MINOCA: One Size Fits All? Probably Not—A Review of Etiology, Investigation, and Treatment
Abstract
1. Introduction
2. Diagnostic Criteria
3. Epidemiology
4. Physiopathology
5. Atherosclerotic Causes
Coronary Plaque Disruption
6. Non-Atherosclerotic Causes
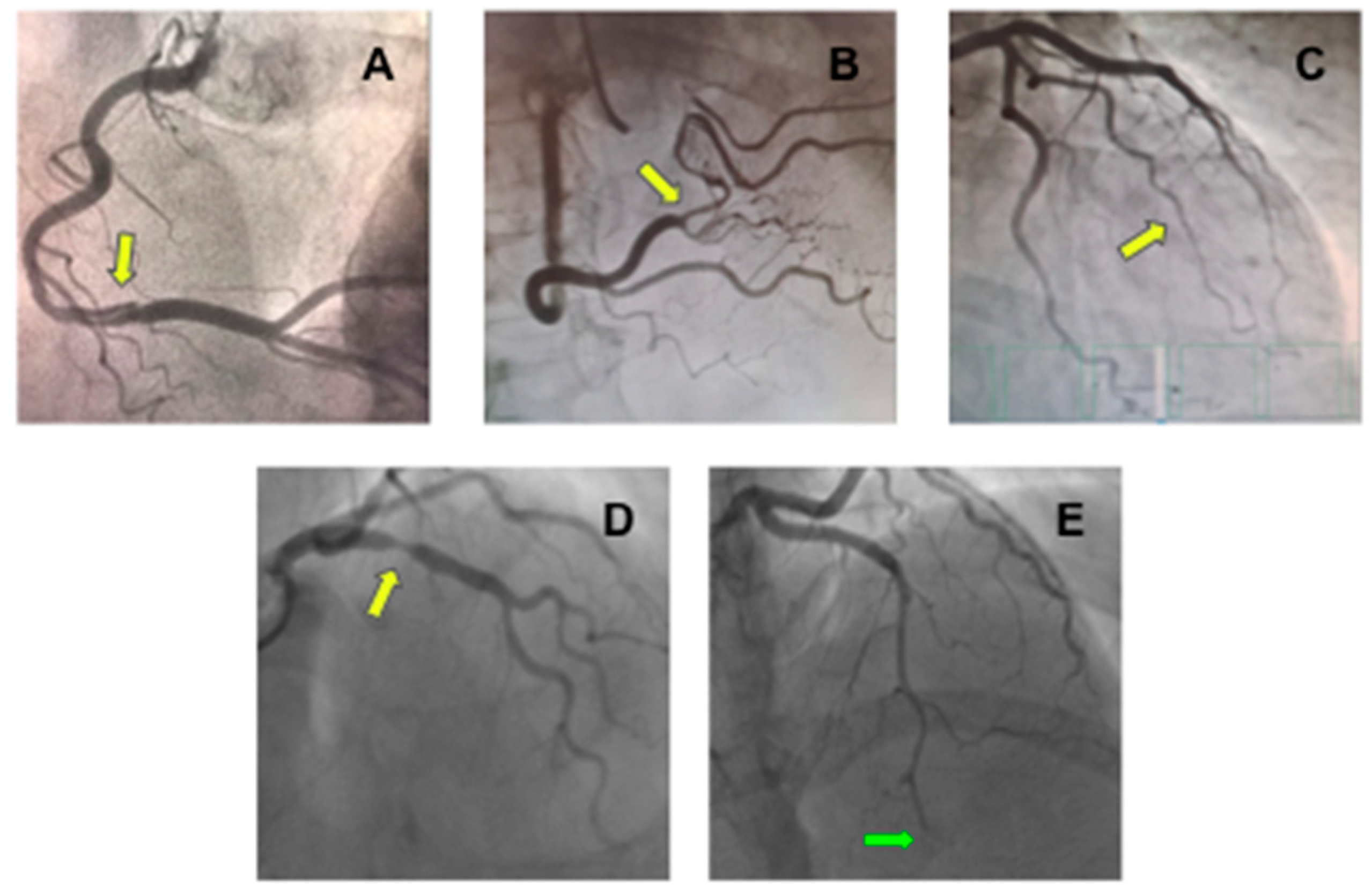
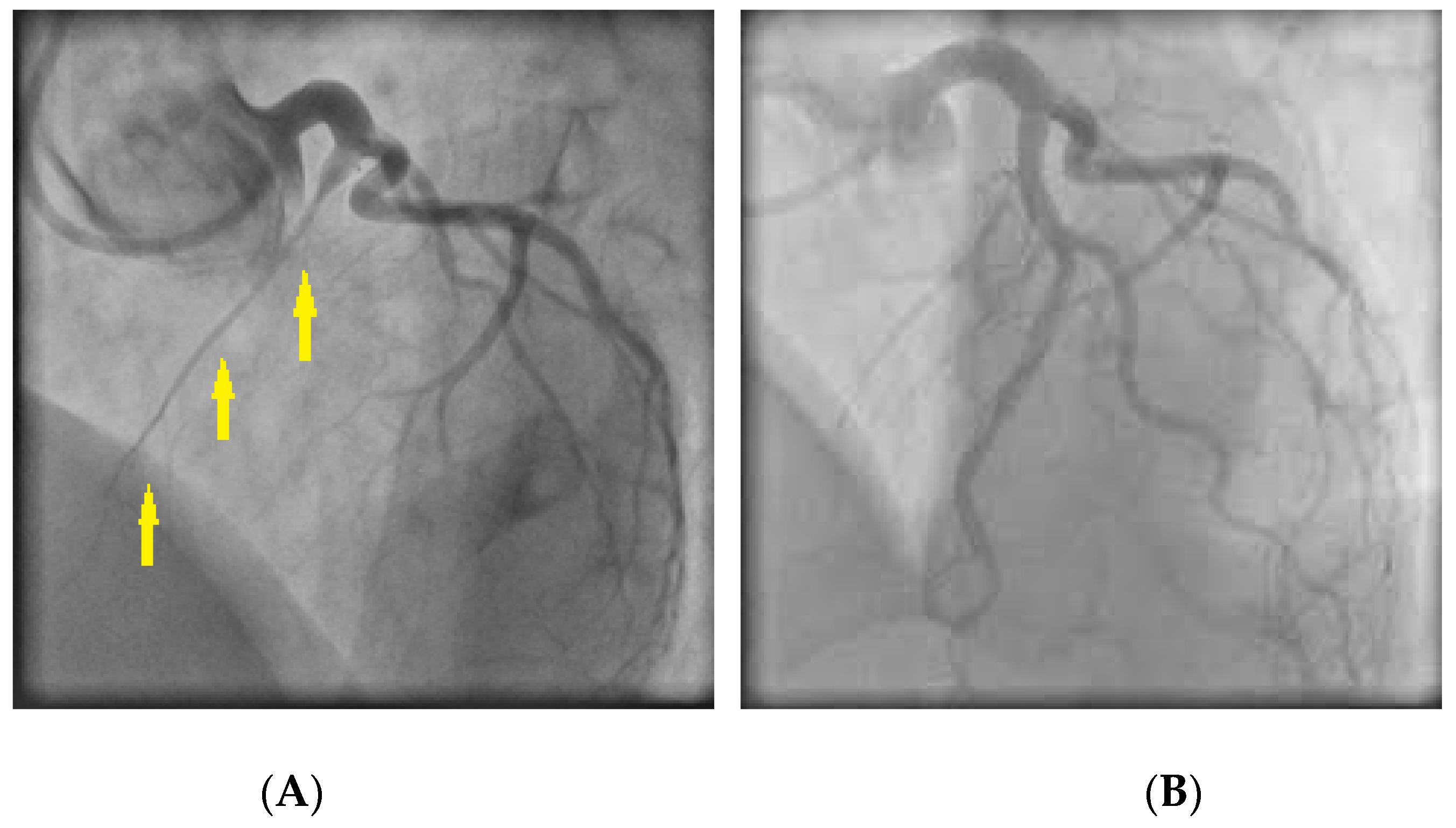
6.1. Spontaneous Coronary Artery Dissection (SCAD)
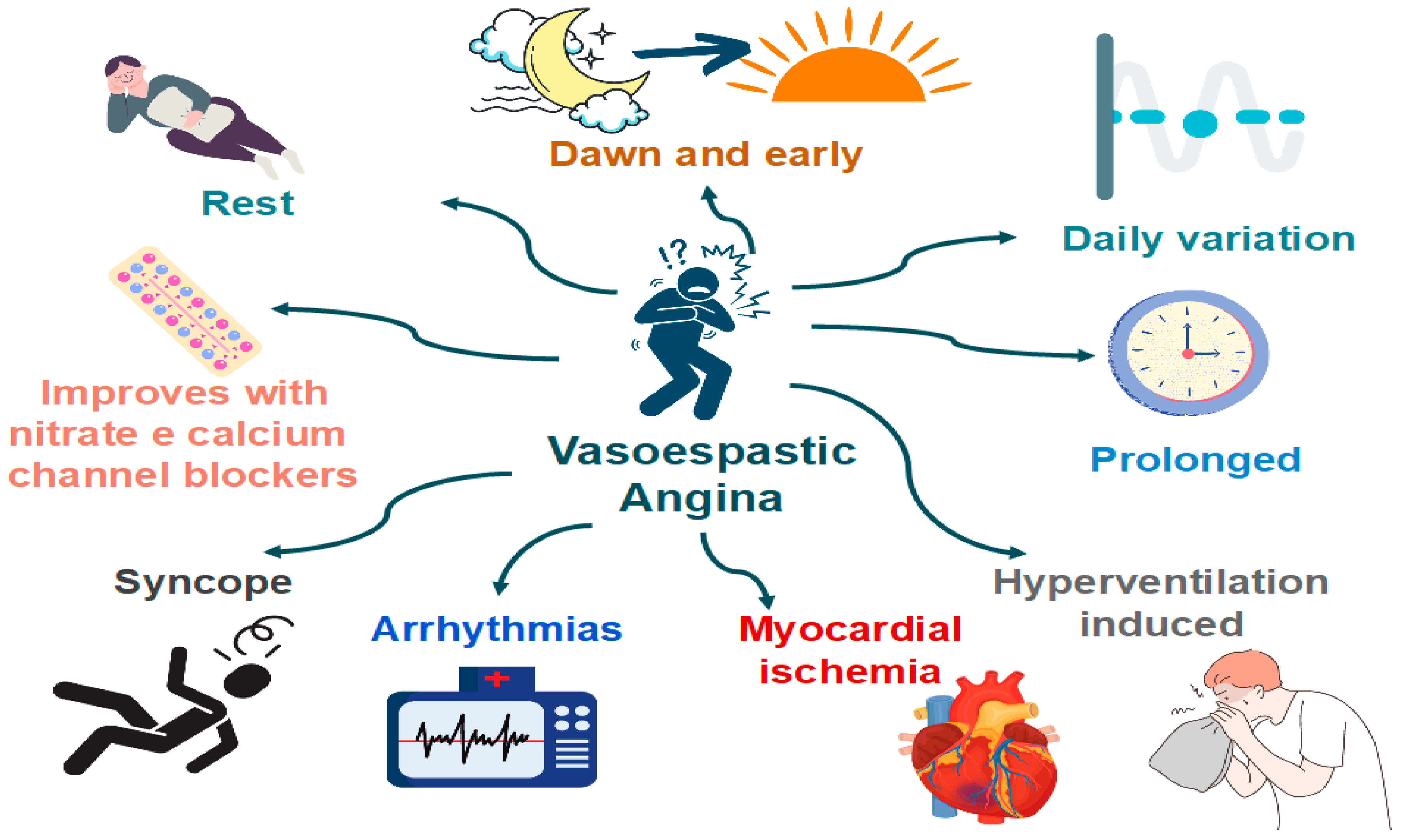
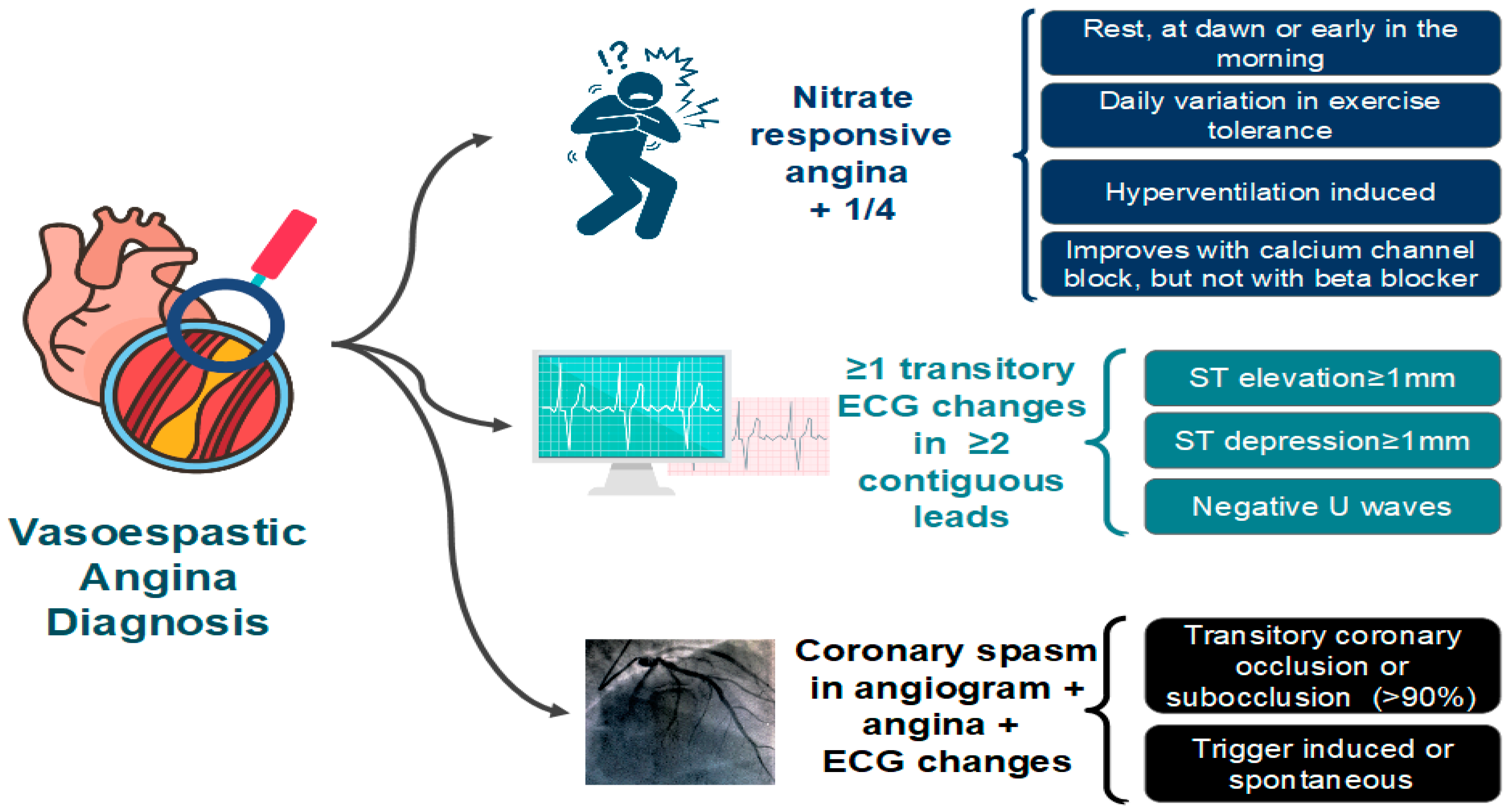
6.2. Coronary Artery Spasm (CAS)
- Angina occurs predominantly at rest and may occur more frequently from midnight to early morning;
