The Effects of Four Weeks of Chiropractic Spinal Adjustments on Blood Biomarkers in Adults with Chronic Stroke: Secondary Outcomes of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Study Participants
2.3. Interventions
2.3.1. Chiropractic Intervention
2.3.2. Sham Chiropractic Intervention
2.3.3. Physical Therapy Intervention
2.4. Outcome Measures
2.5. Randomization and Blinding
2.6. Statistical Analysis
3. Results
3.1. Within and between-Group Comparisons
3.2. Between-Group Differences
3.3. Within-Group Estimates
4. Discussion
Limitations, Implications and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics—2019 update: A report from the American heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Krishnamurthi, R.V.; Barker-Collo, S.; Forouzanfar, M.H.; Naghavi, M.; Connor, M.; Lawes, C.M.; Moran, A.E.; Anderson, L.M.; Roth, G.A.; et al. The Global Burden of Ischemic Stroke: Findings of the GBD 2010 Study. Glob. Heart 2014, 9, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R. Global burden of neurological diseases highlights stroke. Nat. Rev. Neurol. 2019, 15, 371–372. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Carey, L. Neuroplasticity and learning lead a new era in stroke rehabiliation. Int. J. Ther. Rehabil. 2007, 14, 250–251. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef]
- Ng, K.L.; Carmichael, S.T. Chapter 35—Mechanisms of Stroke Recovery. In Primer on Cerebrovascular Diseases, 2nd ed.; Caplan, L.R., Biller, J., Leary, M.C., Lo, E.H., Thomas, A.J., Yenari, M., Zhang, J.H., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 171–174. [Google Scholar]
- Burke, E.; Cramer, S.C. Biomarkers and Predictors of Restorative Therapy Effects After Stroke. Curr. Neurol. Neurosci. Rep. 2013, 13, 329. [Google Scholar] [CrossRef]
- Gandolfi, M.; Smania, N.; Vella, A.; Picelli, A.; Chirumbolo, S. Assessed and Emerging Biomarkers in Stroke and Training-Mediated Stroke Recovery: State of the Art. Neural Plast. 2017, 2017, 1389475. [Google Scholar] [CrossRef]
- Beck, K.D.; Valverde, J.; Alexi, T.; Poulsen, K.; Moffat, B.; Vandlen, R.A.; Rosenthal, A.; Hefti, F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 1995, 373, 339–341. [Google Scholar] [CrossRef]
- Duarte, E.; Curcio, M.; Canzoniero, L.M.; Duarte, C.B. Neuroprotection by GDNF in the ischemic brain. Growth Factors 2012, 30, 242–257. [Google Scholar] [CrossRef]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF signaling and survival of striatal neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.-R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.-G. Intravenous Brain-Derived Neurotrophic Factor Enhances Poststroke Sensorimotor Recovery and Stimulates Neurogenesis. Stroke 2007, 38, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Ploughman, M.; Windle, V.; MacLellan, C.L.; White, N.; Doré, J.J.; Corbett, D. Brain-Derived Neurotrophic Factor Contributes to Recovery of Skilled Reaching after Focal Ischemia in Rats. Stroke 2009, 40, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Alonso, M.; Bramham, C.R.; Pozzo-Miller, L.D. From Acquisition to Consolidation: On the Role of Brain-Derived Neurotrophic Factor Signaling in Hippocampal-Dependent Learning. Learn. Mem. 2002, 9, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kido, Y.; Accili, D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr. Rev. 2001, 22, 818–835. [Google Scholar] [CrossRef]
