Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Definition of Comorbidity and Hospital Complications and Mortality
2.3. Definition of Hemodynamic Parameters
2.4. Definition of Patient-Prosthesis Mismatch
2.5. Definition of Endpoints
2.6. Data Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Hemodynamic Parameters before and after the Operation
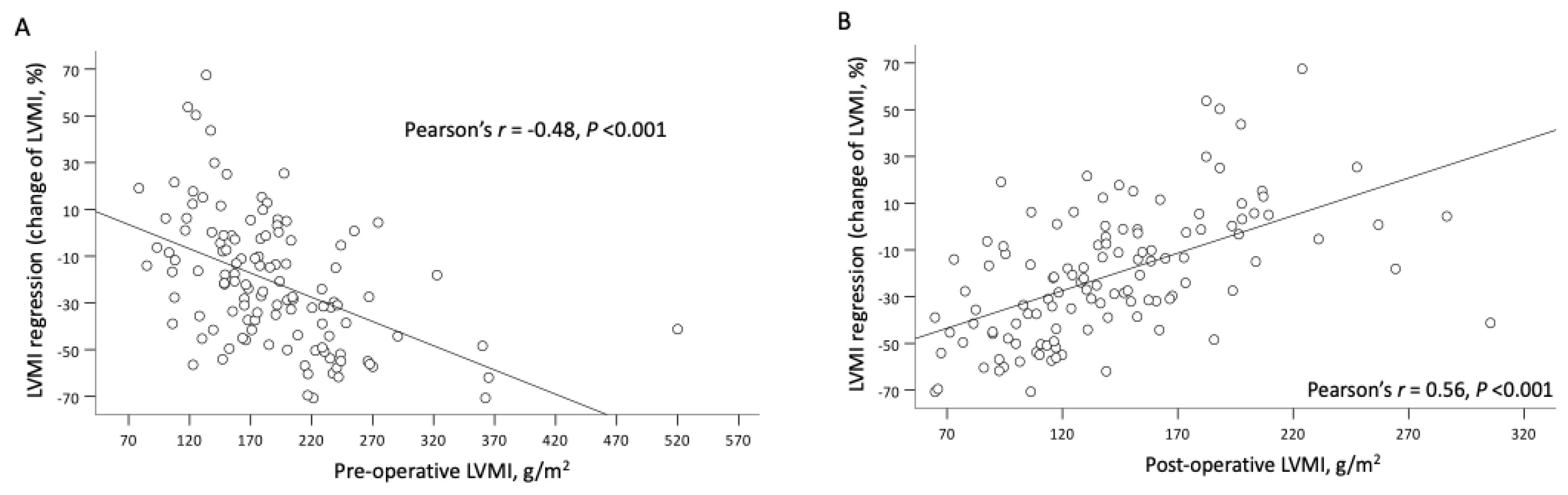
3.3. Association between Follow-Up Hemodynamic Parameters and Relative LVMI Regression
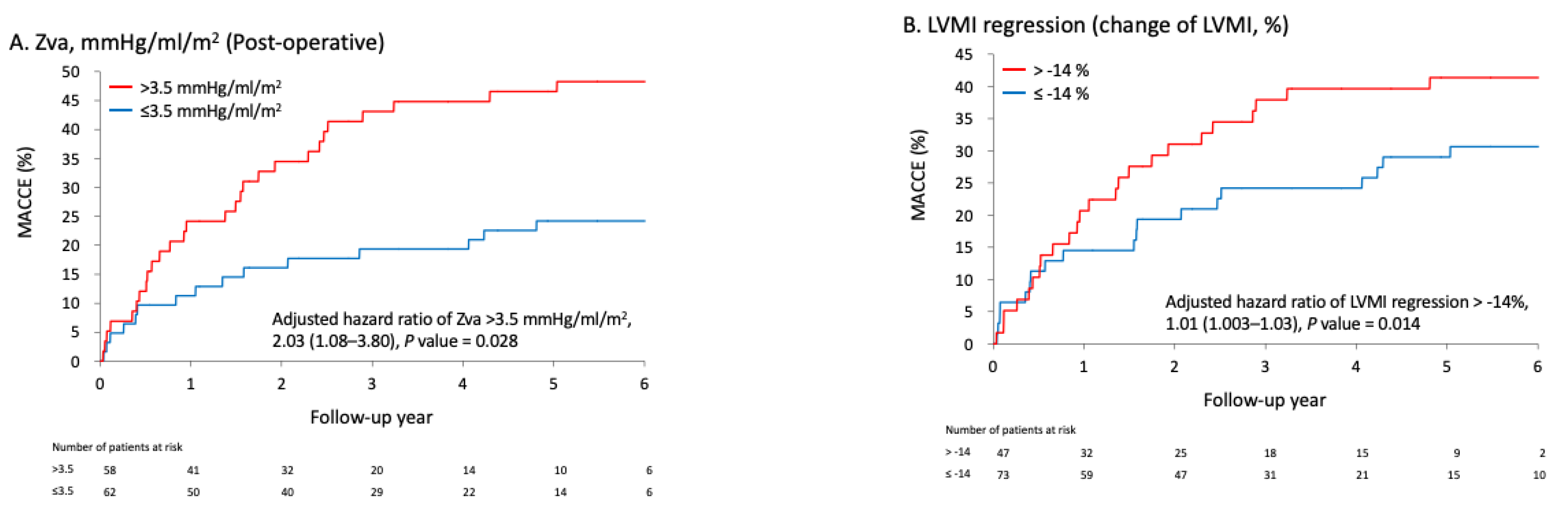
3.4. Association between Follow-Up Hemodynamic Parameters and the Risk of MACCE
3.5. Association between Changes in Hemodynamic Parameters and Relative LVMI Regression and Risk of MACCE
4. Discussion
4.1. Patient-Prosthesis Mismatch
4.2. Reverse Remodeling and Residual Hypertrophy
4.3. Global Left Ventricular Afterload
4.4. Left Ventricular Mass Regression
4.5. Major Adverse Cardiovascular and Cerebral Events
4.6. Strategy for Avoiding PPM and Less Reverse Remodeling
4.7. Clinical Implications
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | Aortic Stenosis |
| AVR | aortic valve replacement |
| PPM | patient-prosthesis mismatch |
| EOA | effective orifice area |
| EOAI | indexed effective orifice area |
| LVOT | left ventricular outflow tract |
| VTI | velocity time integral |
| LVM | left ventricular mass |
| LVMI | indexed left ventricular mass |
| RWT | Relative wall thickness |
| MPG | mean pressure gradient |
| SWL | stroke work loss |
| ZVA | valvulo-arterial impedance |
| MACCE | major adverse cardiovascular and cerebral events |
References
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific aortic stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossman, W.; Jones, D.; McLaurin, L. Wall stress and patterns of hypertrophy in the human left ventricle. J. Clin. Investig. 1975, 56, 56–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, D.; Garrison, R.J.; Savage, D.D.; Kannel, W.B.; Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 1990, 322, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Devereux, R.B.; Casale, P.N.; Savage, D.D.; Laragh, J.H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann. Intern. Med. 1991, 114, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Orsinell, D.A.; Aurigemma, G.P.; Battista, S.; Kerndel, S.; Gaasch, W.H. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. J. Am. Coll. Cardiol. 1993, 22, 1679–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, A.W.; Larson, M.G.; Benjamin, E.J.; Levy, D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J. Am. Coll. Cardiol. 1998, 32, 1454–1459. [Google Scholar] [CrossRef] [Green Version]
- Panidis, I.P.; Kotler, M.N.; Ren, J.-F.; Mintz, G.S.; Ross, J.; Kalman, P. Development and regression of left ventricular hypertrophy. J. Am. Coll. Cardiol. 1984, 3, 1309–1320. [Google Scholar] [CrossRef] [Green Version]