- Effort and exercise tolerance are usually preserved;
- Hyperventilation can precipitate VSA [65];
- Episodes appear in “clusters;”
- VSA often has a more rapid response to sublingual nitroglycerin.
6.3. Coronary Artery Embolism/Thrombosis
6.4. Coronary Microvascular Dysfunction (CMD)
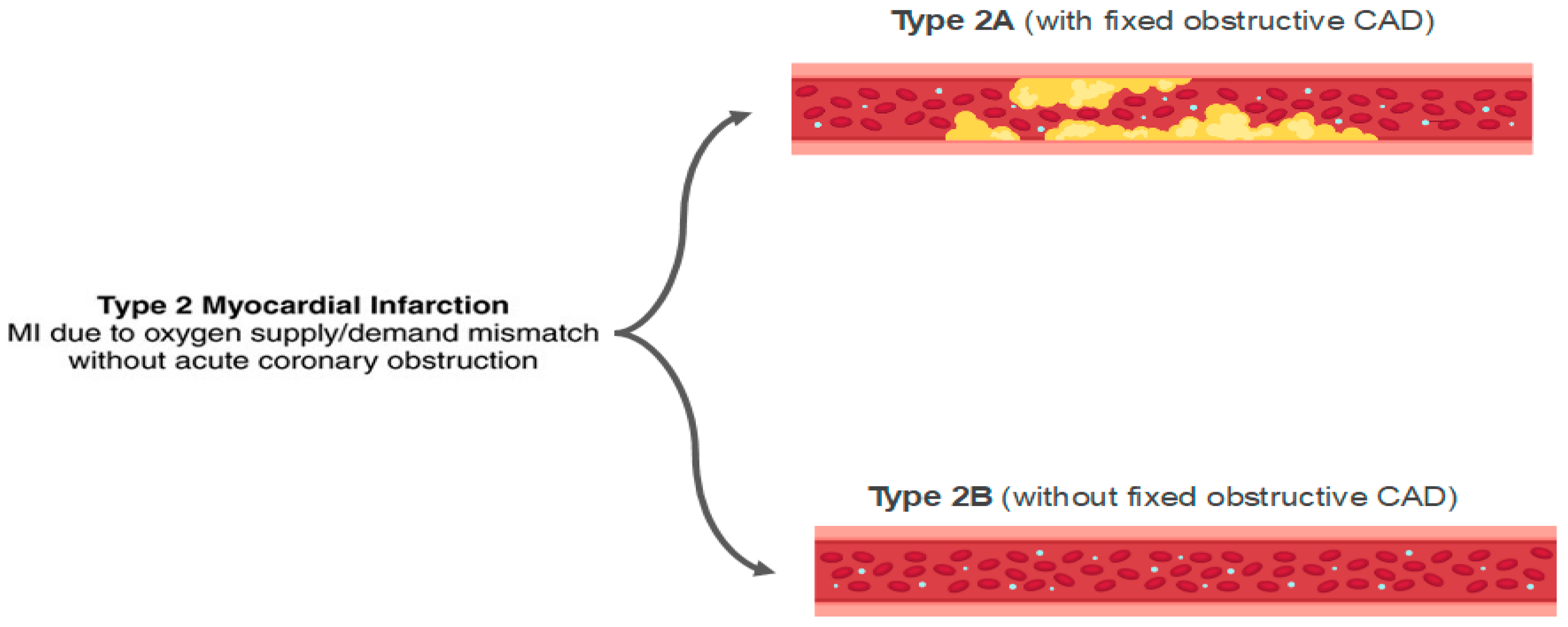
6.5. Supply–Demand Mismatch
7. Non-Schemic Differential Diagnosis
7.1. Takotsubo Cardiomyopathy
7.2. Myocarditis
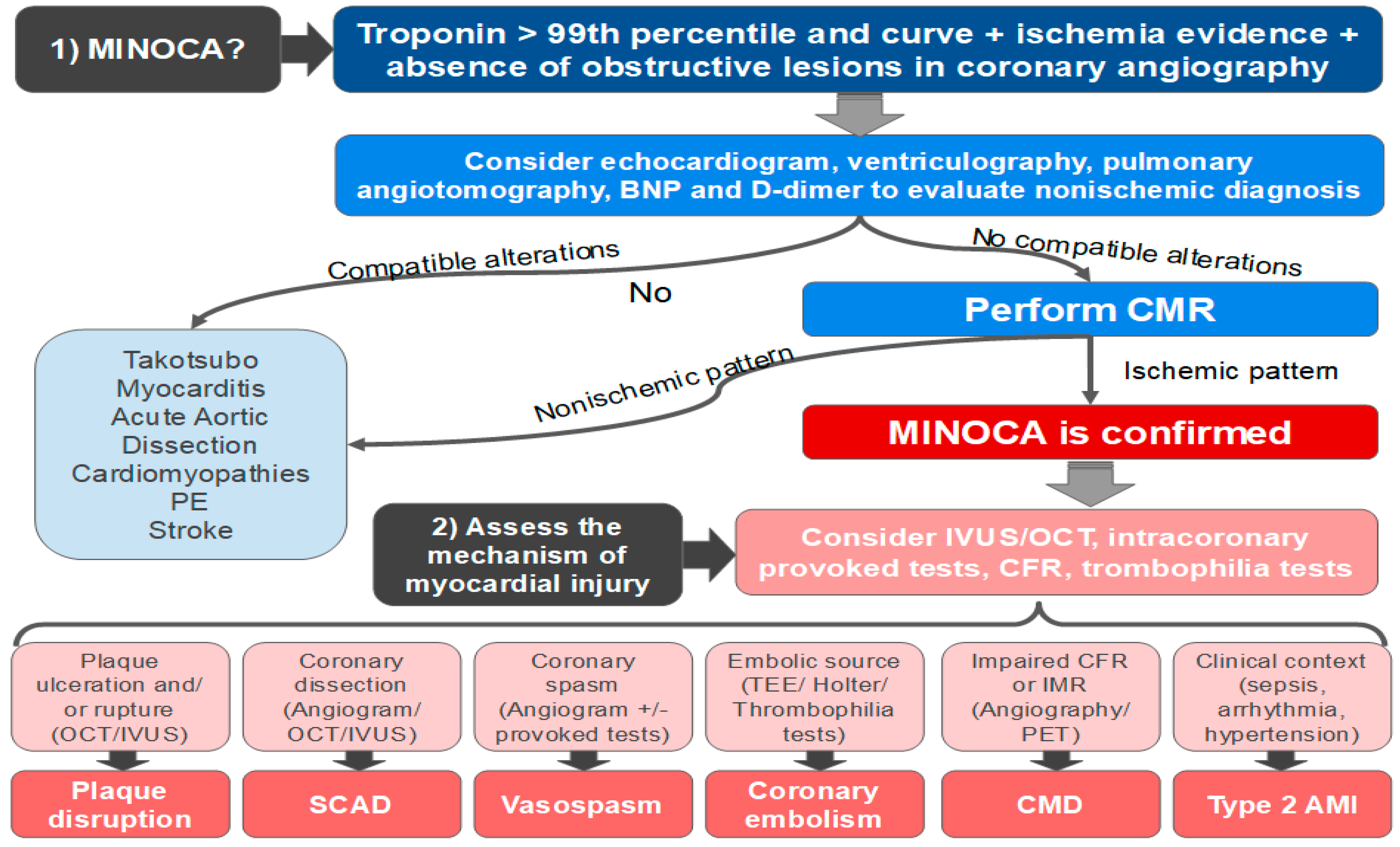
8. Clinical Investigation
- Is this really MINOCA?