- Reinhardt, R.R.; Bondy, C.A. Insulin-like growth factors cross the blood-brain barrier. Endocrinology 1994, 135, 1753–1761. [Google Scholar] [CrossRef]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The Critical Role of Metabolic Pathways in Aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef]
- O’Kusky, J.; Ye, P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocr. 2012, 33, 230–251. [Google Scholar] [CrossRef]
- Baptista, P.; Andrade, J.P. Adult Hippocampal Neurogenesis: Regulation and Possible Functional and Clinical Correlates. Front. Neuroanat. 2018, 12, 44. [Google Scholar] [CrossRef]
- Jiao, S.; Ren, H.; Li, Y.; Zhou, J.; Duan, C.; Lu, L. Differential regulation of IGF-I and IGF-II gene expression in skeletal muscle cells. Mol. Cell. Biochem. 2013, 373, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Gailly, P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell. Mol. Life Sci. 2013, 70, 4117–4130. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.N.; Levison, S.; Wood, T. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat. Rev. Endocrinol. 2015, 11, 161. [Google Scholar] [CrossRef]
- Alberini, C.M.; Chen, D.Y. Memory enhancement: Consolidation, reconsolidation and insulin-like growth factor 2. Trends Neurosci. 2012, 35, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.D.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Høyer, E.; Jahnsen, R.; Stanghelle, J.K.; Strand, L.I. Body weight supported treadmill training versus traditional training in patients dependent on walking assistance after stroke: A randomized controlled trial. Disabil. Rehabil. 2012, 34, 210–219. [Google Scholar] [CrossRef]
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Liepert, J.; Bauder, H.; Miltner, W.H.R.; Taub, E.; Weiller, C. Treatment-Induced Cortical Reorganization After Stroke in Humans. Stroke 2000, 31, 1210–1216. [Google Scholar] [CrossRef]
- Takeuchi, N.; Izumi, S.-I. Rehabilitation with Poststroke Motor Recovery: A Review with a Focus on Neural Plasticity. Stroke Res. Treat. 2013, 2013, 128641. [Google Scholar] [CrossRef]
- Volpe, B.; Krebs, H.I.; Hogan, N.; Edelsteinn, L.; Diels, C.M.; Aisen, M.L. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology 1999, 53, 1874. [Google Scholar] [CrossRef]
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Taub, E.; Uswatte, G.; Morris, D.; Giuliani, C.; Light, K.E.; Nichols-Larsen, D.; EXCITE Investigators. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA 2006, 296, 2095–2104. [Google Scholar] [CrossRef]
- Afzalpour, M.E.; Chadorneshin, H.T.; Foadoddini, M.; Eivari, H.A. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol. Behav. 2015, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, A.; Kokaia, Z.; Airaksinen, M.; Saarma, M.; Lindvall, O. Stroke induces widespread changes of gene expression for glial cell line-derived neurotrophic factor family receptors in the adult rat brain. Neuroscience 2001, 106, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Tillerson, J.L.; Smith, A.D.; Schallert, T.; Zigmond, M.J. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: Possible role of GDNF. J. Neurochem. 2003, 85, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, C.C.; García-Salazar, L.-F.; Silva-Couto, M.A.; Santos, G.L.; Reisman, D.S.; Russo, T. Post-stroke BDNF Concentration Changes Following Physical Exercise: A Systematic Review. Front. Neurol. 2018, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- So, W.-Y.; Han, T.-K. Effect of ten weeks of combined exercise on growth hormone, insulin-like growth factor-2 and myostatin levels in elderly Korean women. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2016, 38, 61–67. [Google Scholar]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctôt, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef]