- Dumesnil, J.G.; Honos, G.N.; Lemieux, M.; Beauchemin, J. Validation of applications of indexed aortic prosthetic valve areas calculated by Doppler echocardiography. J. Am. Coll. Cardiol. 1990, 16, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Rahimtoola, S.H. The problem of valve prosthesis-patient mismatch. Circulation 1978, 58, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Pibarot, P.; Dumesnil, J.G. Hemodynamic and clinical impact of prosthesis–patient mismatch in the aortic valve position and its prevention. J. Am. Coll. Cardiol. 2000, 36, 1131–1141. [Google Scholar] [CrossRef]
- Tasca, G.; Brunelli, F.; Cirillo, M.; DallaTomba, M.; Mhagna, Z.; Troise, G.; Quaini, E. Impact of valve prosthesis-patient mismatch on left ventricular mass regression following aortic valve replacement. Ann. Thorac. Surg. 2005, 79, 505–510. [Google Scholar] [CrossRef]
- Lund, O.; Emmertsen, K.; Dorup, I.; Jensen, F.T.; Flo, C. Regression of left ventricular hypertrophy during 10 years after valve replacement for aortic stenosis is related to the preoperative risk profile. Eur. Heart J. 2003, 24, 1437–1446. [Google Scholar] [CrossRef] [Green Version]
- Dumesnil, J.G.; Pibarot, P. Prosthesis-patient mismatch: An update. Curr. Cardiol. Rep. 2011, 13, 250–257. [Google Scholar] [CrossRef]
- Fuster, R.G.; Argudo, J.A.M.; Albarova, O.G.; Sos, F.H.; Lopez, S.C.; Codoner, M.B.; Minano, J.A.B.; Albarran, I.R. Patient-prosthesis mismatch in aortic valve replacement: Really tolerable? Eur. J. Cardiothorac. Surg. 2005, 27, 441–449; discussion 449. [Google Scholar] [CrossRef] [Green Version]
- Bermejo, J.; Odreman, R.; Feijoo, J.; Moreno, M.M.; Gomez-Moreno, P.; Garcia-Fernandez, M.A. Clinical efficacy of Doppler-echocardiographic indices of aortic valve stenosis:a comparative test-based analysis of outcome. J. Am. Coll. Cardiol. 2003, 41, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Giannini, C.; Petronio, A.S.; Carlo, M.D.; Guarracino, F.; Benedetti, G.; Donne, M.G.D.; Dini, F.L.; Marzilli, M.; Bello, V.D. The incremental value of valvuloarterial impedance in evaluating the results of transcatheter aortic valve implantation in symptomatic aortic stenosis. J. Am. Soc. Echocardiogr. 2012, 25, 444–453. [Google Scholar] [CrossRef]
- Katsanos, S.; Yiu, K.H.; Clavel, M.-A.; Rodes-Cabau, J.; Leong, D.; Kley, F.v.d.; Marsan, N.A.; Bax, J.J.; Pibarot, P.; Delgado, V. Impact of valvuloarterial impedance on 2-year outcome of patients undergoing transcatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 2013, 26, 691–698. [Google Scholar] [CrossRef]
- Devereux, R.B.; Reichek, N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation 1977, 55, 613–618. [Google Scholar] [CrossRef] [Green Version]
