Abstract
Background: Scientific statements recommend multimodality imaging in myocardial infarction with non-obstructive coronary arteries (MINOCA) to define the underlying cause. Aim: We evaluated the diagnostic yield of intravascular optical coherence tomography (OCT) and cardiac magnetic resonance (CMR) in the MINOCA setting. Methods: In this prospective, single center, observational pilot study, we enrolled patients with MINOCA without previous coronary interventions. All patients underwent three vessel OCT, followed by CMR. Imaging results were combined to determine the mechanism of MINOCA, when possible. Results: We enrolled 10 patients in this pilot study. Women constituted 50% of the analyzed population. The mean age of patients was 52 years. ST-segment elevation was found in 30% of patients. A possible culprit lesion was identified by OCT in 70% of participants, most commonly plaque rupture or erosion. An ischemic pattern of CMR abnormalities was identified in 70% of participants. Myocarditis and Tako-Tsubo were identified in 30%. A cause of MINOCA was identified in all patients using multimodality imaging, while using OCT alone identification occurred in only 70% of patients. Conclusion: In this pilot study, multimodality imaging with OCT and CMR identified potential mechanisms in all patients with a diagnosis of MINOCA, and it has the potential to guide medical therapy for secondary prevention.
1. Introduction
Myocardial infarction with nonobstructive coronary arteries (MINOCA) is characterized as acute myocardial infarction (AMI) without evidence of obstructive stenosis in coronary arteries in major coronary arteries (stenosis severity < 50%) []. MINOCA is found in approximately 3% to 15% of patients with AMI and disproportionately affects women [,,]. Although in clinical practice the underlying mechanism of MINOCA is frequently undetermined, its prognosis remains serious, with a 1-year mortality ranging from 5–10%, reaching up to 16% at 5-year follow-up [,]. Even though, elevated troponin levels and chest discomfort are not specific solely for AMI, MINOCA is caused by a vastly heterogeneous group of myocardial or vascular disorders, thus it should always be considered merely as a working diagnosis, which requires further investigation. To confirm MINOCA other underlying causes of elevated troponin levels must be ruled out. Therefore, finding prognostic markers and determining the specific pathophysiological mechanism is crucial to provide appropriate treatment strategies in patients with MINOCA diagnosis. Major pathophysiological mechanisms of MINOCA include plaque rupture or erosion, coronary thromboembolism, coronary artery spasm, spontaneous coronary artery dissection, Tako-Tsubo cardiomyopathy and myocarditis. Importantly, plaque disruption may be missed in coronary angiogram and occur in areas that initially appear normal []. Optical coherence tomography (OCT) due to its high resolution is able to precisely evaluate the morphological characteristics of atherosclerotic plaque []. However, cardiac magnetic resonance (CMR) allows for detection of myocardial fibrosis with a high diagnostic accuracy and is considered the gold standard for in vivo myocardial tissue characterization [].
The purpose of this pilot study was to evaluate in a prospective cohort of patients of MINOCA the diagnostic yield of combined OCT and CMR imaging.
2. Materials and Methods
In this prospective, single-center study, consecutive patients aged 18 years and older who presented with suspected AMI and had no obstructive (>50% stenosis) coronary lesions on angiography and no specific alternative diagnosis for the clinical presentation were enrolled. All patients underwent OCT imaging in all major coronary arteries during the initial procedure or up to 24 h after the initial procedure. Subsequently, all patients underwent CMR during hospital stay. Major exclusion criteria were renal failure in stage ≥3, allergy to contrast media, estimated survival of <2 years, active systemic inflammatory process and pregnancy. The study was approved by Bioethical Committee and conformed to the Declaration of Helsinki. All patients signed informed consent. This study is a pilot phase of a large ongoing registry on MINOCA.