- Knaepen, K.; Goekint, M.; Heyman, E.; Meeusen, R. Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Mackay, C.P.; Kuys, S.S.; Brauer, S.G. The Effect of Aerobic Exercise on Brain-Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural Plast. 2017, 2017, 4716197. [Google Scholar] [CrossRef]
- El-Tamawy, M.S.; Abd-Allah, F.; Ahmed, S.M.; Darwish, M.H.; Khalifa, H.A. Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. Neurorehabilitation 2014, 34, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Rosner, A.L. Chiropractic identity: A neurological, professional, and political assessment. J. Chiropr. Humanit. 2016, 23, 35–45. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haavik, H.; Murphy, B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J. Electromyogr. Kinesiol. 2012, 22, 768–776. [Google Scholar] [CrossRef]
- Haavik, H.; Kumari, N.; Holt, K.; Niazi, I.K.; Amjad, I.; Pujari, A.N.; Türker, K.S.; Murphy, B. The contemporary model of vertebral column joint dysfunction and impact of high-velocity, low-amplitude controlled vertebral thrusts on neuromuscular function. Eur. J. Appl. Physiol. 2021, 121, 2675–2720. [Google Scholar] [CrossRef]
- Vining, R.D.; Salsbury, S.A.; Cooley, W.C.; Gosselin, D.; Corber, L.; Goertz, C.M. Patients receiving chiropractic care in a neurorehabilitation hospital: A descriptive study. J. Multidiscip. Health 2018, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Kohl-Heckl, W.K.; Koch, A.K.; Cramer, H. Complementary medicine use in stroke survivors: A US nationally representative survey. BMC Complement. Med. Ther. 2022, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.D.; Boyle, E.; Côté, P.; He, Y.; Hogg-Johnson, S.; Silver, F.L.; Bondy, S.J. Risk of vertebrobasilar stroke and chiropractic care: Results of a population-based case-control and case-crossover study. J. Manip. Physiol. Ther. 2009, 32, S201–S208. [Google Scholar] [CrossRef]
- Cassidy, J.D.; Boyle, E.; Côté, P.; Hogg-Johnson, S.; Bondy, S.J.; Haldeman, S. Risk of Carotid Stroke after Chiropractic Care: A Population-Based Case-Crossover Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 842–850. [Google Scholar] [CrossRef]
- Whedon, J.M.; Song, Y.; Mackenzie, T.A.; Phillips, R.B.; Lukovits, T.G.; Lurie, J.D. Risk of Stroke After Chiropractic Spinal Manipulation in Medicare B Beneficiaries Aged 66 to 99 Years with Neck Pain. J. Manip. Physiol. Ther. 2015, 38, 93–101. [Google Scholar] [CrossRef]
- Kosloff, T.M.; Elton, D.; Tao, J.; Bannister, W.M. Chiropractic care and the risk of vertebrobasilar stroke: Results of a case–control study in U.S. commercial and Medicare Advantage populations. Chiropr. Man. Ther. 2015, 23, 19. [Google Scholar] [CrossRef]
- Chu, E.C.-P.; Trager, R.J.; Tao, C.; Lee, L.Y.-K. Chiropractic Management of Neck Pain Complicated by Symptomatic Vertebral Artery Stenosis and Dizziness. Am. J. Case Rep. 2022, 23, e937991. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.K.; Türker, K.S.; Flavel, S.; Kinget, M.; Duehr, J.; Haavik, H. Changes in H-reflex and V-waves following spinal manipulation. Exp. Brain Res. 2015, 233, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Pickar, J.; Bolton, P. Spinal manipulative therapy and somatosensory activation. J. Electromyogr. Kinesiol. 2012, 22, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Murphy, B. Subclinical Neck Pain and the Effects of Cervical Manipulation on Elbow Joint Position Sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B.A. Altered cortical integration of dual somatosensory input following the cessation of a 20 min period of repetitive muscle activity. Exp. Brain Res. 2007, 178, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Haavik-Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef]