- Briand, M.; Dumesnil, J.G.; Kadem, L.; Tongue, A.G.; Rieu, R.; Garcia, D.; Pibarot, P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005, 46, 291–298. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereus, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Dumesnil, J.G. Prosthetic heart valves: Selection of the optimal prosthesis and long-term management. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Pearlman, A.S.; Comess, K.A.; Reamer, R.P.; Janko, C.L.; Huntsman, L.L. Determination of the stenotic aortic valve area in adults using Doppler echocardiography. J. Am. Coll. Cardiol. 1986, 7, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Farmer, K.L.; Soto, J.G.; Nelson, J.G.; Quinones, M.A. Accurate noninvasive quantification of stenotic aortic valve area by Doppler echocardiography. Circulation 1986, 73, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Sondergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Casale, P.N.; Devereux, R.B.; Milner, M.; Zullo, G.; Harshfield, G.A.; Pickering, T.G.; Laragh, J.H. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann. Intern. Med. 1986, 105, 173–178. [Google Scholar] [CrossRef]
- Dweck, M.R.; Boon, N.A.; Newby, D.E. Calcific aortic stenosis: A disease of the valve and the myocardium. J. Am. Coll. Cardiol. 2012, 60, 1854–1863. [Google Scholar] [CrossRef] [Green Version]
- Gjesdal, O.; Bluemke, D.A.; Lima, J.A. Cardiac remodeling at the population level—Risk factors, screening, and outcomes. Nat. Rev. Cardiol. 2011, 8, 673–685. [Google Scholar] [CrossRef]
- Hein, S.; Arnon, E.; Kostin, S.; Schonburg, M.; Elsasser, A.; Polyakova, V.; Bauer, E.P.; Klovekorn, W.-P.; Schaper, J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation 2003, 107, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Lindman, B.R.; Stewart, W.J.; Pibarot, P.; Hahn, R.T.; Otto, C.M.; Xu, K.; Devereux, R.B.; Weissman, N.J.; Enriquez-Sarano, M.; Szeto, W.Y.; et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc. Interv. 2014, 7, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Fuster, R.G.A.; Argudo, J.A.M.; Albarova, O.G.; Sos, F.H.; Lopez, S.C.; Codoner, M.B.; Minano, J.A.B.; Albarran, I.R. Left ventricular mass index in aortic valve surgery: A new index for early valve replacement? Eur. J. Cardio-Thorac. Surg. 2003, 23, 696–702. [Google Scholar] [CrossRef] [Green Version]
- Suri, R.M.; Zehr, K.J.; Sundt III, T.M.; Dearani, J.A.; Daly, R.C.; Oh, J.K.; Schaff, H.V. Left ventricular mass regression after porcine versus bovine aortic valve replacement: A randomized comparison. Ann. Thorac. Surg. 2009, 88, 1232–1237. [Google Scholar] [CrossRef]
- Rajappan, K.; Rimoldi, O.E.; Camici, P.G.; Bellenger, N.G.; Pennell, D.J.; Sheridan, D.J. Functional changes in coronary microcirculation after valve replacement in patients with aortic stenosis. Circulation 2003, 107, 3170–3175. [Google Scholar] [CrossRef] [Green Version]