- Which exact mechanism caused the myocardial injury?
8.1. The Role of the CRM
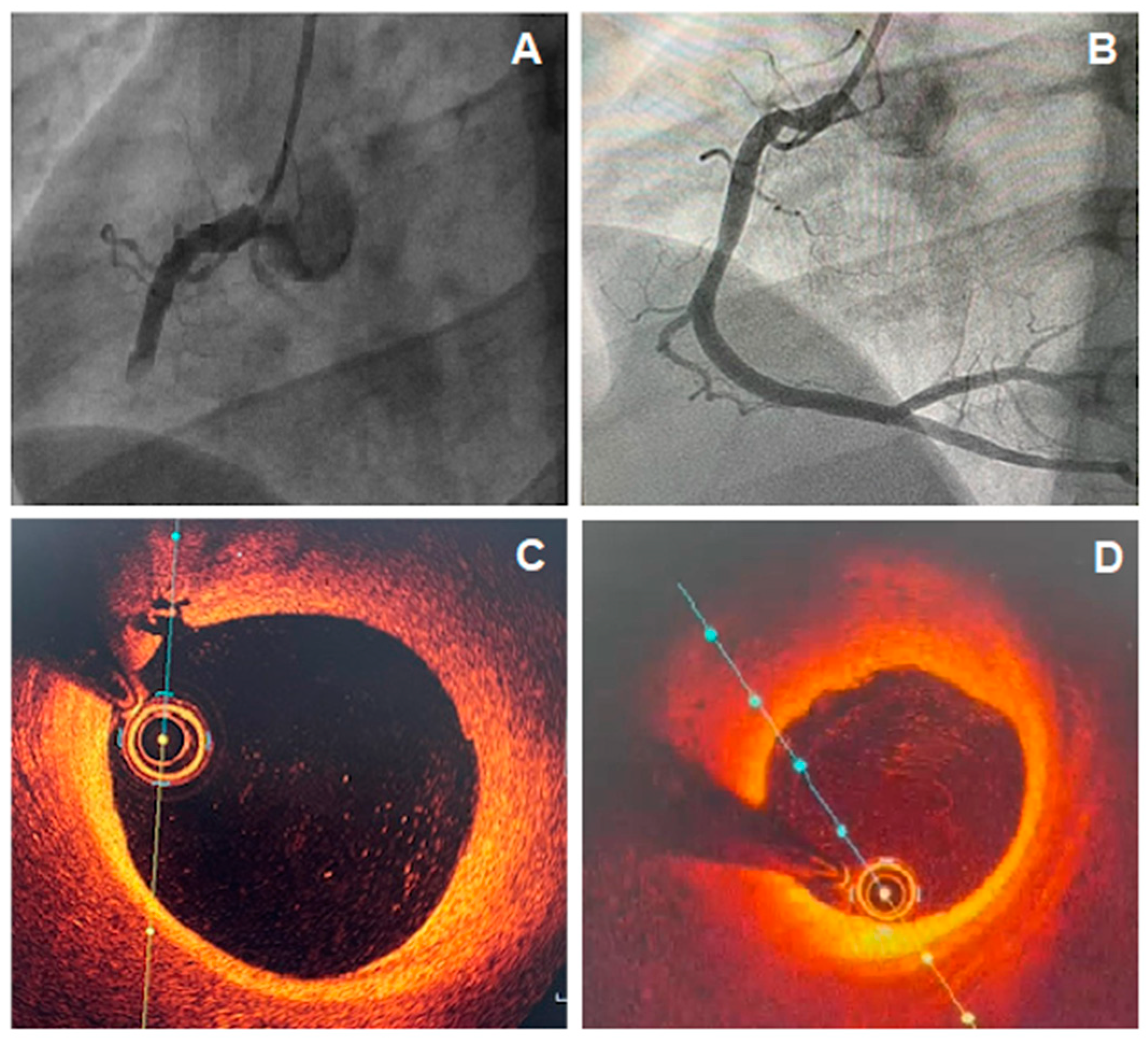
8.2. The Role of Intravascular Imaging
9. Management
9.1. Plaque Disruption
9.2. SCAD
9.3. Coronary Artery Spasm (CAS)
9.4. Coronary Artery Embolism/Thrombosis
9.5. Coronary Microvascular Dysfunction
9.6. Supply–Demand Mismatch
10. Future Directions
- The identification of the MINOCA mechanism has important implications for management and secondary prevention and a multimodality imaging approach should be employed for a prompt recognition of MINOCA.
- To evaluate the best strategy for the management and follow-up of patients with MINOCA in order to prevent cardiovascular events.
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, H.; Steinberg, W.H. Myocardial infarction without significant lesions of coronary arteries. Arch. Intern. Med. 1939, 64, 249–267. [Google Scholar] [CrossRef]
- Safdar, B.; Spatz, E.S.; Dreyer, R.P.; Beltrame, J.F.; Lichtman, J.H.; Spertus, J.A.; Reynolds, H.R.; Geda, M.; Bueno, H.; Dziura, J.D.; et al. Presentation, Clinical Profile, and Prognosis of Young Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Results from the Study. J. Am. Heart Assoc. 2018, 7, e009174. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, R.P.; Tavella, R.; Curtis, J.P.; Wang, Y.; Pauspathy, S.; Messenger, J.; Rumsfeld, J.S.; Maddox, T.M.; Krumholz, H.M.; Spertus, J.A.; et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: Outcomes in a Medicare population. Eur. Heart J. 2020, 41, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Kumar, R.; Armstrong, R.; Murphy, R.; Daly, C. 1 Incidence and prevalence of MINOCA (myocardial infarction with non-obstructive coronary arteries) in STEMI patients: Experience from Irish tertiary care centre. Heart 2021, 107, A1–A2. [Google Scholar] [CrossRef]
- Kilic, S.; Aydın, G.; Çoner, A.; Doğan, Y.; Arican Özlük, Ö.; Çelik, Y.; Ungan, I.; Tascanov, M.B.; Düz, R.; Polat, V.; et al. Prevalence and clinical profile of patients with myocardial infarction with non-obstructive coronary arteries in Turkey (MINOCA-TR): A national multi-center, observational study. Anatol. J. Cardiol. 2020, 23, 176–182. [Google Scholar] [CrossRef]
- Dees, D.; Rahimi, F.; Amann, M.; Nührenberg, T.G.; Löffelhardt, N.; Schmitz, R.; Valina, C.M.; Neumann, F.J.; Hochholzer, W. Prevalence and Causes of Myocardial Infarction with Non-Obstructive Coronary Arteries in a Contemporary Cohort of Patients with Suspected Myocardial Infarction. J. Clin. Med. 2021, 10, 5188. [Google Scholar] [CrossRef]
- Pelliccia, F.; Pasceri, V.; Niccoli, G.; Tanzilli, G.; Speciale, G.; Gaudio, C.; Crea, F.; Camici, P.G. Predictors of mortality in myocardial infarction and nonobstructed coronary arteries: A systematic review and Meta-regression. Am. J. Med. 2020, 133, 73–83. [Google Scholar] [CrossRef]
- Adams, C.; Sawhney, G.; Singh, K. Comparing pharmacotherapy in MINOCA versus medically managed obstructive acute coronary syndrome. Heart Vessel 2022, 37, 705–710. [Google Scholar] [CrossRef]
- Pasupathy, S.; Air, T.; Dreyer, R.P.; Tavella, R.; Beltrame, J.F. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 2015, 131, 861–870. [Google Scholar] [CrossRef]
- Tamis-Holland, J.E.; Jneid, H. Myocardial infarction with nonobstructive coronary arteries (MINOCA): It’s time to face reality! J. Am. Heart Assoc. 2018, 7, e009635. [Google Scholar] [CrossRef]
- Agewall, S.; Beltrame, J.F.; Reynolds, H.R.; Niessner, A.; Rosano, G.; Caforio, A.L.; De Caterina, R.; Zimarino, M.; Roffi, M.; Kjeldsen, K.; et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. 2017, 38, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Tamis-Holland, J.E.; Jneid, H.; Reynolds, H.R.; Agewall, S.; Brilakis, E.S.; Brown, T.M.; Lerman, A.; Cushman, M.; Kumbhani, D.J.; Arslanian-Engoren, C.; et al. Contemporary Diagnosis and Management of Patients with Myocardial Infarction in the Absence of Obstructive Coronary Artery Disease: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e891–e908. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; Hjort, M.; Baron, T.; Jernberg, T.; Nordenskjöld, A.M.; Tornvall, P.; Lindahl, B. Morbidity and cause-specific mortality in first-time myocardial infarction with nonobstructive coronary arteries. J. Intern. Med. 2019, 285, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.M.; Lagerqvist, B.; Baron, T.; Jernberg, T.; Hadziosmanovic, N.; Reynolds, H.R.; Tornvall, P.; Lindahl, B. Reinfarction in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Coronary Findings and Prognosis. Am. J. Med. 2019, 132, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Srichai, M.B.; Iqbal, S.N.; Slater, J.N.; Macini, G.B.J.; Feit, F.; Pena-Sing, I.; Axel, L.; Attubato, M.J.; Yatskar, L.; et al. Mechanisms of Myocardial Infarction in Women without Angiographically Obstructive Coronary Artery Disease. Circulation 2011, 124, 1414–1425. [Google Scholar] [CrossRef]
- Ouldzein, H.; Elbaz, M.; Roncalli, J.; Cagnac, R.; Carrié, D.; Puel, J.; Alibelli-Chermarin, M.-J. Plaque rupture and morphological characteristics of the culprit lesion in acute coronary syndromes without significant angiographic lesion: Analysis by intravascular ultrasound. Ann. Cardiol. Angeiol. 2012, 61, 20–26. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary optical coherence tomography and cardiac magnetic resonance imaging to determine underlying causes of myocardial infarction with nonobstructive coronary arteries in women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, A.L.; Kyaw, T.S.; Bobik, A.; Peter, K. Atherosclerotic Plaque Rupture. Identifying the Straw That Breaks the Camel’s Back. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e63–e72. [Google Scholar] [CrossRef]
- Vergallo, R.; Ren, X.; Yonetsu, T.; Kato, K.; Uemura, S.; Yu, B.; Jia, H.; Abtahian, F.; Aguirre, A.D.; Tian, J.; et al. Pancoronary plaque vulnerability in patients with acute coronary syndrome and ruptured culprit plaque: A 3-vessel optical coherence tomography study. Am. Heart J. 2014, 167, 59–67. [Google Scholar] [CrossRef]
- Dugan, K.E.; Maehara, A.; Kwong, R.Y.; Mahajan, A.M.; Reynolds, H.R. Calcified nodule as a cause of myocardial infarction with non-obstructive coronary artery disease. Int. J. Case Rep. Images 2016, 7, 388–391. [Google Scholar] [CrossRef]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of vulnerable/unstable plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Okafor, O.N.; Gorog, D.A. Endogenous Fibrinolysis: An Important Mediator of Thrombus Formation and Cardiovascular Risk. J. Am. Coll. Cardiol. 2015, 65, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Pretty, H.C. Dissecting aneurysm of coronary artery in a woman aged 42: Rupture. Br. Med. J. 1931, 1, 667. [Google Scholar] [CrossRef]