- Haavik Taylor, H.; Murphy, B. Transient modulation of intracortical inhibition following spinal manipulation. Chiropr. J. Aust. 2007, 37, 106–116. [Google Scholar]
- Taylor, H.H.; Murphy, B. Altered Sensorimotor Integration with Cervical Spine Manipulation. J. Manip. Physiol. Ther. 2008, 31, 115–126. [Google Scholar] [CrossRef]
- Haavik Taylor, H.; Murphy, B. Altered Central Integration of Dual Somatosensory Input Following Cervical Spine Manipulation. J. Manip. Physiol. Ther. 2010, 33, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Haavik Taylor, H.; Murphy, B. The effects of spinal manipulation on central integration of dual somatosensory input observed following motor training: A crossover study. J. Manip. Physiol. Ther. 2010, 33, 261–272. [Google Scholar] [CrossRef]
- Marshall, P.; Murphy, B. The Effect of Sacroiliac Joint Manipulation on Feed-Forward Activation Times of the Deep Abdominal Musculature. J. Manip. Physiol. Ther. 2006, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Cunha, M.; Velasques, B.; Minc, D.; Teixeira, S.; Domingues, C.A.; Silva, J.G.; Bastos, V.H.; Budde, H.; Cagy, M.; et al. Sensorimotor integration: Basic concepts, abnormalities related to movement disorders and sensorimotor training-induced cortical reorganization. Rev. Neurol. 2010, 51, 427–436. [Google Scholar] [PubMed]
- Treleaven, J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man. Ther. 2008, 13, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Jax, S.A.; Coslett, H.B. Disorders of the perceptual-motor system. In Progress in Motor Control; Springer: New York, NY, USA, 2009; pp. 377–391. [Google Scholar]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, M.; Zantedeschi, M. Plasticity and Awareness of Bodily Distortion. Neural Plast. 2016, 2016, 9834340. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.E.; Licari, M.K.; Elliott, C.; Lay, B.S.; Williams, J. Motor imagery ability and internal representation of movement in children with probable developmental coordination disorder. Hum. Mov. Sci. 2015, 44, 287–298. [Google Scholar] [CrossRef]
- Holt, K.; Niazi, I.K.; Nedergaard, R.W.; Duehr, J.; Amjad, I.; Shafique, M.; Anwar, M.N.; Ndetan, H.; Turker, K.S.; Haavik, H. The effects of a single session of chiropractic care on strength, cortical drive, and spinal excitability in stroke patients. Sci. Rep. 2019, 9, 2673. [Google Scholar] [CrossRef]
- Holt, K.; Niazi, I.; Amjad, I.; Kumari, N.; Rashid, U.; Duehr, J.; Navid, M.; Shafique, M.; Haavik, H. The Effects of 4 Weeks of Chiropractic Spinal Adjustments on Motor Function in People with Stroke: A Randomized Controlled Trial. Brain Sci. 2021, 11, 676. [Google Scholar] [CrossRef]
- Duarte, F.C.; Funabashi, M.; Starmer, D.; Partata, W.A.; West, D.W.; Kumbhare, D.A.; Injeyan, S. Effects of Distinct Force Magnitude of Spinal Manipulative Therapy on Blood Biomarkers of Inflammation: A Proof of Principle Study in Healthy Young Adults. J. Manip. Physiol. Ther. 2022, 45, 20–32. [Google Scholar] [CrossRef]
