Associations of Circulating Irisin and Fibroblast Growth Factor-21 Levels with Measures of Energy Homeostasis in Highly Trained Adolescent Rhythmic Gymnasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Research Design
2.2. Measurements
2.2.1. Body Composition
2.2.2. Resting Energy Expenditure and Aerobic Performance
2.2.3. Blood Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jürimäe, J.; Mäestu, J.; Jürimäe, T.; Mangus, B.; von Duvillard, S.P. Peripheral signals of energy homeostasis as possible markers of training stress in athletes: A review. Metab. Clin. Exp. 2011, 60, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef]
- Kurgan, N.; Logan-Sprenger, H.; Falk, B.; Klentrou, P. Bone and inflammatory responses to training in female rowers over an Olympic year. Med. Sci. Sports Exerc. 2018, 50, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Gruodyte, R.; Jürimäe, J.; Cicchella, A.; Stefanelli, C.; Pasariello, C.; Jürimäe, T. Adipocytokines and bone mineral density in adolescent female athletes. Acta Paediatr. 2010, 99, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, J.; Tillmann, V.; Cicchella, A.; Stefanelli, A.; Võsoberg, K.; Tamm, A.L.; Jürimäe, T. Increased sclerostin and preadipocyte factor-1 levels in prepubertal rhythmic gymnasts: Associations with bone mineral density, body composition, and adipocytokine values. Osteoporos. Int. 2016, 27, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Roupas, N.D.; Mamali, I.; Armeni, A.K.; Markantes, G.K.; Theodoropoulou, A.; Alexandrides, T.K.; Leglise, M.; Markou, K.B.; Georgopoulos, N.A. The influence of intensive physical training on salivary adipokine levels in elite rhythmic gymnasts. Horm. Metab. Res. 2012, 44, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Roupas, N.D.; Maimoun, L.; Mamali, I.; Coste, O.; Tsouka, A.; Mahadea, K.K.; Mura, T.; Philibert, P.; Gaspari, L.; Mariano-Goulart, D.; et al. Saliva adiponectin levels are associated with training intensity but not with bone mass or reproductive function in elite rhythmic gymnasts. Peptides 2014, 51, 80–85. [Google Scholar] [CrossRef]
- Jürimäe, J.; Gruodyte-Racience, R.; Baxter-Jones, A.D.G. Effects of gymnastics activities on bone accrual during growth: A systematic review. J. Sports Sci. Med. 2018, 17, 245–258. [Google Scholar]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchala, M.; Guzik, P. Effect of various exercise regimens on selected exercise-induced cytokines in healthy people. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Maimoun, L.; Mariano-Goulart, D.; Huguet, H.; Renard, E.; Lefebvre, P.; Picot, M.C.; Dupuy, A.M.; Cristol, J.P.; Courtet, P.; Boudousq, V.; et al. In patients with anorexia nervosa, myokine levels are altered but are associated with bone mineral density loss and bone turnover alteration. Endocr. Connect. 2022, 11, e210488. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Prado Neto, E.V.; De Alvares Goulart, R.; Bechara, M.D.; Baisi Chagas, E.F.; Audi, M.; Guissoni Campos, L.M.; Landgraf Guiger, E.; Buchaim, R.L.; Buchaim, D.V.; et al. Myokines: A descriptive review. J. Sports Med. Phys. Fit. 2020, 60, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Rämson, R.; Jürimäe, J.; Jürimäe, T.; Mäestu, J. The influence of increased training volume on cytokines and ghrelin concentration in college level male rowers. Eur. J. Appl. Physiol. 2008, 104, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Sliwicka, E.; Cison, T.; Pilaczyriska-Szczesniak, L.; Ziemba, A.; Straburzynska-Lupa, A. Effect of marathon race on selected myokines and sclerostin in middle-aged male amateur runners. Sci. Rep. 2021, 11, 2813. [Google Scholar] [CrossRef]