- McConkey, H.Z.R.; Marber, M.; Chiribiru, A.; Pibarot, P.; Redwood, S.R.; Prendergast, B.D. Coronary Microcirculation in Aortic Stenosis. Circ. Cardiovasc. Interv. 2019, 12, e007547. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Levy, D. Why Is Left Ventricular Hypertrophy So Predictive of Morbidity and Mortality? Am. J. Med. Sci. 1999, 317, 168–175. [Google Scholar] [CrossRef]
- Mehta, R.H.; Bruckman, D.; Das, S.; Tsai, T.; Russman, P.; Karavite, D.; Monaghan, H.; Sonnad, S.; Shea, M.J.; Eagle, K.A.; et al. Implications of increased left ventricular mass index on in-hospital outcomes in patients undergoing aortic valve surgery. J. Thorac. Cardiovasc. Surg. 2001, 122, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.A.; Olson, E.N. Cardiac Plasticity. N. Engl. J. Med. 2008, 358, 1370–1380. [Google Scholar] [CrossRef]
- Pibarot, P.; Magne, J.; Leipsic, J.; Cote, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch after Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- Fallon, J.M.; DeSimone, J.P.; Brennan, J.M.; O’Brien, S.; Thibault, D.P.; DiScipio, A.W.; Pibarot, P.; Jacobs, J.P.; Malenka, D.J. The Incidence and Consequence of Prosthesis-Patient Mismatch after Surgical Aortic Valve Replacement. Ann. Thorac. Surg. 2018, 106, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díez, J.; Gonzalez, A.; Lopez, B.; Querejeta, R. Mechanisms of disease: Pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Herrmann, S.; Stork, S.; Niemann, M.; Frantz, S.; Lange, V.; Beer, M.; Gattenlohner, S.; Voelker, W.; Ertl, G.; et al. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation 2009, 120, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohty, D.; Dumesnil, J.G.; Echahidi, N.; Mathieu, P.; Dagenais, F.; Voisine, P.; Pibarot, P. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: Influence of age, obesity, and left ventricular dysfunction. J. Am. Coll. Cardiol. 2009, 53, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flameng, W.; Herregods, M.-C.; Vercalsteren, M.; Herijgers, P.; Bogaerts, K.; Meuris, B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation 2010, 121, 2123–2129. [Google Scholar] [CrossRef] [Green Version]
- Pisano, C.; D’Amico, T.; Palmeri, C.; Franchino, R.; Fattouch, K.; Bianco, G.; Ruvolo, G. Valve prosthesis-patient mismatch: Hemodynamic, echocardiographic and clinical consequences. Interact Cardiovasc. Thorac. Surg. 2011, 13, 606–610. [Google Scholar] [CrossRef] [Green Version]
- Capoulade, R.; Clavel, M.-A.; Ven, F.L.; Dahou, A.; Thebault, C.; Tastet, L.; Shen, M.; Arsenault, M.; Bedard, E.; Beaudoin, J.; et al. Impact of left ventricular remodelling patterns on outcomes in patients with aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1378–1387. [Google Scholar] [CrossRef] [Green Version]
- Bluemke, D.A.; Kronmal, R.A.; Lima, J.A.C.; Liu, K.; Olson, J.; Burke, G.L.; Folsom, A.R. The relationship of left ventricular mass and geometry to incident cardiovascular events: The MESA (Multi-Ethnic Study of Atherosclerosis) study. J. Am. Coll. Cardiol. 2008, 52, 2148–2155. [Google Scholar] [CrossRef] [Green Version]