2.1. Coronary Angiography and Optical Coherence Tomography
Coronary angiography was performed using standard techniques through the transradial or transfemoral approach. Angiographic views were acquired in optimal projection angels using 6-Fr diagnostic catheters with manual contrast injections. Patients were excluded from participation if the angiogram showed any coronary stenosis ≥50% or excessive tortuosity and/or abnormal origin of the coronary artery that, in the opinion of the operator, increased the risk of OCT imaging. Subsequently, OCT of all major coronary arteries was performed during the initial coronary angiography or within 24 h using the iLumien OPTIS Medical system (Abbott Vascular, Santa Clara, CA, USA). OCT was performed using 6-Fr guiding catheters with manual contrast injections. Following imaging, operator performed interpretation of the OCT imaging in order to choose the most optimal treatment strategy. Subsequently OCT images were stored digitally and analyzed by 3 experienced and blinded investigators (J.F.-W., M.M., and M.R.-D.). The evaluation of the OCT images was performed according to consensus document on the acquisition, measurement and reporting of OCT studies, reported by Tearney et al. []. Plaque rupture (PR) was defined as the discontinuity of the fibrous cap with the evidence of presence of cavity inside the plaque [,]. Plaque erosion (PE) was defined as thrombus presence overlying an irregular surface without evidence of fibrous cap disruption []. Eruptive calcific nodule was identified in case of evident fibrous cap disruption and/or thrombus presence on the surface of calcified plaque protruding into the lumen []. Intraluminal thrombus was defined as irregular mass attached to the arterial wall or floating inside the lumen [,].
2.2. Cardiac Magnetic Resonance
The CMR images were acquired on 1.5T system Optima MR450w (GE Healthcare). All cardiac CMR imaging were electrocardiographically gated and based on the protocols developed according to guidelines and routinely used in clinical practice.
CMR study protocol included: (1) functional sequences using cine imagining; (2) edema imaging with T2-weighted short-tau inversion recovery; and (3) viability imaging utilizing late gadolinium enhancement (LGE) imagining. At the time of interpretation, physicians responsible for CMR interpretation were not provided with results of the other imaging test. Final diagnosis was established based on CMR findings: (1) AMI: presence of subendocardial or transmural abnormalities located in the distribution of a single coronary artery, or (2) myopericarditis presence of subepicardial and/or midwall abnormalities and lack of subendocardial LGE [].
2.3. Statistical Analysis
Statistical analysis was performed using Statistica (Statistica v. 13, Tibco Software Inc., Palo Alto, CA, USA). Continuous variables were expressed as mean ± SD with the median and interquartile range used for variables with non-normal distributions. Categorical variables were described with percentages and counts.
3. Results
3.1. Study Population
A total of 10 patients underwent OCT imaging of the major epicardial arteries and CMR during hospitalization. The mean age of patients was 52 ± 6 years and woman constituted half of the patients. Typical angina on admission was present in 60% of patients. At admission, 40% of patients had regional wall motion abnormalities on echocardiography and the mean left ventricle ejection fraction (LVEF) was 51 ± 13%. None of the patients had a severe valvular heart disease. Mean troponin level at admission was 0.24 ± 0.18 ng/mL, while maximal level was (0.35 ± 0.22 ng/mL). Mean low-density lipoprotein level at admission was 97.60 ± 30.03 mg/dL. In total, 70% of patients presented as NSTEMI. The baseline characteristics are summarized in Table 1. In Supplement S1, baseline characteristics are summarized for each patient.Coronary arteries were normal in 40% of patients and the remaining patients presented with mild to moderate (up to 50%) coronary lesions.

Table 1.
Baseline characteristics.
3.2. OCT and CMR Findings
The number of OCT pullback acquisitions per patient was 5.2 ± 0.8. OCT provided clear substrate for AMI in 70% of patients and remaining patients had negative result. Plaque rupture, plaque erosion, and spontaneous coronary artery dissection (SCAD) were found in 20%, 30% and 20% patients, respectively. Thrombus was present in all patients with plaque rupture and in one patient with plaque erosion. No eruptive calcific nodules were found in any patient. Average contrast volume used per patient was 102 ± 8 mL. There were no complications during OCT examination. In the CMR imaging, AMI was evident in 70% of patients. In addition, substrate for AMI in OCT was found in all coronary arteries supplying the infarct-related territory as confirmed by CMR. There was a complete concordance between new ischemic lesions in CMR and presence of positive OCT findings. In 90% of patients, T2 hyperintensity was found. The mean number of segments with LGE was 1.7 ± 0.7. Myocarditis was diagnosed using CMR in two patients and Tako-Tsubo in one subject. One patient had microvascular obstruction. Pericardial effusion was found in two patients. OCT and CMR findings are summarized in Table 2. In Supplement S2, OCT and CMR findings are summarized for each patient. A representative picture of multimodality imaging is presented in Figure 1.