- Kim, E.S.H. Spontaneous Coronary-Artery Dissection. N. Engl. J. Med. 2020, 383, 2358–2370. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.; Gulati, R.; Williamson, E.E.; Vrtiska, T.J.; Hayes, S.H. Multimodality Imaging for Spontaneous Coronary Artery Dissection in Women. JACC Cardiovasc. Imaging 2016, 9, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Mancini, G.B.J.; Humphries, K.; Fung, A.; Boone, R.; Starovoytov, A.; Aymong, E. Angiographic appearance of spontaneous coronary artery dissection with intramural hematoma proven on intracoronary imaging. Catheter. Cardiovasc. Interv. 2016, 87, E54–E61. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Starovoytov, A.; Humphries, K.; Sheth, T.; So, D.; Minhas, K.; Brass, N.; Lavoie, A.; Bishop, H.; Lavi, S.; et al. Canadian spontaneous coronary artery dissection cohort study: In-hospital and 30-day outcomes. Eur. Heart J. 2019, 40, 1188–1197. [Google Scholar] [CrossRef]
- Eleid, M.F.; Guddeti, R.R.; Tweet, M.S.; Lerman, A.; Singh, M.; Best, P.J.; Prasad, M.; Rihal, C.S.; Hayes, S.N.; Gulati, R. Coronary artery tortuosity in spontaneous coronary artery dissection: Angiographic characteristics and clinical implications. Circ. Cardiovasc. Interv. 2014, 7, 656–662. [Google Scholar] [CrossRef]
- Saw, J.; Aymong, E.; Sedlak, T.; Buller, C.E.; Starovoytov, A.; Ricci, D.; Robinson, S.; Vuurmans, T.; Gao, M.; Humphries, K.; et al. Spontaneous coronary artery dissection: Association with predisposing arteriopathies and precipitating stressors and cardiovascular outcomes. Circ. Cardiovasc. Interv. 2014, 7, 645–655. [Google Scholar] [CrossRef]
- Michelis, K.C.; Olin, J.W.; Kadian-Dodov, D.; d’Escamard, V.; Kovacic, J.C. Coronary artery manifestations of fibromuscular dysplasia. J. Am. Coll. Cardiol. 2014, 64, 1033–1046. [Google Scholar] [CrossRef]
- Saw, J.; Humphries, K.; Aymong, E.; Sedlak, T.; Prakash, R.; Starovoytov, A.; Mancini, G.B.J. Spontaneous Coronary Artery Dissection: Clinical Outcomes and Risk of Recurrence. J. Am. Coll. Cardiol. 2017, 70, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Hayes, S.N.; Pitta, S.R.; Simari, R.D.; Lerman, A.; Lennon, R.J.; Gersh, B.J.; Khambatta, S.; Best, P.J.M.; Rihal, C.S.; et al. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012, 126, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Kim, E.S.H.; Saw, J.; Adlam, D.; Arslanian-Engoren, C.; Economy, K.E.; Ganesh, S.K.; Gulati, R.; Lindsay, M.E.; Mieres, J.H.; et al. Spontaneous Coronary Artery Dissection: Current State of the Science: A Scientific Statement from the American Heart Association. Circulation 2018, 137, e523–e557. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Noguchi, T.; Haruta, S.; Yamamoto, Y.; Oshima, S.; Nakao, K.; Taniguchi, Y.; Yamaguchi, J.; Tsuchihashi, K.; Seki, A.; et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the Angina Pectoris—Myocardial Infarction Multicenter Investigators in Japan. Int. J. Cardiol. 2016, 207, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, S.; Maeder, M.T.; Weilenmann, D.; Haager, P.K.; Ammann, P.; Rohner, F.; Joerg, L.; Rickli, H. Spontaneous coronary artery dissection: Angiographic follow-up and long-term clinical outcome in a predominantly medically treated population. Catheter. Cardiovasc. Interv. 2017, 89, 59–68. [Google Scholar] [CrossRef]
- Elkayam, U.; Jalnapurkar, S.; Barakkat, M.N.; Khatri, N.; Kealey, A.J.; Mehra, A.; Roth, A. Pregnancy-associated acute myocardial infarction: A review of contemporary experience in 150 cases between 2006 and 2011. Circulation 2014, 129, 1695–1702. [Google Scholar] [CrossRef]
- Tweet, M.S.; Hayes, S.N.; Codsi, E.; Gulati, R.; Rose, C.H.; Best, P.J.M. Spontaneous coronary artery dissection associated with pregnancy. J. Am. Coll. Cardiol. 2017, 70, 426–435. [Google Scholar] [CrossRef]
- Faden, M.S.; Bottega, N.; Benjamin, A.; Brown, R.N. A nationwide evaluation of spontaneous coronary artery dissection in pregnancy and the puerperium. Heart 2016, 102, 1974–1979. [Google Scholar] [CrossRef]
- Cade, J.R.; Szarf, G.; de Siqueira, M.E.M.; Chaves, A.; Andréa, J.C.M.; Figueira, H.R.; Gomes, M.M.; Freitas, B.P.; Medeiros, J.F.; dos Santos, M.R.; et al. Pregnancy-associated spontaneous coronary artery dissection: Insights from a case series of 13 patients. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 54–61. [Google Scholar] [CrossRef]
- Kamel, H.; Roman, M.J.; Pitcher, A.; Devereux, R.B. Pregnancy and the risk of aortic dissection or rupture: A cohort-crossover analysis. Circulation 2016, 134, 527–533. [Google Scholar] [CrossRef]
- Saw, J. Pregnancy-associated spontaneous coronary artery dissection represents an exceptionally high-risk spontaneous coronary artery dissection cohort. Circ. Cardiovasc. Interv. 2017, 10, e005119. [Google Scholar] [CrossRef] [PubMed]
- Tweet, M.S.; Eleid, M.F.; Best, P.J.; Lennon, R.J.; Lerman, A.; Rihal, C.S.; Holmes, D.R., Jr.; Hayes, A.N.; Gulati, R. Spontaneous coronary artery dissection: Revascularization versus conservative therapy. Circ. Cardiovasc. Interv. 2014, 7, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.N.Z.; Wong, D.T.L.; Wijesekera, H.; Gutman, S.J.; Shanmugam, V.B.; Gulati, R.; Malaipan, Y.; Meredith, I.T.; Psaltis, P.J. Incidence and characterization of spontaneous coronary artery dissection as a cause of acute coronary syndrome: A single-centre Australian experience. Int. J. Cardiol. 2016, 202, 336–338. [Google Scholar] [CrossRef]
- Prakash, R.; Starovoytov, A.; Heydari, M.; Mancini, G.B.; Saw, J. Catheter-induced iatrogenic coronary artery dissection in patients with spontaneous coronary dissection. JACC Cardiovasc. Interv. 2016, 9, 1851–1853. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.N.; Tweet, M.S.; Adlam, D.; Kim, E.S.H.; Gulati, R.; Price, J.E.; Rose, C.H. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Mintz, G.S.; Kadohira, T.; Fall, K.N.; Brogno, D.A.; Maehara, A. Spontaneous coronary artery dissection with intra-adventitial hematoma detected by high-definition intravascular ultrasound. Coron. Artery Dis. 2016, 27, 707–708. [Google Scholar] [CrossRef]
- Tweet, M.S.; Akhtar, N.J.; Hayes, S.N.; Best, P.J.; Gulati, R.; Araoz, P.A. Spontaneous coronary artery dissection: Acute findings on coronary computed tomography angiography. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 467–475. [Google Scholar] [CrossRef]
- Pozo-Osinalde, E.; Garcia-Guimaraes, M.; Bastante, T.; Aguilera, M.C.; Rodríguez-Alcudia, D.; Rivero, F.; Hernández, S.; Jiménez-Borregero, L.J.; Alfonso, F. Characteristic findings of acute spontaneous coronary artery dissection by cardiac computed tomography. Coron. Artery Dis. 2020, 31, 293–299. [Google Scholar] [CrossRef]
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina pectoris I. A variant form of angina pectoris: Preliminary report. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef]
- Prinzmetal, M.; Ekmekci, A.; Kennamer, R.; Kwoczynski, J.K.; Shubin, H.; Toyoshima, H. Variant Form of Angina Pectoris. JAMA 1960, 174, 1794–1800. [Google Scholar] [CrossRef]
- Aziz, A.; Hansen, H.S.; Sechtem, U.; Prescott, E.; Ong, P. Sex-related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J. Am. Coll. Cardiol. 2017, 70, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Matta, A.; Nader, V.; Canitrot, R.; Delmas, C.; Bouisset, F.; Lhermusier, T.; Blanco, S.; Campelo-Parada, F.; Elbaz, M.; Carrie, D.; et al. Myocardial bridging is significantly associated to myocardial infarction with non-obstructive coronary arteries. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 501–507. [Google Scholar] [CrossRef]