- Gaasch, W.H.; Zile, M.R. Left ventricular structural remodeling in health and disease: With special emphasis on volume, mass, and geometry. J. Am. Coll. Cardiol. 2011, 58, 1733–1740. [Google Scholar] [CrossRef] [Green Version]
- Burwash, I.G.; Hay, K.M.; Chan, K.L. Hemodynamic stability of valve area, valve resistance, and stroke work loss in aortic stenosis: A comparative analysis. J. Am. Soc. Echocardiogr. 2002, 15, 814–822. [Google Scholar] [CrossRef]
- Imanaka, K.; Kohmoto, O.; Nishimura, S.; Yokote, Y.; Kyo, S. Impact of postoperative blood pressure control on regression of left ventricular mass following valve replacement for aortic stenosis. Eur. J. Cardiothorac. Surg. 2005, 27, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Kadem, L.; Dumesnil, J.G.; Rieu, R.; Durand, L.-G.; Garcia, D.; Pibarot, P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart 2005, 91, 354–361. [Google Scholar] [CrossRef] [Green Version]
- Pibarot, P.; Dumesnil, J.G. Assessment of aortic stenosis severity: Check the valve but don’t forget the arteries! Heart 2007, 93, 780–782. [Google Scholar] [CrossRef]
- Little, S.H.; Chan, K.L.; Burwash, I.G. Impact of blood pressure on the Doppler echocardiographic assessment of severity of aortic stenosis. Heart 2007, 93, 848–855. [Google Scholar] [CrossRef]
- Ito, H.; Mizumoto, t.; Shomura, Y.; Sawada, Y.; Kajiyama, K.; Shimpo, H. The impact of global left ventricular afterload on left ventricular reverse remodeling after aortic valve replacement. J. Card. Surg. 2017, 32, 530–536. [Google Scholar] [CrossRef]
- Herrmann, S.; Stork, S.; Niemann, M.; Lange, V.; Strotmann, J.M.; Frantz, S.; Beer, M.; Gattenlohner, S.; Voelker, W.; Ertl, G.; et al. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J. Am. Coll. Cardiol. 2011, 58, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Chau, K.H.; Douglas, P.S.; Pibarot, P.; Hahn, R.t.; Khalique, O.K.; Jaber, W.A.; Cremer, P.; Weissman, N.J.; Asch, F.M.; Zhang, Y.; et al. Regression of Left Ventricular Mass After Transcatheter Aortic Valve Replacement: The PARTNER Trials and Registries. J. Am. Coll. Cardiol. 2020, 75, 2446–2458. [Google Scholar] [CrossRef]
- Tomoeda, H.; Ueda, T.; Teshima, H.; Arinaga, K.; Tayama, K.; Fukunaga, S.; Aoyagi, S. Postoperative left ventricular mass regression after aortic valve replacement for aortic stenosis. Ann. Thorac. Surg. 2010, 89, 745–750. [Google Scholar] [CrossRef]
- Tasca, G.; Brunelli, F.; Cirillo, M.; Tomba, M.D.; Mhagna, Z.; Troise, G.; Quaini, E. Impact of the improvement of valve area achieved with aortic valve replacement on the regression of left ventricular hypertrophy in patients with pure aortic stenosis. Ann. Thorac. Surg. 2005, 79, 1291–1296; discussion 1296. [Google Scholar] [CrossRef]