Table 2.
OCT and CMR findings.
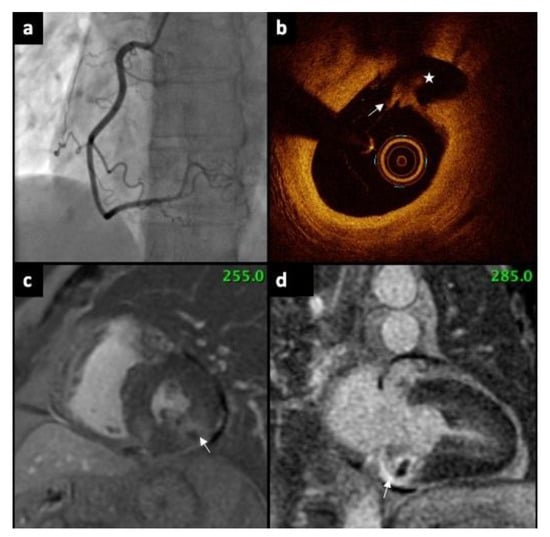
Figure 1.
(a) Coronary angiography showing normal right coronary artery, without any stenosis. (b) OCT revealed ruptured plaque (arrow). Rupture cavity (asterisk) is formed behind fibrous cap disruption (c) CMR (short axis) presents subendocardial myocardial LGE (arrow). (d) CMR (LV two-chamber, long axis) demonstrate subendocardial scar in the basal segment of inferior wall (arrow). OCT—Optical Coherence Tomography, CMR—Cardiac magnetic resonance, LGE—late gadolinium enhancement, LV—left ventricle.
3.3. Treatment at Discharge
All patients received Aspirin at discharge and P2Y12 inhibitors were prescribed in 80% of patients. No patient received oral anticoagulants, but one patient received low molecular weight heparin at discharge. Long-acting nitrates were prescribed in one case. Eight patients received angiotensin-converting enzyme inhibitors and all included subjects were prescribed with beta-blockers at discharge. All patients received statins. The summary of medications at discharge is presented in Table 3.

Table 3.
Medications at discharge.
4. Discussion
The results of our analysis demonstrates that OCT combined with CMR imaging ensures identification of MINOCA substrate in all patients presenting with AMI without obstructive lesions in coronary arteries. Furthermore, OCT findings well corresponded with CMR confirmed myocardial injury. Our results emphasize the benefit of using additional testing for more detailed evaluation MINOCA patients, which may potentially lead to better tailored treatment based on the actual underlying pathophysiological mechanism.
It must be stressed that MINOCA is not a benign condition; it has a 3-year mortality reaching up to 16% []. The European Association of Percutaneous Coronary Interventions recently published an expert consensus document, which strongly recommends adoption of intracoronary imaging to complete diagnosis in cases where uncertainty exists based solely on angiography in the AMI setting []. The most frequent event leading to AMI is coronary thrombosis, and while filling defects and haziness observed in angiography may suggest present of plaque disruption, OCT is able to provide definite diagnosis due to its superior resolution. Furthermore, a large thrombus usually causes significant stenosis or even occlusion of coronary artery, whereas smaller thrombi are usually associated with insignificant narrowing (not visible in angiography) or distal segment embolization [,]. Information regarding the exact pathological mechanism responsible for MINOCA presentation often cannot be obtained based on angiographical evaluation alone. In the previously published studies PR rates varied between 24–34% among patients with working diagnosis of MINOCA, which is similar to the presented study (20%) [,]. However, existing evidence suggest that PR is not the only cause that may lead to acute vessel thrombosis. Recent studies demonstrated that in up to 30% of patients with ST-segment elevation myocardial infarction there was no evidence of PR, but rather presented with PE [,,,]. In our study, the rate of PE (20%) was similar to previously published results ranging from 11–31% in patients with suspected MINOCA [,]. Furthermore, in our study, the prevalence of coronary artery dissection was 20%, which is higher than in a recently published OCT and CMR study (5%) []. Spontaneous coronary artery dissection may be missed or even misdiagnosed as vasospasm on angiogram due to low resolution of this modality, even though it might be a life-threating condition []. The main advantage of the presented analysis is that all patients underwent three vessel OCT together with CMR imaging.