- Nam, P.; Choi, B.G.; Choi, S.Y.; Byun, J.K.; Mashaly, A.; Park, Y.; Jang, W.Y.; Kim, W.; Choi, J.Y.; Park, E.J.; et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis 2018, 270, 8–12. [Google Scholar] [CrossRef]
- Montone, R.A.; Gurgoglione, F.L.; Del Buono, M.G.; Rinaldi, R.; Meucci, M.C.; Iannaccone, G.; La Vecchia, G.; Camilli, M.; D’Amario, D.; Leone, A.M.; et al. Interplay Between Myocardial Bridging and Coronary Spasm in Patients with Myocardial Ischemia and Non-Obstructive Coronary Arteries: Pathogenic and Prognostic Implications. J. Am. Heart Assoc. 2021, 10, e020535. [Google Scholar] [CrossRef]
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of Coronary Artery Spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H. Endothelium-derived relaxing factor and coronary vasospasm. Circulation 1989, 80, 1–9. [Google Scholar] [CrossRef]
- Kaski, J.C.; Tousoulis, D.; Gavrielides, S.; McFadden, E.; Galassi, A.R.; Crea, F.; Maseri, A. Comparison of epicardial coronary artery tone and reactivity in Prinzmetal’s variant angina and chronic stable angina pectoris. J. Am. Coll. Cardiol. 1991, 17, 1058–1062. [Google Scholar] [CrossRef]
- Bertrand, M.E.; LaBlanche, J.M.; Tilmant, P.Y.; Thieuleux, F.A.; Delforge, M.R.; Carre, A.G.; Asseman, P.; Berzin, B.; Libersa, C.; Laurent, J.M. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary angiography. Circulation 1982, 65, 1299–1306. [Google Scholar] [CrossRef]
- Kaski, J.C.; Maseri, A.; Vejar, M.; Crea, F.; Hackett, D. Spontaneous coronary artery spasm in variant angina results from a local hyperreactivity to a generalized constrictor stimulus. J. Am. Coll. Cardiol. 1989, 14, 1456. [Google Scholar] [CrossRef]
- Yasue, H.; Horio, Y.; Nakamura, N.; Fujii, H.; Sonoda, R.; Kugiyama, K.; Obata, K.; Morikami, Y.; Kimura, T. Induction of coronary artery spasm by acetylcholine in patients with variant angina: Possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation 1986, 74, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Chierchia, S.; Kaski, J.C.; Davies, G.J.; Margonato, A.; Miran, D.O.; Maseri, A. Provocation of CAS by dopamine in patients with active variant angina pectoris. Circulation 1986, 74, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Previtali, M.; Ardissino, D.; Barberis, P.; Panciroli, C.; Chimienti, M.; Salerno, J.A. Hyperventilation and ergonovine tests in Prinzmetal’s variant angina pectoris in men. Am. J. Cardiol. 1989, 63, 17–20. [Google Scholar] [CrossRef]
- Zaya, M.; Mehta, P.K.; Merz, N.B. Provocative Testing for Coronary Reactivity and Spasm. J. Am. Coll. Cardiol. 2014, 63, 103–109. [Google Scholar] [CrossRef]
- Takagi, Y.; Yasuda, S.; Takahashi, J.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; Tanabe, Y.; et al. Clinical implications of provocation tests for coronary artery spasm: Safety, arrhythmic complications, and prognostic impact: Multicentre registry study of the Japanese Coronary Spasm Association. Eur. Heart J. 2013, 34, 258–267. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Vokshi, I.; Bastiaenen, R.; Kubik, S.; Hill, S.; Schäufele, T.; Mahrholdt, H.; Kaski, J.C.; et al. Clinical Usefulness, Angiographic Characteristics, and Safety Evaluation of Intracoronary Acetylcholine Provocation Testing Among 921 Consecutive White Patients with Unobstructed Coronary Arteries. Circulation 2014, 129, 1723–1730. [Google Scholar] [CrossRef]
- Montone, R.A.; Niccoli, G.; Fracassi, F.; Russo, M.; Gurgoglione, F.; Cammà, G.; Lanza, G.A.; Crea, F. Patients with acute myocardial infarction and non-obstructive coronary arteries: Safety and prognostic relevance of invasive coronary provocative tests. Eur. Heart J. 2018, 39, 91–98. [Google Scholar] [CrossRef]
- Shibata, T.; Kawakami, S.; Noguchi, T.; Tanaka, T.; Asaumi, Y.; Kanaya, T.; Nagai, T.; Nakao, K.; Fujino, M.; Nagatsuka, K.; et al. Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation 2015, 132, 241–250. [Google Scholar] [CrossRef]
- Cannon, R.O., 3rd; Epstein, S.E. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol. 1988, 61, 1338–1343. [Google Scholar] [CrossRef]
- Camici, P.G.; Crea, F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007, 356, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.J.; Lerman, A.; Bech, J.W.; De Bruyne, B.; Eeckhout, E.; Fearon, W.F.; Higano, S.T.; Lim, M.J.; Meuwissen, M.; Piek, J.J.; et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: A scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 2006, 114, 1321–1341. [Google Scholar] [CrossRef]
- Melikian, N.; Vercauteren, S.; Fearon, W.F.; Cuisset, T.; MacCarthy, P.A.; Davidavicius, G.; Aarnoudse, W.; Bartunek, J.; Vanderheyden, M.; Wyffels, E.; et al. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention 2010, 5, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Long, M.; Hu, X.; Huang, Z.; Hu, C.; Gao, X.; Du, Z. Thermodilution-Derived Coronary Microvascular Resistance and Flow Reserve in Patients with Cardiac Syndrome X. Circ. Cardiovasc. Interv. 2014, 7, 43–48. [Google Scholar] [CrossRef]
- Solberg, O.G.; Ragnarsson, A.; Kvarsnes, A.; Endresen, K.; Kongsgârd, E.; Aakhus, S.; Gullestad, L.; Stavem, K.; Aaberge, L. Reference interval for the index of coronary microvascular resistance. EuroIntervention 2014, 9, 1069–1075. [Google Scholar] [CrossRef]
- Rahman, H.; Ryan, M.; Lumley, M.; Modi, B.; McConkey, H.; Ellis, H.; Scannell, C.; Clapp, B.; Marber, M.; Webb, A.; et al. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation 2019, 140, 1805–1816. [Google Scholar] [CrossRef]
- Rahman, H.; Demir, O.M.; Khan, F.; Ryan, M.; Ellis, H.; Mills, M.T.; Chiribiri, A.; Webb, A.; Perera, D. Physiological Stratification of Patients with Angina Due to Coronary Microvascular Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 2538–2549. [Google Scholar] [CrossRef]
- Shaw, L.J.; Merz, N.B.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights From the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: Gender Differences in Traditional and Novel Risk Factors, Symptom Evaluation, and Gender-Optimized Diagnostic Strategies. J. Am. Coll. Cardiol. 2006, 47, S4–S20. [Google Scholar] [CrossRef]
- Johnson, B.D.; Shaw, L.J.; Buchthal, S.D.; Merz, C.N.B.; Kim, H.-W.; Scott, K.N.; Olson, M.B.; Pepine, C.J.; Hollander, J.; Sharaf, B.; et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease; results from the WISE. Circulation 2004, 109, 2993–2999. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef] [PubMed]
- Godo, S.; Suda, A.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Coronary Microvascular Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1625–1637. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.T.; Kalra, D. Coronary Microvascular Dysfunction and the Role of Noninvasive Cardiovascular Imaging. Diagnostics 2020, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.E.; Holubkov, R.; Smith, A.J.C.; Kelsey, S.F.; Sharaf, B.L.; Reichek, N.; Rogers, W.J.; Merz, C.N.; Sopko, G.; Pepine, C.J.; et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001, 141, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel index for invasively assessing the coronary microcirculation. Circulation 2003, 107, 3129–3132, Erratum in Circulation 2003, 108, 3165. [Google Scholar] [CrossRef]