- Gevers-Montoro, C.; Romero-Santiago, M.; Losapio, L.; Conesa-Buendía, F.M.; Newell, D.; Álvarez-Galovich, L.; Piché, M.; Mues, A.O.-D. Presence of Tumor Necrosis Factor-Alpha in Urine Samples of Patients with Chronic Low Back Pain Undergoing Chiropractic Care: Preliminary Findings from a Prospective Cohort Study. Front. Integr. Neurosci. 2022, 16, 879083. [Google Scholar] [CrossRef]
- López-Herradón, A.; Fujikawa, R.; Gómez-Marín, M.; Stedile-Lovatel, J.P.; Mulero, F.; Ardura, J.A.; Ruiz, P.; Muñoz, I.; Esbrit, P.; Mahíllo-Fernández, I.; et al. Impact of Chiropractic Manipulation on Bone and Skeletal Muscle of Ovariectomized Rats. Calcif. Tissue Res. 2017, 101, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Maltese, P.E.; Dautaj, A.; Paolacci, S.; Kurti, D.; Picotti, P.M.; Bertelli, M. Neurobiological basis of chiropractic manipulative treatment of the spine in the care of major depression. Acta Bio Med. Atenei Parm. 2020, 91, e2020006. [Google Scholar] [CrossRef]
- Greisberger, A.; Aviv, H.; Garbade, S.; Diermayr, G. Clinical relevance of the effects of reach-to-grasp training using trunk restraint in individuals with hemiparesis poststroke: A systematic review. J. Rehabil. Med. 2016, 48, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Wist, S.; Clivaz, J.; Sattelmayer, K.M. Muscle strengthening for hemiparesis after stroke: A meta-analysis. Ann. Phys. Rehabil. Med. 2016, 59, 114–124. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R. Post-stroke hemiplegia assessment of physical properties. Scand. J. Rehabil. Med. Suppl. 1980, 7, 85–93. [Google Scholar]
- Triano, J.J.; Budgell, B.; Bagnulo, A.; Roffey, B.; Bergmann, T.; Cooperstein, R.; Gleberzon, B.; Good, C.; Perron, J.; Tepe, R. Review of methods used by chiropractors to determine the site for applying manipulation. Chiropr. Man. Ther. 2013, 21, 36. [Google Scholar] [CrossRef]
- Holt, K.; Russell, D.; Cooperstein, R.; Younes, M.; Sherson, M.; Haavik, H. Interexaminer reliability of a multidimensional battery of tests used to assess for vertebral subluxations. Chiropr. J. Aust. 2018, 46, 101–117. [Google Scholar]
- Cooperstein, R.; Gleberzon, B. Technique Systems in Chiropractic; Churchill Livingstone: London, UK, 2004. [Google Scholar]
- Hancock, M.J.; Maher, C.G.; Latimer, J.; McAuley, J.H. Selecting an appropriate placebo for a trial of spinal manipulative therapy. Aust. J. Physiother. 2006, 52, 135–138. [Google Scholar] [CrossRef]
- Rosner, A.L. Evidence-based medicine: Revisiting the pyramid of priorities. J. Bodyw. Mov. Ther. 2012, 16, 42–49. [Google Scholar] [CrossRef]
- Ashraf, M.; Saeed, H.; Saleem, Z.; Rathore, H.A.; Rasool, F.; Tahir, E.; Bhatti, T.; Khalid, J.; Bhatti, I.; Tariq, A. A cross-sectional assessment of knowledge, attitudes and self-perceived effectiveness of complementary and alternative medicine among pharmacy and non-pharmacy university students. BMC Complement. Altern. Med. 2019, 19, 95. [Google Scholar] [CrossRef]
- Pollock, A.; St George, B.; Fenton, M.; Firkins, L. Top 10 research priorities relating to life after stroke–consensus from stroke survivors, caregivers, and health professionals. Int. J. Stroke 2014, 9, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Saghaei, M.; Saghaei, S. Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials. J. Biomed. Sci. Eng. 2011, 4, 734–739. [Google Scholar] [CrossRef]
- The R Development Core Team. R: A Language and Environment for Statistical Computing; GBIF: Copenhagen, Denmark, 2020. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models 2020; R Core Team: Copenhagen, Denmark, 2020. [Google Scholar]