The clinical use of CMR is increasingly growing due to its ability to confirm MI and accurately categorize patients according to the contemporary definition of MINOCA []. Furthermore, CMR is a noninvasive technique and can precisely differentiate MI from other conditions including myocarditis and various cardiomyopathies, which significantly enhances patient diagnosis, personalized treatment pathways, and assessment of prognosis. In our study, CMR showed high rate of ischemic LGE (70%), which is similar to the results previously reported in the MINOCA population (77.5%) []. In addition, in this prospective study, OCT provided clear substrate for AMI in all coronaries supplying the infarct-related region as confirmed by CMR. Our results demonstrated that high-risk lesions identified by OCT might indicate atherosclerosis as an underlying factor of AMI confirmed by CMR in the MINOCA population.
There is a paucity of data on recommended secondary prevention therapies for patients with MINOCA [,,]. Guidelines stress the need for individualized treatment based on the underlying mechanism of MINOCA []. A recent, large, retrospective study demonstrated that use of statins, ACEI/ARB and beta blockers was associated with a reduction in the adverse events rate []. In our study, all patient received b-blockers and statins and in a majority of cases, ACEI/ARB were prescribed upon discharge. Furthermore, it needs to be stressed that randomized trials investigating secondary prevention in MINOCA setting are of utmost clinical importance, especially when considering the uncertainty regarding underlying pathophysiological mechanisms and the large number of patients with adverse long-term prognosis.
The major finding of this study is fact that combination of CMR and OCT provides a clear substrate and/or diagnosis in all patients with MINOCA. Therefore, the widespread adoption of the multimodality imaging can facilitate diagnostic process and allows the physicians to choose individualized treatment strategies, which might be challenging in daily practice in some regions due to limited CMR and OCT availability. In addition, providing patient with a definite diagnosis could also improve compliance with therapeutic strategies. However, whether combination of CMR and OCT-guided therapy may improve outcomes in patients presenting with a working diagnosis of MINOCA has to be further investigated in dedicated large randomized studies.
Study Limitations
This study has some important limitations that need to be stressed. First, this prospective, observational, single center pilot study included a small number of patients. Second, patient selection bias cannot be excluded as we were unable to enroll all eligible patients due to logistical problems. Third, OCT was not systematically performed during the initial coronary angiography. Finally, invasive physiological evaluation was not included in our research protocol.
5. Conclusions
In this pilot study, OCT coupled with CMR provided a clear substrate and/or diagnosis in all patients presenting with MINOCA, emphasizing the need for multimodality imaging for tailored treatment in this population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11247495/s1, Supplement S1. Baseline characteristics; Supplement S2. OCT and CMR findings.
Author Contributions
Conceptualization, P.G. and J.F.-W.; methodology, P.G. and J.F.-W.; formal analysis, J.F.-W., M.M., M.R.-D. and M.H.; writing—original draft preparation, J.F.-W., M.M. and M.R.-D.; writing—review and editing, P.G., M.H., A.O. and W.W.; supervision, P.G. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Ethics Committee of The Medical University of Silesia (Approval Code: KNW/0022/KB1/114/17 Approval Date: 17 OCT 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Gasior, P.; Desperak, A.; Gierlotka, M.; Milewski, K.; Wita, K.; Kalarus, Z.; Fluder, J.; Kazmierski, M.; Buszman, P.E.; Gasior, M.; et al. Clinical Characteristics, Treatments, and Outcomes of Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Results from a Multicenter National Registry. J. Clin. Med. 2020, 9, 2779. [Google Scholar] [CrossRef] [PubMed]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction with Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic Review of Patients Presenting with Suspected Myocardial Infarction and Nonobstructive Coronary Arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef]