- Ng, M.K.; Yeung, A.C.; Fearon, W.F. Invasive assessment of the coronary microcirculation: Superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 2006, 113, 2054–2061. [Google Scholar] [CrossRef]
- Aarnoudse, W.; Fearon, W.F.; Manoharan, G.; Geven, M.; van de Vosse, F.; Rutten, M.; De Bruyne, B.; Pijls, N.H.J. Epicardial Stenosis Severity Does Not Affect Minimal Microcirculatory Resistance. Circulation 2004, 110, 2137–2142. [Google Scholar] [CrossRef]
- Teunissen, P.F.; de Waard, G.A.; Hollander, M.R.; Robbers, L.F.; Danad, I.; Biesbroek, P.S.; Amier, R.P.; Echavarría-Pinto, M.; Quirós, A.; Broyd, C.; et al. Doppler-derived intracoronary physiology indices predict the occurrence of microvascular injury and microvascular perfusion deficits after angiographically successful primary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2015, 8, e001786. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.M.; Park, J.; Choi, K.H.; Hwang, D.; Doh, J.H.; Nam, C.W.; Shin, E.S.; Hoshino, M.; Murai, T.; et al. Prognostic Implications of Resistive Reserve Ratio in Patients with Coronary Artery Disease. J. Am. Heart Assoc. 2020, 9, e015846. [Google Scholar] [CrossRef]
- De Bruyne, B.; Pijls, N.H.J.; Gallinoro, E.; Candreva, A.; Fournier, S.; Keulards, D.C.J.; Sonck, J.; Van’t Veer, M.; Barbato, E.; Bartunek, J.; et al. Microvascular Resistance Reserve for Assessment of Coronary Microvascular Function: JACC Technology Corner. J. Am. Coll. Cardiol. 2021, 78, 1541–1549. [Google Scholar] [CrossRef]
- Morris, P.D.; Gosling, R.; Zwierzak, I.; Evans, H.; Aubiniere-Robb, L.; Czechowicz, K.; Evans, P.C.; Hose, D.R.; Lawford, P.V.; Narracott, A.J.; et al. A novel method for measuring absolute coronary blood flow and microvascular resistance in patients with ischaemic heart disease. Cardiovasc. Res. 2021, 117, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Mangiacapra, F.; Viscusi, M.M.; Paolucci, L.; Nusca, A.; Melfi, R.; Ussia, G.P.; Grigioni, F. The Pivotal Role of Invasive Functional Assessment in Patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA). Front. Cardiovasc. Med. 2021, 8, 781485. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Albertucci, M.; Prati, F. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention 2021, 17, 875–887. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Murphy, S.; Cohen, J.A.; Rehman, S.; Jones-O’Connor, M.; Olshan, D.S.; Singh, A.; Vaduganathan, M.; Januzzi, J.L., Jr.; Wasfy, J.H. Misclassification of Myocardial Injury as Myocardial Infarction: Implications for Assessing Outcomes in Value-Based Programs. JAMA Cardiol. 2019, 4, 460–464. [Google Scholar] [CrossRef] [PubMed]
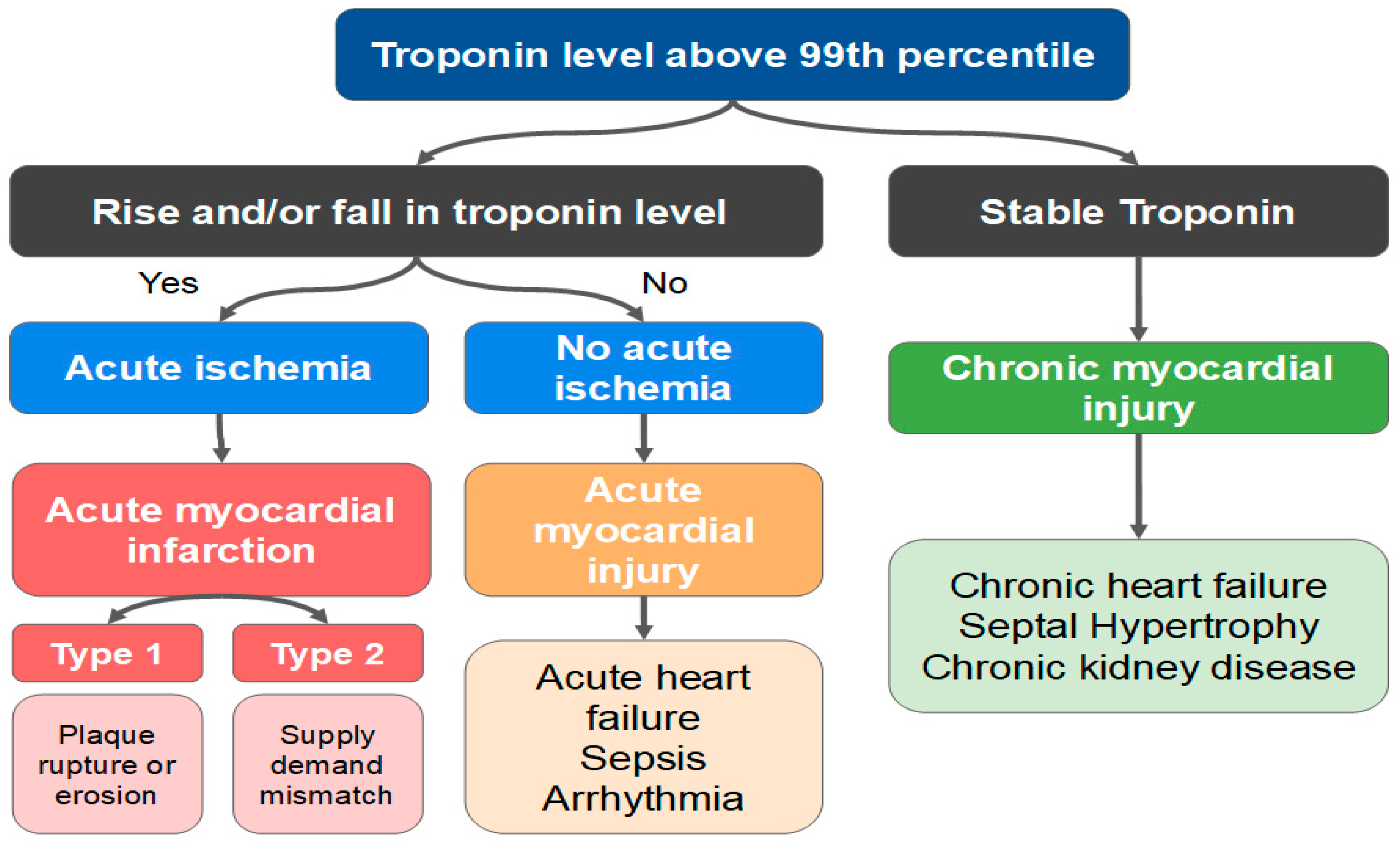
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tateishi, H.; Uchida, T. Takotsubo type cardiomyopathy due to multivessel spasm. In Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure; Kodama, K., Haze, K., Hon, M., Eds.; Kagaku Hyoronsha: Tokyo, Japan, 1990; pp. 56–64. [Google Scholar]
- Pilgrim, T.M.; Wyss, T.R. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: A systematic review. Int. J. Cardiol. 2008, 124, 283–292. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Takotsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Farid, A.; Dufresne, W.; Farid, B.; Amsterdam, E.A. A stressful situation: Takotsubo cardiomyopathy. Am. J. Med. 2018, 13, 253–256. [Google Scholar] [CrossRef]
- De Chazal, H.M.; Del Buono, M.G.; Keyser-Marcus, L.; Ma, L.; Moeller, F.G.; Berrocal, D.; Abbate, A. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018, 72, 1955–1971. [Google Scholar] [CrossRef]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent long-term structural, functional, and metabolic changes after stress-induced (Takotsubo) cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef] [PubMed]
- Pustjens, T.F.S.; Appelman, Y.; Damman, P.; Berg, J.M.T.; Jukema, J.W.; de Winter, R.J.; Agema, W.R.P.; van der Wielen, M.L.J.; Arslan, F.; Rasoul, S.; et al. Guidelines for the management of myocardial infarction/injury with non-obstructive coronary arteries (MINOCA): A position paper from the Dutch ACS working group. Neth. Heart J. 2020, 28, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Marcotte, F. Cardiac Magnetic Resonance Assessment of Myocarditis. Circ. Cardiovasc. Imaging 2013, 6, 833–839. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Montone, R.A.; Iannaccone, G.; Meucci, M.C.; Rinaldi, R.; D’Amario, D.; Niccoli, G. Diagnostic work-up and therapeutic implications in MINOCA: Need for a personalized approach. Future Cardiol. 2021, 17, 149–154. [Google Scholar] [CrossRef]
- Gudenkauf, B.; Hays, A.G.; Tamis-Holland, J.; Trost, J.; Ambinder, D.I.; Wu, K.C.; Arbab-Zadeh, A.; Blumenthal, R.S.; Sharma, G. Role of Multimodality Imaging in the Assessment of Myocardial Infarction with Nonobstructive Coronary Arteries: Beyond Conventional Coronary Angiography. J. Am. Heart Assoc. 2022, 11, e022787. [Google Scholar] [CrossRef]
- Wu, K.C. CMR of microvascular obstruction and hemorrhage in myocardial infarction. J. Cardiovasc. Magn. Reson. 2012, 14, 68. [Google Scholar] [CrossRef]
- Abbas, A.; Matthews, G.H.; Brown, I.W.; Shambrook, J.S.; Peebles, C.R.; Harden, S.P. Cardiac MR assessment of microvascular obstruction. Br. J. Radiol. 2015, 88, 20140470. [Google Scholar] [CrossRef]
- Gatti, M.; Carisio, A.; D’Angelo, T.; Darvizeh, F.; Dell’Aversana, S.; Tore, D.; Centonze, M.; Faletti, R. Cardiovascular magnetic resonance in myocardial infarction with non-obstructive coronary arteries patients: A review. World J. Cardiol. 2020, 12, 248–261. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Caliuto, L.; Desmet, W.; et al. International expert consensus document on takotsubo syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1497. [Google Scholar] [CrossRef] [PubMed]
- De Cobelli, F.; Pieroni, M.; Esposito, A.; Chimenti, C.; Belloni, E.; Mellone, R.; Canu, G.; Perseghin, G.; Gaudio, C.; Maseri, A.; et al. Cardiac magnetic resonance imaging with delayed gadolinium in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. J. Am. Coll. Cardiol. 2006, 47, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Von Knobelsdorff-Brenkenhoff, F.; Schuler, J.; Dogangüzel, S.; Dieringer, M.A.; Greiser, A.; Kellman, P.; Schulz-Menger, J. Detection and Monitoring of Acute Myocarditis Applying Quantitative Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2017, 10, e005242. [Google Scholar] [CrossRef] [PubMed]