- De Morais, V.A.C.; Tourino, M.F.D.S.; Almeida, A.C.D.S.; Albuquerque, T.B.D.; Linhares, R.C.; Christo, P.P.; Martinelli, P.M.; Scalzo, P.L. A single session of moderate intensity walking increases brain-derived neurotrophic factor (BDNF) in the chronic post-stroke patients. Top. Stroke Rehabil. 2018, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.C.; Alcantara, C.C.; French, M.; Li, X.; Matt, K.S.; Kim, H.E.; Morton, S.M.; Reisman, D.S. A single exercise bout and locomotor learning after stroke: Physiological, behavioural, and computational outcomes. J. Physiol. 2018, 596, 1999–2016. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.C.; Helm, E.E.; Lau, K.A.; Morton, S.M.; Reisman, D.S. The feasibility of an acute high-intensity exercise bout to promote locomotor learning after stroke. Top. Stroke Rehabil. 2017, 25, 83–89. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Cunningham, D.; Carl, D.; Jansen, C.; Khoury, J.C.; et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. J. Appl. Physiol. 2019, 126, 431–443. [Google Scholar] [CrossRef]
- King, M.; Kelly, L.P.; Wallack, E.M.; Hasan, S.M.M.; Kirkland, M.C.; Curtis, M.E.; Chatterjee, T.; McCarthy, J.; Ploughman, M. Serum levels of insulin-like growth factor-1 and brain-derived neurotrophic factor as potential recovery biomarkers in stroke. Neurol. Res. 2019, 41, 354–363. [Google Scholar] [CrossRef]
- Luo, W.; Liu, T.; Li, S.; Wen, H.; Zhou, F.; Zafonte, R.; Luo, X.; Xu, M.; Black-Schaffer, R.; Wood, L.J.; et al. The Serum BDNF Level Offers Minimum Predictive Value for Motor Function Recovery after Stroke. Transl. Stroke Res. 2019, 10, 342–351. [Google Scholar] [CrossRef]
- Koroleva, E.; Tolmachev, I.; Alifirova, V.; Boiko, A.; Levchuk, L.; Loonen, A.; Ivanova, S. Serum BDNF’s Role as a Biomarker for Motor Training in the Context of AR-Based Rehabilitation after Ischemic Stroke. Brain Sci. 2020, 10, 623. [Google Scholar] [CrossRef]
- Panja, D.; Bramham, C.R. BDNF mechanisms in late LTP formation: A synthesis and breakdown. Neuropharmacology 2014, 76, 664–676. [Google Scholar] [CrossRef]
- Pikula, A.; Beiser, A.S.; Chen, T.C.; Preis, S.R.; Vorgias, D.; DeCarli, C.; Au, R.; Kelly-Hayes, M.; Kase, C.S.; Wolf, P.A.; et al. Serum brain-derived neurotrophic factor and vascular endothelial growth factor levels are associated with risk of stroke and vascular brain injury: Framingham Study. Stroke 2013, 44, 2768–2775. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, L.A.; Altar, C.A.; Blue, M.E.; Kaplan, D.R.; Tessarollo, L.; Lyons, W.E. BDNF Promotes the Regenerative Sprouting, But Not Survival, of Injured Serotonergic Axons in the Adult Rat Brain. J. Neurosci. 2000, 20, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, A.; Overman, J.J.; Zhong, S.; Mueller, R.; Lynch, G.; Carmichael, S.T. AMPA Receptor-Induced Local Brain-Derived Neurotrophic Factor Signaling Mediates Motor Recovery after Stroke. J. Neurosci. 2011, 31, 3766–3775. [Google Scholar] [CrossRef] [PubMed]
- Limaye, N.S.; Carvalho, L.B.; Kramer, S. Effects of Aerobic Exercise on Serum Biomarkers of Neuroplasticity and Brain Repair in Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1633–1644. [Google Scholar] [CrossRef]
- Barbey, A.K.; Colom, R.; Paul, E.J.; Grafman, J. Architecture of fluid intelligence and working memory revealed by lesion mapping. Brain Struct. Funct. 2014, 219, 485–494. [Google Scholar] [CrossRef]
- Ploughman, M.; Granter-Button, S.; Chernenko, G.; Attwood, Z.; Tucker, B.; Mearow, K.M.; Corbett, D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007, 1150, 207–216. [Google Scholar] [CrossRef]