- Tasca, G.; Mhagna, Z.; Perotti, S.; Centurini, P.B.; Sabatini, T.; Amaducci, A.; Brunelli, F.; Cirillo, M.; Tomba, M.D.; Quiani, E.; et al. Impact of prosthesis-patient mismatch on cardiac events and midterm mortality after aortic valve replacement in patients with pure aortic stenosis. Circulation 2006, 113, 570–576. [Google Scholar] [CrossRef]
- Shalabi, A.; Spiegelstein, D.; Sternik, L.; Feinberg, M.S.; Kogan, A.; Levin, S.; Orlov, B.; Nachum, E.; Lipey, A.; Raanani, E. Sutureless Versus Stented Valve in Aortic Valve Replacement in Patients with Small Annulus. Ann. Thorac. Surg. 2016, 102, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluschi, I.; Moriggia, S.; Giacomini, A.; Forno, B.D.; Sanzo, S.D.; Blasio, A.; Scafuri, A.; Alfieri, O. Can Perceval sutureless valve reduce the rate of patient-prosthesis mismatch? Eur. J. Cardiothorac. Surg. 2017, 51, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
| Patient-Prosthesis Mismatch | ||||
|---|---|---|---|---|
| Variable | Total (n = 120) | Moderate/Severe (n = 59) | None (n = 61) | p |
| Baseline characteristics | ||||
| Age, year | 67.7 ± 10.2 | 68.1 ± 11.4 | 67.4 ± 8.9 | 0.685 |
| Body mass index, kg/m2 | 25.6 ± 4.7 | 25.7 ± 5.3 | 25.4 ± 4.0 | 0.720 |
| Male sex | 66 (55.0) | 32 (54.2) | 34 (55.7) | 0.869 |
| Body surface area, m2 | 1.66 ± 0.17 | 1.67 ± 0.16 | 1.65 ± 0.19 | 0.506 |
| Smoking | 26 (21.7) | 14 (23.7) | 12 (19.7) | 0.590 |
| Surgery-related variables | ||||
| Previous AVR surgery | 8 (6.7) | 4 (6.8) | 4 (6.6) | 0.961 |
| Concomitant CABG | 23 (19.2) | 15 (25.4) | 8 (13.1) | 0.087 |
| Mini-invasive surgery | 82 (68.3) | 36(30) | 46(38.3) | 0.090 |
| Comorbidities | ||||
| Coronary artery disease | 51 (42.5) | 27 (45.8) | 24 (39.3) | 0.477 |
| Hypertension | 91 (75.8) | 47 (79.7) | 44 (72.1) | 0.335 |
| Diabetes | 48 (40.0) | 26 (44.1) | 22 (36.1) | 0.371 |
| Hyperlipidemia | 79 (65.8) | 37 (62.7) | 42 (68.9) | 0.478 |
| Stroke | 18 (15.0) | 11 (18.6) | 7 (11.5) | 0.272 |
| Chronic kidney disease (including dialysis) | 33 (27.5) | 18 (30.5) | 15 (24.6) | 0.468 |
| Valve-related features | ||||
| Bicuspid | 46 (38.3) | 18 (30.5) | 28 (45.9) | 0.083 |
| Annulus size <21 mm | 71 (59.2) | 41 (69.5) | 30 (49.2) | 0.024 |
| Ascending aortic >30 mm | 69 (57.5) | 34 (57.6) | 35 (57.4) | 0.978 |
| Pre-operation left ventricular status | ||||
| LVEF <50 % | 22 (18.3) | 11 (18.6) | 11 (18.0) | 0.931 |
| LVESD >40 mm | 24 (20.2) | 10 (17.2) | 14 (23.0) | 0.438 |
| LVEDD >55 mm | 26 (21.7) | 12 (20.3) | 14 (23.0) | 0.728 |
| Remodel mode | 0.479 | |||
| Normal | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Physiologic | 7 (5.8) | 5 (8.5) | 2 (3.3) | |
| Eccentric | 23 (19.2) | 11 (18.6) | 12 (19.7) | |
| Concentric | 90 (75.0) | 43 (72.9) | 47 (77.0) | |
| Valve type | 0.244 | |||
| Mechanical | 3 (2.5) | 0 (0.0) | 3 (4.9) | |
| Bioprosthesis | 117 (97.5) | 59 (100.0) | 58 (95.1) | |
| Porcine | 48 (41.0) | 36 (61.0) | 12 (20.7) | <0.001 |
