.; Hu, Y. Metabolic reprogramming and redox adaptation in sorafenib-resistant leukemia cells: Detected by untargeted metabolomics and stable isotope tracing analysis. Cancer Commun. 2019, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.E.Z.; Lu, X.; Talebi, Z.; Jeon, J.Y.; Buelow, D.R.; Gibson, A.A.; Uddin, M.E.; Brinton, L.T.; Nguyen, J.; Collins, M. Gilteritinib inhibits glutamine uptake and utilization in flt3-itd–positive aml. Mol. Cancer Ther. 2021, 20, 2207–2217. [Google Scholar] [CrossRef]
- Tyner, J.W.; Tognon, C.E.; Bottomly, D.; Wilmot, B.; Kurtz, S.E.; Savage, S.L.; Long, N.; Schultz, A.R.; Traer, E.; Abel, M. Functional genomic landscape of acute myeloid leukaemia. Nature 2018, 562, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Mengucci, C.; Padella, A.; Fonzi, E.; Picone, G.; Delpino, C.; Nanni, J.; De Tommaso, R.; Franchini, E.; Papayannidis, C. Integrated genomic-metabolic classification of acute myeloid leukemia defines a subgroup with npm1 and cohesin/DNA damage mutations. Leukemia 2021, 35, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).