- Ploughman, M.; Granter-Button, S.; Chernenko, G.; Tucker, B.; Mearow, K.; Corbett, D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience 2005, 136, 991–1001. [Google Scholar] [CrossRef]
- Briken, S.; Rosenkranz, S.C.; Keminer, O.; Patra, S.; Ketels, G.; Heesen, C.; Hellweg, R.; Pless, O.; Schulz, K.-H.; Gold, S.M. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J. Neuroimmunol. 2016, 299, 53–58. [Google Scholar] [CrossRef]
- Ieraci, A.; Madaio, A.I.; Mallei, A.; Lee, F.S.; Popoli, M. Brain-Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology 2016, 41, 3070–3079. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Kim, D.Y.; Quinlan, E.B.; Gramer, R.; Cramer, S.C. BDNF Val66Met Polymorphism Is Related to Motor System Function after Stroke. Phys. Ther. 2016, 96, 533–539. [Google Scholar] [CrossRef]
- Cramer, S.; Procaccio, V.; Americas, G.; Investigators, G.I.S. Correlation between genetic polymorphisms and stroke recovery: Analysis of the GAIN Americas and GAIN International Studies. Eur. J. Neurol. 2012, 19, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, M.; Van Der Meij, A.; Van Deurzen, P.; Janzing, J.; Arias-Vasquez, A.; Buitelaar, J.; Franke, B. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: Effects of gender and ethnicity. Mol. Psychiatry 2010, 15, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.A.; Sykes, D.; Gilbert, K.L.; Chen, J.-W.; Frystyk, J. Brief, high intensity exercise alters serum ghrelin and growth hormone concentrations but not IGF-I, IGF-II or IGF-I bioactivity. Growth Horm. IGF Res. 2010, 20, 289–294. [Google Scholar] [CrossRef]
- Bang, P.; Brandt, J.; Degerblad, M.; Enberg, G.; Kaijser, L.; Thorén, M.; Hall, K. Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur. J. Clin. Investig. 1990, 20, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, A.J.; Brasel, J.; Hintz, R.L.; Mohan, S.; Cooper, D. Acute effect of brief low-and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J. Clin. Endocrinol. Metab. 1996, 81, 3492–3497. [Google Scholar] [PubMed]
- Bailey, D.M.; Evans, K.A.; McEneny, J.; Young, I.S.; Hullin, D.A.; James, P.E.; Ogoh, S.; Ainslie, P.N.; Lucchesi, C.; Rockenbauer, A.; et al. Exercise-induced oxidative-nitrosative stress is associated with impaired dynamic cerebral autoregulation and blood-brain barrier leakage. Exp. Physiol. 2011, 96, 1196–1207. [Google Scholar] [CrossRef]
- McCullough, M.; Gyorkos, A.; Spitsbergen, J. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 2013, 240, 258–268. [Google Scholar] [CrossRef]
- Kitagawa, H.; Hayashi, T.; Mitsumoto, Y.; Koga, N.; Itoyama, Y.; Abe, K. Reduction of Ischemic Brain Injury by Topical Application of Glial Cell Line–Derived Neurotrophic Factor after Permanent Middle Cerebral Artery Occlusion in Rats. Stroke 1998, 29, 1417–1422. [Google Scholar] [CrossRef][Green Version]
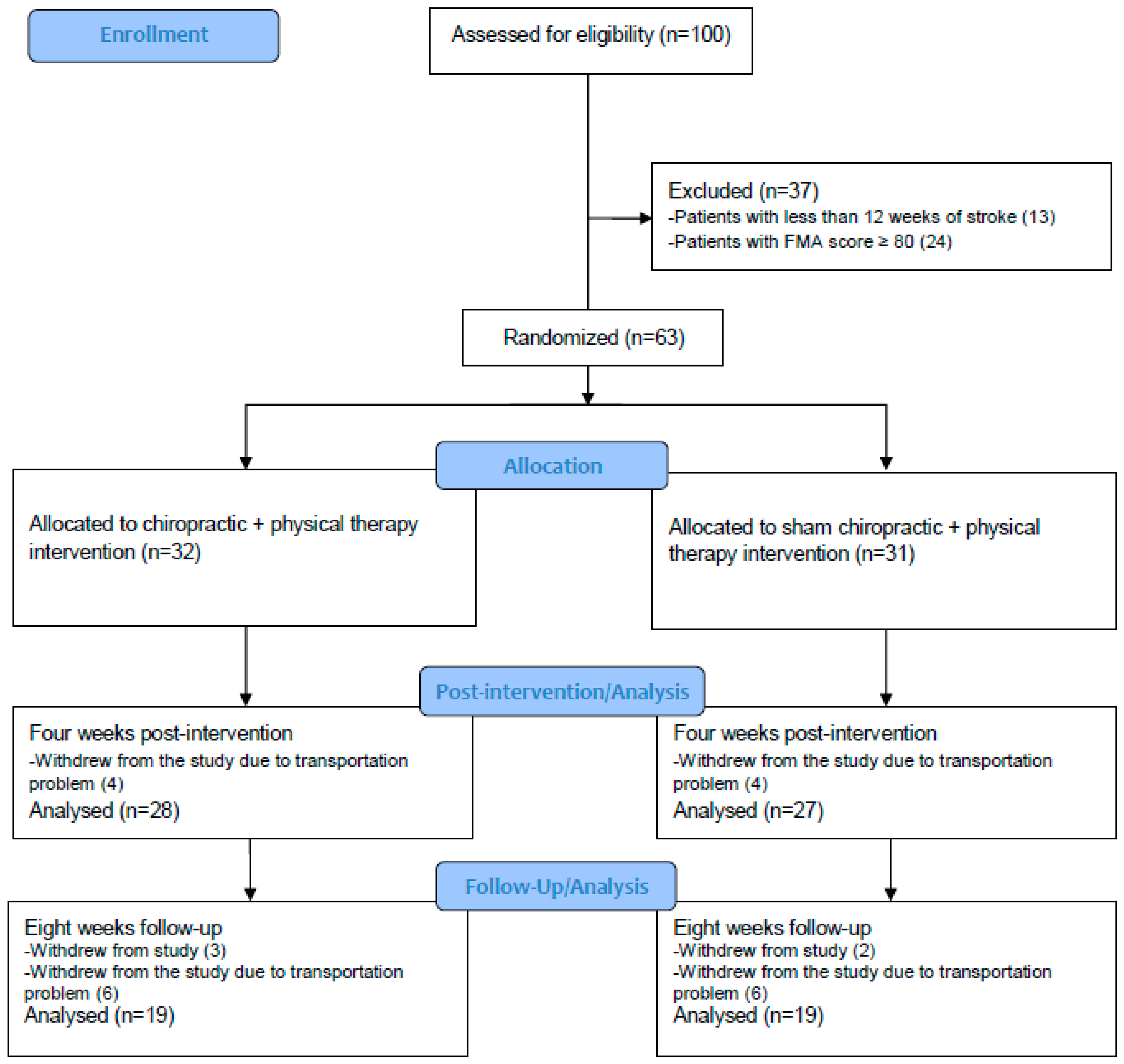
| Variables | Chiro + PT | Sham + PT |
|---|---|---|
| Gender | ||
| Male (n) | 18 | 16 |
| Female (n) | 10 | 11 |
| Age, years (mean ± SD) | 53.3 ± 14.0 | 58.5 ± 11.3 |
| Side of body affected by stroke | ||
| Left (n) | 14 | 12 |
| Right (n) | 14 | 15 |
| Time since stroke, months (mean ± SD) | 30.0 ± 36.6 | 27.3 ± 31.5 |
| 12–24 weeks, n | 5 | 4 |
| >24 weeks, n | 23 | 23 |
| Type of stroke | ||
| Ischemic (n) | 24 | 25 |
| Hemorrhagic (n) | 4 | 2 |
| Independent Variable | DFnum | DFden | F-Value | p-Value |
|---|---|---|---|---|
| Outcome | 3 | 276 | 1836.18 | <0.001 |
| Time | 2 | 276 | 3.66 | 0.027 |