- Sörensson, P.; Ekenbäck, C.; Lundin, M.; Agewall, S.; Brolin, E.B.; Caidahl, K.; Cederlund, K.; Collste, O.; Daniel, M.; Sensen, J.; et al. Early Comprehensive Cardiovascular Magnetic Resonance Imaging in Patients with Myocardial Infarction with Nonobstructive Coronary Arteries. JACC Cardiovasc. Imaging 2021, 14, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Pathik, B.; Raman, B.; Amin, N.H.M.; Mahadavan, D.; Rajendran, S.; McGavigan, A.D.; Grover, S.; Smith, E.; Mazhar, J.; Bridgman, C.; et al. Troponin-positive chest pain with unobstructed coronary arteries: Incremental diagnostic value of cardiovascular magnetic resonance imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1146–1152. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.-M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the international working group for intravascular optical coherence tomography standardization and validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Sucato, V.; Testa, G.; Puglisi, S.; Evola, S.; Galassi, A.R.; Novo, G. Myocardial infarction with non-obstructive coronary arteries (MINOCA): Intracoronary imaging-based diagnosis and management. J. Cardiol. 2021, 77, 444–451. [Google Scholar] [CrossRef]
- Mariani, J., Jr.; Guedes, C.; Soares, P.; Zalc, S.; Campos, C.M.; Lopes, A.C.; Spadaro, A.G.; Perin, M.A.; Filho, A.E.; Takimura, C.K.; et al. Intravascular ultrasound guidance to minimize the use of iodine contrast in percutaneous coronary intervention the MOZART (minimizing contrast utilization with IVUS guidance in coronary angioplasty) randomized controlled trial. JACC Cardiovasc. Interv. 2014, 7, 1287–1293. [Google Scholar] [CrossRef]
- Malik, A.O.; Spertus, J.A.; Grantham, J.A.; Peri-Okonny, P.; Gosch, K.; Sapontis, J.; Moses, J.; Lombardi, W.; Karmpaliotis, D.; Nicholson, W.J.; et al. Outcomes of chronic total occlusion percutaneous coronary intervention in patients with renal dysfunction. Am. J. Cardiol. 2020, 125, 1046–1053. [Google Scholar] [CrossRef]
- Gerbaud, E.; Arabucki, F.; Nivet, H.; Barbey, C.; Cetran, L.; Chassaing, S.; Seguy, B.; Lesimple, A.; Cochet, H.; Montaudon, M.; et al. OCT and CMR for the Diagnosis of Patients Presenting with MINOCA and Suspected Epicardial Causes. JACC Cardiovasc. Imaging 2020, 13, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Van der Sijde, J.N.; Karanasos, A.; van Ditzhuijzen, N.S.; Okamura, T.; van Geuns, R.J.; Valgimigli, M.; Ligthart, J.M.; Witberg, K.T.; Wemelsfelder, S.; Fam, J.M.; et al. Safety of optical coherence tomography in daily practice: A comparison with intravascular ultrasound. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, B.; Baron, T.; Erlinge, D.; Hadziosmanovic, N.; Nordenskjöld, A.; Gard, A.; Jernberg, T. Medical Therapy for Secondary Prevention and Long-Term Outcome in Patients with Myocardial Infarction with Nonobstructive Coronary Artery Disease. Circulation 2017, 135, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Nordenskjöld, A.M.; Agewall, S.; Atar, D.; Baron, T.; Beltrame, J.; Bergström, O.; Erlinge, D.; Gale, C.P.; López-Pais, J.; Jernberg, T.; et al. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment in patients with myocardial infarction with non-obstructive coronary arteries (MINOCA-BAT): Rationale and design. Am. Heart J. 2021, 231, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef]
- Lobo, L.M.; Masson, W.; Molinero, G.; Cobo, A.L.; Benincasa, F.; Wehit, G.; Delgado, J.; Losada, P.; Suarez, F.; Barbagelata, L. Role of statins in patients with MINOCA. A systematic review and meta-analysis. Eur. Heart J. 2021, 42, ehab724-2534. [Google Scholar] [CrossRef]
- Cannon, C.P.; Weintraub, W.S.; Demopoulos, L.A.; Vicari, R.; Frey, M.J.; Lakkis, N.; Neumann, F.J.; Robertson, D.H.; DeLucca, P.T.; DiBattiste, P.M.; et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N. Engl. J. Med. 2001, 344, 1879–1887. [Google Scholar] [CrossRef]
- Fox, K.A.; Poole-Wilson, P.; Clayton, T.C.; Henderson, R.A.; Shaw, T.R.; Wheatley, D.J.; Knight, R.; Pocock, S.J. 5-year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: The British Heart Foundation RITA 3 randomised trial. Lancet 2005, 366, 914–920. [Google Scholar] [CrossRef]
- Mokashi, S.A.; Svensson, L.G. Guidelines for the management of thoracic aortic disease in 2017. Gen. Thorac. Cardiovasc. Surg. 2019, 67, 59–65. [Google Scholar] [CrossRef]
- Alfonso, F.; de la Torre Hernández, J.M.; Ibáñez, B.; Sabaté, M.; Pan, M.; Gulati, R.; Saw, J.; Angiolillo, D.J.; Adlam, D.; Sánchez-Madrid, F. Rationale and design of the BA-SCAD (Beta-blockers and Antiplatelet agents in patients with Spontaneous Coronary Artery Dissection) randomized clinical trial. Rev. Esp. Cardiol. 2022, 75, 515–522. [Google Scholar] [CrossRef]
- Adlam, D.; Alfonso, F.; Maas, A.; Vrints, C. European Society of Cardiology, Acute Cardiovascular Care Association, SCAD Study Group: A position paper on spontaneous coronary artery dissection. Eur. Heart J. 2018, 39, 3353–3368. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, E.; Giacobbe, F.; Quadri, G.; Macaya, F.; Bianco, M.; Mori, R.; Biolè, C.A.; Boi, A.; Bettari, L.; Rolfo, C.; et al. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur. Heart J. 2021, 42, 3161–3171. [Google Scholar] [CrossRef] [PubMed]
- Zghouzi, M.; Pacha, H.M.; Sattar, Y.; Alraies, M.C. Successful Treatment of Spontaneous Coronary Artery Dissection With Cutting Balloon Angioplasty. Cureus 2021, 13, e13706. [Google Scholar] [CrossRef] [PubMed]
- Main, A.; Lombardi, W.L.; Saw, J. Cutting balloon coronary angioplasty for treatment of spontaneous artery dissection: Case report, literature review, and recommended technical approaches. Cardiovasc. Diagn. Ther. 2019, 9, 50–54. [Google Scholar] [CrossRef]
- Tweet, M.S.; Young, K.A.; Rose, C.H.; Best, P.J.; Gulati, R.; Hayes, S.N. Abstract 13043: Pregnancy After Spontaneous Coronary Artery Dissection: A Case Series of 22 Women and 31 Pregnancies. Circulation 2019, 140, A13043. [Google Scholar]
- Cauldwell, M.; Steer, P.J.; von Klemperer, K.; Kaler, M.; Grixti, S.; Hale, J.; O’Heney, J.; Warriner, D.; Curtis, S.; Mohan, A.R.; et al. Maternal and neonatal outcomes in women with history of coronary artery disease. Heart 2020, 106, 380–386. [Google Scholar] [CrossRef]
- Tweet, M.S.; Young, K.A.; Best, P.J.M.; Hyun, M.; Gulati, R.; Rose, C.H.; Hayes, S.N. Association of Pregnancy with Recurrence of Spontaneous Coronary Artery Dissection Among Women with Prior Coronary Artery Dissection. JAMA Netw. Open 2020, 3, e2018170. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Mizuno, Y.; Harada, E.; Katoh, D.; Kashiwagi, Y.; Morita, S.; Yoshimura, M.; Uemura, S.; Saito, Y.; Yasue, H. Aerobic interval exercise training in the afternoon reduces attacks of coronary spastic angina in conjunction with improvement in endothelial function, oxidative stress, and inflammation. Coron. Artery Dis. 2013, 24, 177–182. [Google Scholar] [CrossRef]
- Chahine, R.A.; Feldman, R.L.; Giles, T.D.; Nicod, P.; Raizner, A.E.; Weiss, R.J.; Vanov, S.K. Randomized placebo-controlled trial of amlodipine in vasospastic angina. J. Am. Coll. Cardiol. 1993, 21, 1365–1370. [Google Scholar] [CrossRef]
- Hung, M.J.; Cherng, W.J.; Cheng, C.W.; Yang, N.I. Effect of antispastic agents (calcium antagonists and/or isosorbide dinitrate) on high-sensitivity C-reactive protein in patients with vasospastic angina pectoris and no hemodynamically significant coronary artery disease. Am. J. Cardiol. 2005, 95, 84–87. [Google Scholar] [CrossRef]