- Iqbal, S.N.; Feit, F.; Mancini, G.B.; Wood, D.; Patel, R.; Pena-Sing, I.; Attubato, M.; Yatskar, L.; Slater, J.N.; Hochman, J.S.; et al. Characteristics of plaque disruption by intravascular ultrasound in women presenting with myocardial infarction without obstructive coronary artery disease. Am. Heart J. 2014, 167, 715–722. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measure- ment, and reporting of intravascular optical coherence tomography studies: A report from the international working group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Prati, F.; Guagliumi, G.; Mintz, G.S.; Costa, M.; Regar, E.; Akasaka, T.; Barlis, P.; Tearney, G.J.; Jang, I.-K.; Arbustini, E.; et al. Expert review document part 2: Methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J. 2012, 33, 2513–2520. [Google Scholar] [CrossRef]
- Jia, H.; Abtahian, F.; Aguirre, A.D.; Lee, S.; Chia, S.; Lowe, H.; Kato, K.; Yonetsu, T.; Vergallo, R.; Hu, S.; et al. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J. Am. Coll. Cardiol. 2013, 62, 1748–1758. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef]
- Johnson, T.W.; Räber, L.; di Mario, C.; Bourantas, C.; Jia, H.; Mattesini, A.; Gonzalo, N.; de la Torre Hernandez, J.M.; Prati, F.; Koskinas, K.; et al. Clinical use of intracoronary imaging. Part 2: Acute coronary syndromes, ambiguous coronary angiography findings, and guiding interventional decision-making: An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions: Endorsed by the Chinese Society of Cardiology, the Hong Kong Society of Transcatheter Endocardiovascular Therapeutics (HKSTENT) and the Cardiac Society of Australia and New Zealand. Eur. Heart J. 2019, 40, 2566–2584. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Higuma, T.; Wang, Z.; Aguirre, A.D.; Mizuno, K.; Takano, M.; Dauerman, H.L.; Park, S.J.; Jang, Y.; Kim, C.J.; et al. Clinical Significance of Lipid-Rich Plaque Detected by Optical Coherence Tomography: A 4-Year Follow-Up Study. J. Am. Coll. Cardiol. 2017, 69, 2502–2513. [Google Scholar] [CrossRef] [PubMed]
- Opolski, M.P.; Spiewak, M.; Marczak, M.; Debski, A.; Knaapen, P.; Schumacher, S.P.; Staruch, A.D.; Grodecki, K.; Chmielak, Z.; Lazarczyk, H.; et al. Mechanisms of Myocardial Infarction in Patients with Nonobstructive Coronary Artery Disease: Results from the Optical Coherence Tomography Study. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 2210–2221. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, E.; Arabucki, F.; Nivet, H.; Barbey, C.; Cetran, L.; Chassaing, S.; Seguy, B.; Lesimple, A.; Cochet, H.; Montaudon, M.; et al. OCT and CMR for the Diagnosis of Patients Presenting with MINOCA and Suspected Epicardial Causes. JACC Cardiovasc. Imaging 2020, 13, 2619–2631. [Google Scholar] [CrossRef]
- Terada, K.; Kubo, T.; Kameyama, T.; Matsuo, Y.; Ino, Y.; Emori, H.; Higashioka, D.; Katayama, Y.; Khalifa, A.K.M.; Takahata, M.; et al. NIRS-IVUS for Differentiating Coronary Plaque Rupture, Erosion, and Calcified Nodule in Acute Myocardial Infarction. JACC Cardiovasc. Imaging 2021, 14, 1440–1450. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- van den Hoogen, I.J.; Gianni, U.; Al Hussein Alawamlh, O.; Wijeratne, R.; Jinnouchi, H.; Finn, A.; Earls, J.P.; Virmani, R.; Lin, F.Y. What atherosclerosis findings can CT see in sudden coronary death: Plaque rupture versus plaque erosion. J. Cardiovasc. Comput. Tomogr. 2020, 14, 214–218. [Google Scholar] [CrossRef]
- Saw, J.; Mancini, G.B.J.; Humphries, K.; Fung, A.; Boone, R.; Starovoytov, A.; Aymong, E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter. Cardiovasc. Interv. 2016, 87, E54–E61. [Google Scholar] [CrossRef]
- Buccheri, D.; Piraino, D.; Orrego, P.S.; Cortese, B. Is vasospasm overestimated in acute coronary syndromes presenting with non-obstructive coronary artery disease? The case for intravascular imaging. Int. J. Cardiol. 2016, 203, 1125–1126. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.P.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2016, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 1082. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjöld, A.; Gard, A.; Jernberg, T. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).