| Group | 1 | 40 | 0.76 | 0.39 |
| Outcome × Time | 4 | 276 | 16.56 | <0.001 |
| Outcome × Group | 2 | 276 | 1.52 | 0.22 |
| Time × Group | 2 | 276 | 1.18 | 0.31 |
| Outcome × Time × Group | 4 | 276 | 0.49 | 0.75 |
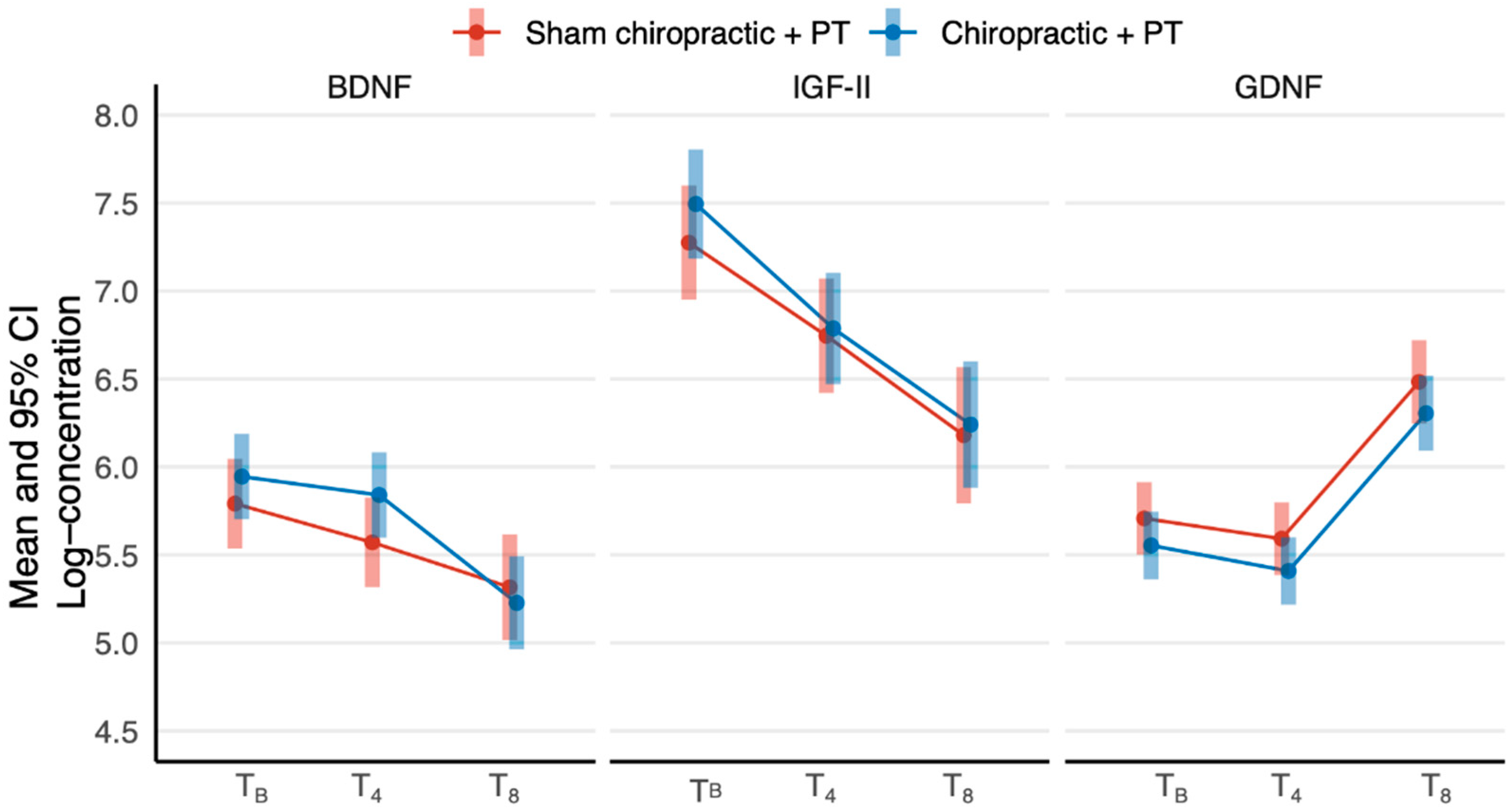
| Outcome | Group | Time | Mean ± SE | 95% CI |
|---|---|---|---|---|
| BDNF | Sham chiropractic + PT | Baseline | 5.8 ± 0.1 | 5.5, 6.0 |
| Chiropractic + PT | Baseline | 5.9 ± 0.1 | 5.7, 6.2 | |
| Sham chiropractic + PT | At 4 weeks | 5.6 ± 0.1 | 5.3, 5.8 | |
| Chiropractic + PT | At 4 weeks | 5.8 ± 0.1 | 5.6, 6.1 | |
| Sham chiropractic + PT | At 8 weeks | 5.3 ± 0.2 | 5.0, 5.6 | |
| Chiropractic + PT | At 8 weeks | 5.2 ± 0.1 | 5.0, 5.5 | |
| IGF-II | Sham chiropractic + PT | Baseline | 7.3 ± 0.2 | 7.0, 7.6 |
| Chiropractic + PT | Baseline | 7.5 ± 0.2 | 7.2, 7.8 | |
| Sham chiropractic + PT | At 4 weeks | 6.7 ± 0.2 | 6.4, 7.1 | |
| Chiropractic + PT | At 4 weeks | 6.8 ± 0.2 | 6.5, 7.1 | |
| Sham chiropractic + PT | At 8 weeks | 6.2 ± 0.2 | 5.8, 6.6 | |
| Chiropractic + PT | At 8 weeks | 6.2 ± 0.2 | 5.9, 6.6 | |
| GDNF | Sham chiropractic + PT | Baseline | 5.7 ± 0.1 | 5.5, 5.9 |
| Chiropractic + PT | Baseline | 5.6 ± 0.1 | 5.4, 5.7 | |
| Sham chiropractic + PT | At 4 weeks | 5.6 ± 0.1 | 5.4, 5.8 | |
| Chiropractic + PT | At 4 weeks | 5.4 ± 0.1 | 5.2, 5.6 | |
| Sham chiropractic + PT | At 8 weeks | 6.5 ± 0.1 | 6.2, 6.7 | |
| Chiropractic + PT | At 8 weeks | 6.3 ± 0.1 | 6.1, 6.5 |
| Outcome | Time | Mean Difference ± SE | 95% CI | H0: Mean Difference = 0, t [df], p-Value |
|---|---|---|---|---|
| BDNF | At 4 weeks | 0.12 ± 0.21 | −0.31, 0.54 | 0.54 [276], 0.59 |
| At 8 weeks | −0.24 ± 0.23 | −0.70, 0.22 | −1.03 [276], 0.30 | |
| IGF-II | At 4 weeks | −0.18 ± 0.29 | −0.75, 0.40 | −0.61 [276], 0.54 |
| At 8 weeks | −0.16 ± 0.32 | −0.79, 0.47 | −0.50 [276], 0.62 | |
| GDNF | At 4 weeks | −0.03 ± 0.16 | −0.35, 0.29 | −0.18 [276], 0.86 |
| At 8 weeks | −0.03 ± 0.18 | −0.38, 0.33 | −0.14 [276], 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haavik, H.; Niazi, I.K.; Amjad, I.; Kumari, N.; Rashid, U.; Duehr, J.; Navid, M.S.; Trager, R.J.; Shafique, M.; Holt, K. The Effects of Four Weeks of Chiropractic Spinal Adjustments on Blood Biomarkers in Adults with Chronic Stroke: Secondary Outcomes of a Randomized Controlled Trial. J. Clin. Med. 2022, 11, 7493. https://doi.org/10.3390/jcm11247493
Haavik H, Niazi IK, Amjad I, Kumari N, Rashid U, Duehr J, Navid MS, Trager RJ, Shafique M, Holt K. The Effects of Four Weeks of Chiropractic Spinal Adjustments on Blood Biomarkers in Adults with Chronic Stroke: Secondary Outcomes of a Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(24):7493. https://doi.org/10.3390/jcm11247493
Chicago/Turabian StyleHaavik, Heidi, Imran Khan Niazi, Imran Amjad, Nitika Kumari, Usman Rashid, Jens Duehr, Muhammad Samran Navid, Robert J. Trager, Muhammad Shafique, and Kelly Holt. 2022. "The Effects of Four Weeks of Chiropractic Spinal Adjustments on Blood Biomarkers in Adults with Chronic Stroke: Secondary Outcomes of a Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 24: 7493. https://doi.org/10.3390/jcm11247493
APA StyleHaavik, H., Niazi, I. K., Amjad, I., Kumari, N., Rashid, U., Duehr, J., Navid, M. S., Trager, R. J., Shafique, M., & Holt, K. (2022). The Effects of Four Weeks of Chiropractic Spinal Adjustments on Blood Biomarkers in Adults with Chronic Stroke: Secondary Outcomes of a Randomized Controlled Trial. Journal of Clinical Medicine, 11(24), 7493. https://doi.org/10.3390/jcm11247493