- Slavich, M.; Patel, R.S. Coronary artery spasm: Current knowledge and residual uncertainties. Int. J. Cardiol. Heart Vasc. 2016, 10, 47–53. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 2014, 78, 2779–2801. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Münzel, T. Organic Nitrate Therapy, Nitrate Tolerance, and Nitrate-Induced Endothelial Dysfunction: Emphasis on Redox Biology and Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 899–942. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.M.; Wood, A.J.; Vaughn, W.K.; Robertson, D. Exacerbation of vasotonic angina pectoris by propranolol. Circulation 1982, 65, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Akkus, N.I.; Rajpal, S.; Peter, E. Resolution of nebivolol-induced coronary vasospasm by intracoronary nitroglycerin during a coronary angiogram. Rev. Port. Cardiol. 2012, 31, 825–828. [Google Scholar] [CrossRef]
- Yasue, H.; Mizuno, Y.; Harada, E.; Itoh, T.; Nakagawa, H.; Nakayama, M.; Ogawa, H.; Tayama, S.; Honda, T.; Hokimoto, S.; et al. Effects of a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, fluvastatin, on coronary spasm after withdrawal of calcium-channel blockers. J. Am. Coll. Cardiol. 2008, 51, 1742–1748. [Google Scholar] [CrossRef]
- Ishii, M.; Kaikita, K.; Sato, K.; Yamanaga, K.; Miyazaki, T.; Akasaka, T.; Tabata, N.; Arima, Y.; Sueta, D.; Sakamoto, K.; et al. Impact of Statin Therapy on Clinical Outcome in Patients with Coronary Spasm. J. Am. Heart Assoc. 2016, 5, e003426. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.W.; Jo, S.H.; Kim, S.E.; Han, S.H.; Lee, K.Y.; Her, S.H.; Lee, M.H.; Cho, S.S.; Baek, S.H. Clinical impact of statin therapy on vasospastic angina: Data from a Korean nation-wide cohort study. Heart Vessel. 2020, 35, 1051–1059. [Google Scholar] [CrossRef]
- Mori, H.; Takahashi, J.; Sato, K.; Miyata, S.; Takagi, Y.; Tsunoda, R.; Sumiyoshi, T.; Matsui, M.; Tanabe, Y.; Sueda, S.; et al. Impact of statins in patients with vasospastic angina: A multicenter registry study of the Japanese Coronary Spasm Association. J. Cardiol. 2022, 80, 226–231. [Google Scholar] [CrossRef]
- Kishida, H.; Murao, S. Effect of a new coronary vasodilator, nicorandil, on variant angina pectoris. Clin. Pharmacol. Ther. 1987, 42, 166–174. [Google Scholar] [CrossRef]
- Teragawa, H.; Kato, M.; Yamagata, T.; Matsuura, H.; Kajiyama, G. The preventive effect of magnesium on coronary spasm in patients with vasospastic angina. Chest 2000, 118, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Motoyama, T.; Hirai, N.; Kugiyama, K.; Ogawa, H.; Yasue, H. Estradiol supplementation suppresses hyperventilation-induced attacks in postmenopausal women with variant angina. J. Am. Coll. Cardiol. 2001, 37, 735–740. [Google Scholar] [CrossRef]
- Kugiyama, K.; Motoyama, T.; Hirashima, O.; Ohgushi, M.; Soejima, H.; Misumi, K.; Kawano, H.; Miyao, Y.; Yoshimura, M.; Ogawa, H.; et al. Vitamin C attenuates abnormal vasomotor reactivity in spasm coronary arteries in patients with coronary spastic angina. J. Am. Coll. Cardiol. 1998, 32, 103–109. [Google Scholar] [CrossRef]
- Miwa, K.; Kambara, H.; Kawai, C. Effect of aspirin in large doses on attacks of variant angina. Am. Heart J. 1983, 105, 351–355. [Google Scholar] [CrossRef]
- Park, J.Y.; Rha, S.W.; Poddar, K.L.; Ramasamy, S.; Chen, K.Y.; Li, Y.J.; Choi, B.G.; Ryu, S.K.; Choi, J.W.; Park, S.H.; et al. Impact of low-dose aspirin on coronary artery spasm as assessed by intracoronary acetylcholine provocation test in Korean patients. J. Cardiol. 2012, 60, 187–191. [Google Scholar] [CrossRef]
- Jolly, S.S.; James, S.; Džavík, V.; Cairns, J.A.; Mahmoud, K.D.; Zijlstra, F.; Yusuf, S.; Olivecrona, G.K.; Renlund, H.; Gao, P.; et al. Thrombus aspiration in ST-segment-elevation myocardial infarction: An individual patient meta-analysis. Thrombectomy Trialists Collaboration. Circulation 2017, 135, 143–152. [Google Scholar] [CrossRef]
- Nadir, M.A. Letter by Nadir regarding article, Prevalence, Clinical Features, and Prognosis of Acute Myocardial Infarction Attributable to Coronary Artery Embolism. Circulation 2016, 133, e378. [Google Scholar] [CrossRef]
- Raphael, C.E.; Heit, J.A.; Reeder, G.S.; Bois, M.C.; Maleszewski, J.J.; Tilbury, R.T.; Holmes, D.R., Jr. Coronary Embolus. An Underappreciated Cause of Acute Coronary Syndromes. J. Am. Coll. Cardiol. Intv. 2018, 11, 172–180. [Google Scholar] [CrossRef]
- Marinescu, M.A.; Loffler, A.I.; Ouellette, M.; Smith, L.; Kramer, C.M.; Bourque, J.M. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc. Imaging 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]


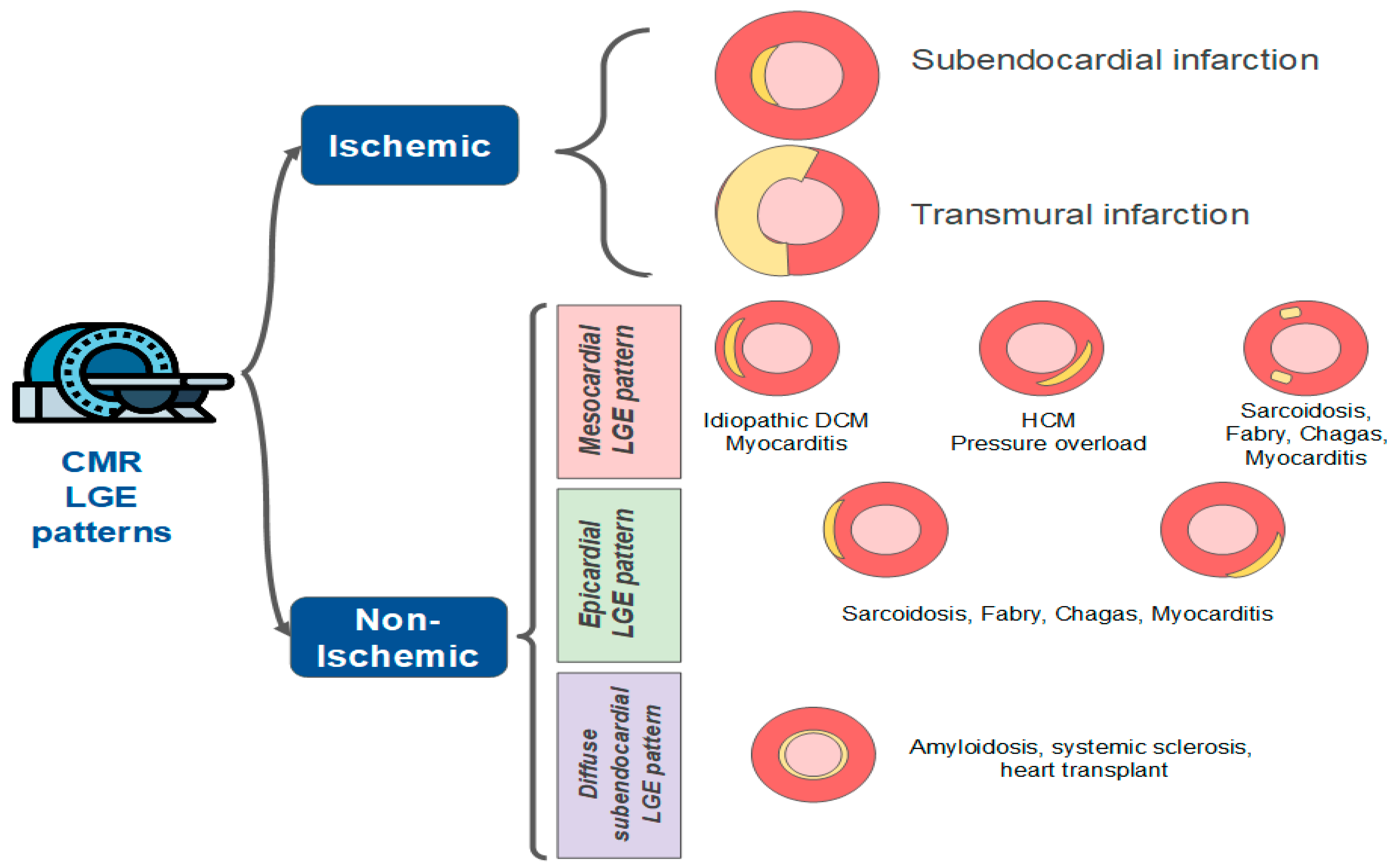
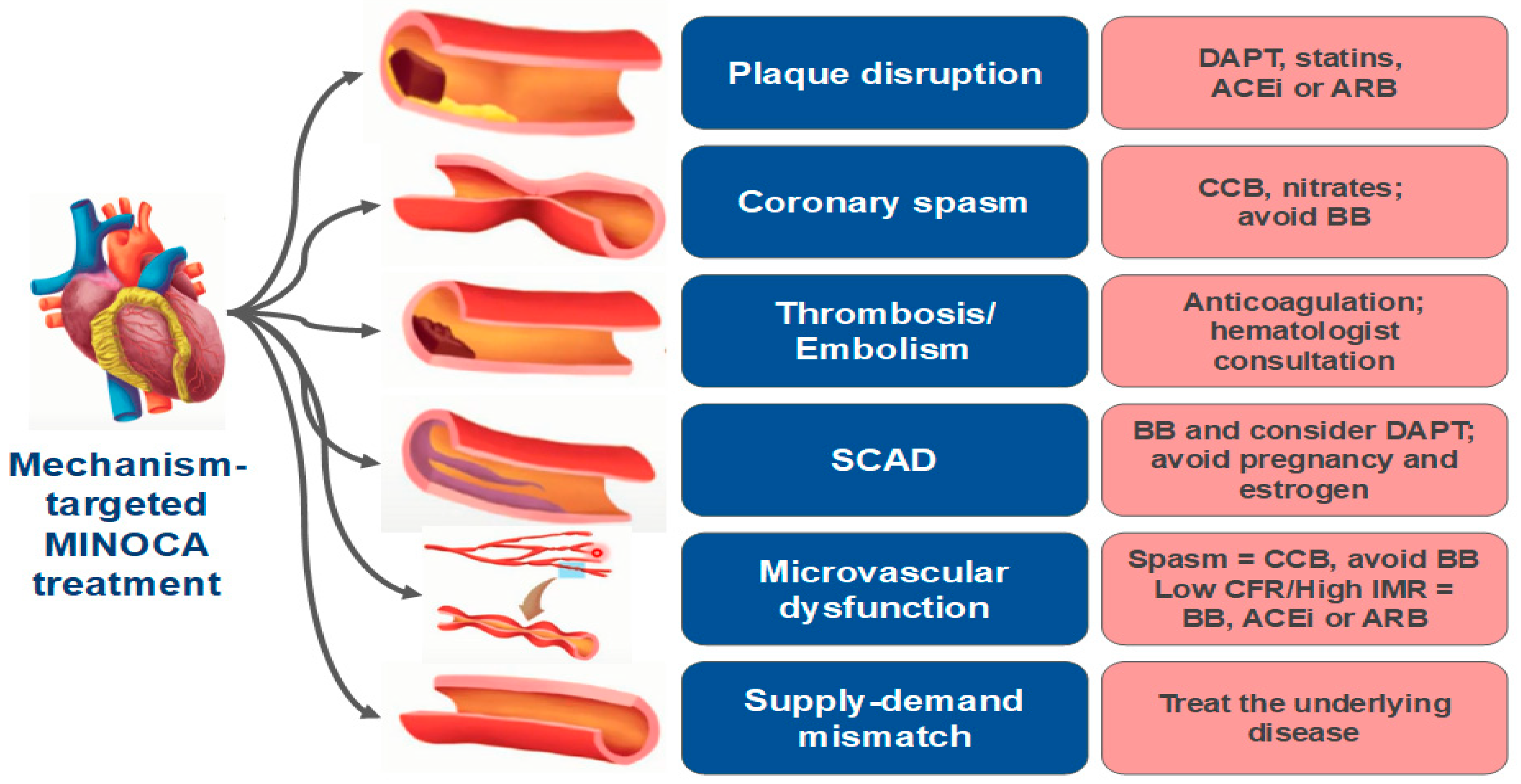
|
|
|
| Major criteria |
|
| Minor criteria |
|
| Definite CE |
|
| Probable CE |
|
| A diagnosis of CE should not be made if there is: |
|
|
|
|
|
| Tests | Acute Myocardial Infarction | Takotsubo Cardiomyopathy | Myocarditis |
|---|---|---|---|
| Transthoracic Echocardiogram | Segmental ventricular dysfunction (respect coronary anatomy) | Regional ventricular dysfunction (does not respect coronary anatomy) | Segmental, regional or global ventricular dysfunction |
| Coronary angiography | Culprit obstructive lesion or absence of obstructive lesion (MINOCA) | Absence of obstructive lesion (more common) or not culprit obstructive lesion | Absence of obstructive lesion (more common) or not culprit obstructive lesion |
| Cardiac Magnetic Resonance | Subendocardial or transmural late gadolinium enhancement (LGE) | LGE absent | Patch and epicardial LGE are common than subendocardial or transmural |
In the setting of clinically suspected myocarditis, CMR findings are consistent with myocardial inflammation, if at least two of the following criteria are present:
|
| A CMR study is consistent with myocyte injury and/or scar caused by myocardial inflammation if criterion 3 is present |
A repeat CMR study between 1 and 2 weeks after the initial CMR study is recommended if:
|
| The presence of LV dysfunction or pericardial effusion provides additional, supportive evidence for myocarditis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herling de Oliveira, L.L.; Correia, V.M.; Nicz, P.F.G.; Soares, P.R.; Scudeler, T.L. MINOCA: One Size Fits All? Probably Not—A Review of Etiology, Investigation, and Treatment. J. Clin. Med. 2022, 11, 5497. https://doi.org/10.3390/jcm11195497
Herling de Oliveira LL, Correia VM, Nicz PFG, Soares PR, Scudeler TL. MINOCA: One Size Fits All? Probably Not—A Review of Etiology, Investigation, and Treatment. Journal of Clinical Medicine. 2022; 11(19):5497. https://doi.org/10.3390/jcm11195497
Chicago/Turabian StyleHerling de Oliveira, Lucas Lentini, Vinícius Machado Correia, Pedro Felipe Gomes Nicz, Paulo Rogério Soares, and Thiago Luis Scudeler. 2022. "MINOCA: One Size Fits All? Probably Not—A Review of Etiology, Investigation, and Treatment" Journal of Clinical Medicine 11, no. 19: 5497. https://doi.org/10.3390/jcm11195497
APA StyleHerling de Oliveira, L. L., Correia, V. M., Nicz, P. F. G., Soares, P. R., & Scudeler, T. L. (2022). MINOCA: One Size Fits All? Probably Not—A Review of Etiology, Investigation, and Treatment. Journal of Clinical Medicine, 11(19), 5497. https://doi.org/10.3390/jcm11195497