| Bovine | 44 (37.6) | 23 (39.0) | 21 (36.2) | 0.757 |
| Sutureless | 25 (21.4) | 0 (0.0) | 25 (43.1) | <0.001 |
| Patient-prosthesis mismatch | <0.001 | |||
| None | 61 (50.8) | 0 (0.0) | 61 (100.0) | |
| Moderate | 53 (44.2) | 53 (89.8) | 0 (0.0) | |
| Severe | 6 (5.0) | 6 (10.2) | 0 (0.0) | |
| Follow-up year | 3.6 ± 2.0 | 4.1 ± 2.2 | 3.1 ± 1.6 | 0.004 |
| Hemodynamic Parameter | Pre-Operation | Follow Up | p Value |
|---|---|---|---|
| Dimension (geometry) | |||
| IVST, mm | 13.9 ± 3.0 | 12.5 ± 2.8 | <0.001 |
| PWT, mm | 13.1 ± 2.4 | 11.9 ± 2.4 | <0.001 |
| LVEDD, mm | 50.5 ± 7.5 | 45.4 ± 6.6 | <0.001 |
| LVESD, mm | 33.1 ± 9.4 | 28.0 ± 6.7 | <0.001 |
| LVEDV, mL | 123.7 ± 43.7 | 96.9 ± 34.4 | <0.001 |
| LVESV, mL | 50.1 ± 36.6 | 32.5 ± 22.2 | <0.001 |
| Hemodynamic function | |||
| LVEF, % | 62.9 ± 16.2 | 67.4 ± 12.6 | 0.003 |
| ZVA, mmHg/mL/m2 | 4.4 ± 1.9 | 3.5 ± 1.0 | <0.001 |
| Stroke work loss, % | 0.23 ± 0.09 | 0.09 ± 0.04 | <0.001 |
| EOAI, cm2/m2 | 0.49 ± 0.19 | 0.96 ± 0.31 | <0.001 |
| Mean PG, mmHg | 41.9 ± 19.0 | 13.5 ± 7.0 | <0.001 |
| RWT, % | 0.53 ± 0.14 | 0.54 ± 0.13 | 0.704 |
| LVMI, g/m2 | 188.0 ± 64.0 | 140.2 ± 47.9 | <0.001 |
| Hemodynamic | Unadjusted Analysis | Adjusted Analysis * | ||
|---|---|---|---|---|
| Parameter | B (95% CI) | p | B (95% CI) | p |
| Pre-operative LVMI, g/m2 | 0.21 (0.29, 0.12) | <0.001 | 0.22 (0.30, 0.13) | <0.001 |
| LVEF, % | 0.02 (0.38, 0.41) | 0.924 | 0.001 (0.48, 0.48) | 0.998 |
| ZVA, mmHg/mL/m2 | 1.15 (3.80, 6.10) | 0.648 | 0.99 (3.86, 5.84) | 0.688 |
| ZVA, mmHg/mL/m2 | ||||
| ≤3.5 | Reference | Reference | ||
| >3.5 | 1.83 (8.05, 11.71) | 0.716 | 1.99 (7.63, 11.61) | 0.685 |
| Stroke work loss, % | 0.89 (0.08, 1.87) | 0.072 | 0.97 (0.001, 1.95) | 0.0497 |
| EOAI, cm2/m2 | 15.61 (29.41, 1.80) | 0.027 | 16.40 (30.08, 2.71) | 0.019 |
| EOAI, cm2/m2 | ||||
| ≥0.85 | Reference | Reference | ||
| <0.85 | 10.76 (1.06, 20.46) | 0.030 | 10.66 (1.42, 19.89) | 0.024 |
| Mean PG, mmHg | 0.66 (0.12, 1.19) | 0.016 | 0.65 (0.12, 1.19) | 0.017 |
| Mean PG, mmHg | ||||
| <20 | Reference | Reference | ||
| ≥20 | 6.95 (3.10, 16.99) | 0.175 | 6.64 (3.04, 16.32) | 0.179 |
| RWT, per 10% | 2.10 (1.04, 5.25) | 0.190 | 1.61 (1.50, 4.71) | 0.310 |
| RWT | ||||
| ≤0.42 | Reference | Reference | ||
| >0.42 | 13.23 (0.05, 26.52) | 0.051 | 10.55 (3.31, 24.41) | 0.136 |
| LVMI, g/m2 | 0.32 (0.20, 0.44) | <0.001 | 0.34 (0.23, 0.45) | <0.001 |
| LV remodel mode | ||||
| Normal or physiologic | Reference | Reference | ||
| Concentric or eccentric | 27.02 (16.87, 37.18) | <0.001 | 26.79 (17.26, 36.32) | <0.001 |
| Hemodynamic | Unadjusted Analysis | Adjusted Analysis * | ||
|---|---|---|---|---|
| Parameter | HR (95% CI) | p | HR (95% CI) | p |
| Pre-operative LVMI, g/m2 | 1.002 (0.998–1.007) | 0.284 | 1.00 (0.995–1.005) | 0.929 |
| LVEF, % | 0.99 (0.97–1.01) | 0.486 | 1.00 (0.98–1.03) | 0.823 |
| ZVA, mmHg/mL/m2 | 1.59 (1.23–2.06) | <0.001 | 1.65 (1.21–2.25) | 0.002 |
| ZVA, mmHg/mL/m2 | ||||
| ≤3.5 | Reference | Reference | ||
| >3.5 | 2.28 (1.22–4.26) | 0.010 | 2.03 (1.08–3.80) | 0.028 |
| Stroke work loss, % | 1.11 (1.03–1.20) | 0.005 | 1.13 (1.06–1.22) | 0.001 |
| EOAI, cm2/m2 | 0.08 (0.02–0.38) | 0.001 | 0.06 (0.01–0.35) | 0.001 |
| EOAI, cm2/m2 | ||||
| ≥0.85 | Reference | Reference | ||
| <0.85 | 2.62 (1.35–5.11) | 0.005 | 2.75 (1.38–5.46) | 0.004 |
| Mean PG, mmHg | 1.06 (1.02–1.10) | 0.001 | 1.07 (1.03–1.11) | <0.001 |
| Mean PG, mmHg | ||||
| <20 | Reference | Reference | ||
| ≥20 | 2.21 (1.17–4.20) | 0.015 | 2.43 (1.26–4.71) | 0.008 |
| RWT, per 10% | 1.36 (1.10–1.67) | 0.004 | 1.43 (1.14–1.79) | 0.002 |
| RWT | ||||
| ≤0.42 | Reference | Reference | ||
| >0.42 | 1.34 (0.53–3.39) | 0.543 | 1.07 (0.42–2.74) | 0.885 |
| LVMI, g/m2 | 1.01 (1.004–1.01) | 0.001 | 1.01 (1.004–1.02) | 0.002 |
| LV remodel mode | ||||
| Normal or physiologic | Reference | Reference | ||
| Concentric or eccentric | 2.72 (1.07–6.92) | 0.036 | 3.32 (1.24–8.89) | 0.017 |
| Unadjusted Analysis | Adjusted Analysis * | |||
|---|---|---|---|---|
| Outcome/ Parameters | B or HR (95% CI) | p | B or HR (95% CI) | p |
| LVM regression | ||||
| EOAI, cm2/m2 | 10.57 (24.76, 3.62) | 0.144 | 12.74 (25.57, 0.10) | 0.052 |
| Mean PG, mmHg | 0.44 (0.23, 0.65) | <0.001 | 0.46 (0.26, 0.67) | <0.001 |
| ZVA, mmHg/mL/m2 | 0.75 (3.36, 1.85) | 0.572 | 1.06 (1.53, 3.65) | 0.423 |
| Stroke work loss, % | 0.92 (0.48, 1.36) | <0.001 | 0.95 (0.52, 1.38) | <0.001 |
| MACCE | ||||
| EOAI, cm2/m2 | 0.19 (0.08–0.44) | <0.001 | 0.27 (0.11–0.66) | 0.004 |
| Mean PG, mmHg | 1.02 (1.003–1.04) | 0.021 | 1.02 (1.002–1.04) | 0.031 |
| ZVA, mmHg/mL/m2 | 1.41 (1.15–1.73) | 0.001 | 1.35 (1.06–1.72) | 0.015 |
| Stroke work loss, % | 1.05 (1.02–1.09) | 0.005 | 1.05 (1.01–1.09) | 0.011 |
| LVMI regression (change of LVMI, %) | 1.008 (0.998–1.018) | 0.127 | 1.01 (1.003–1.03) | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-S.; Huang, J.-H.; Chiu, K.-M.; Chiang, C.-Y. Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement. J. Clin. Med. 2022, 11, 7482. https://doi.org/10.3390/jcm11247482
Chen J-S, Huang J-H, Chiu K-M, Chiang C-Y. Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement. Journal of Clinical Medicine. 2022; 11(24):7482. https://doi.org/10.3390/jcm11247482
Chicago/Turabian StyleChen, Jer-Shen, Jih-Hsin Huang, Kuan-Ming Chiu, and Chih-Yao Chiang. 2022. "Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement" Journal of Clinical Medicine 11, no. 24: 7482. https://doi.org/10.3390/jcm11247482
APA StyleChen, J.-S., Huang, J.-H., Chiu, K.-M., & Chiang, C.-Y. (2022). Extent of Left Ventricular Mass Regression and Impact of Global Left Ventricular Afterload on Cardiac Events and Mortality after Aortic Valve Replacement. Journal of Clinical Medicine, 11(24), 7482. https://doi.org/10.3390/jcm11247482