- He, Z.; Tian, Y.; Valenzuela, P.L.; Huang, C.; Zhao, J.; Hong, P. Myokine response to high-intensity interval vs. resistance exercise: An individual approach. Front. Physiol. 2018, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, A.; Rapisarda, R.; Xourafa, A.; Zanoli, L.; Manfre, V.; Catalano, A.; Singorelli, S.S.; Castellino, P. Effects of competitive physical activity on serum irisin levels and bone turnover markers. J. Endocrinol. Investig. 2021, 44, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, J.; Vaiksaar, S.; Purge, P.; Tillmann, V. Irisin, fibroplast growth factor-21, and follistatin responses to endurance rowing training session in female rowers. Front. Physiol. 2021, 12, 689696. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.B.; Kim, H.J.; Kang, J.H.; Park, S.I.; Park, K.H.; Lee, H.J. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metab. Clin. Exp. 2017, 73, 100–108. [Google Scholar] [CrossRef]
- Lawson, E.A.; Ackerman, K.E.; Slattery, M.; Marengi, D.A.; Clarker, H.; Misra, M. Oxytocin secretion is related to measures of energy homeostasis in young amenorrheic athletes. J. Clin. Endocrinol. Metab. 2014, 99, E881–E885. [Google Scholar] [CrossRef]
- Martinez Munoz, I.Y.; Del Socorro Camarillo Romero, E.; Correa Padillo, T.; Guadalupe Santillan Benitez, J.; Del Socorro Camarillo Romero, M.; Montenegr Morales, L.P.; Huitron Bravo, G.G.; De Jesus Garduno Garcia, J. Association of irisin serum concentration and muscle strength in normal-weight and overweight young women. Front. Endocrinol. 2019, 10, 621. [Google Scholar] [CrossRef]
- Biniaminov, N.; Bandt, S.; Roth, A.; Haertel, S.; Neumann, R.; Bub, A. Irisin, physical activity and fitness status in healthy humans: No association under resting conditions in a cross-sectional study. PLoS ONE 2018, 13, e0189254. [Google Scholar] [CrossRef] [PubMed]
- Singhal, V.; Lawson, E.A.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin levels are lower in young amenorrheic athletes compared with eumenorrheic athletes and non-anthletes and are associated with bone density and strength estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef] [PubMed]
- Khalafi, M.; Alamdari, K.A.; Symonds, M.E.; Nobari, H.; Carlos-Vivas, J. Impact of acute exercise on immediate and following early post-exercise FGF-21 concentration in adults: Systematic review and meta-analysis. Hormones 2021, 20, 23–33. [Google Scholar] [CrossRef]
- Vaiksaar, S.; Jürimäe, J.; Mäestu, J.; Purge, P.; Kalytka, S.; Shakhlina, L.; Jürimäe, T. No effect of menstrual cycle phase on fuel oxidation during exercise in rowers. Eur. J. Appl. Physiol. 2011, 111, 1027–1034. [Google Scholar] [CrossRef]
- Lätt, E.; Jürimäe, J.; Haljuaste, K.; Cicchella, A.; Purge, P.; Jürimäe, T. Physical development and swimming performance during biological maturation in young female swimmers. Coll. Antropol. 2009, 33, 117–122. [Google Scholar] [PubMed]
- Melin, A.; Tornberg, A.B.; Skouby, S.; Moller, S.S.; Sundgot-Borgen, J.; Faber, J.; Sidelmann, J.J.; Aziz, M.; Sjödin, A. Energy availability and the female athlete triad in elite endurance athletes. Scand. J. Med. Sci. Sports 2015, 25, 610–622. [Google Scholar] [CrossRef]
- Weir, J.V.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 4521. [Google Scholar] [CrossRef] [PubMed]
- Remmel, L.; Tamme, R.; Tillmann, V.; Mäestu, E.; Purge, P.; Mengel, E.; Riso, E.M.; Jürimäe, J. Pubertal physical activity and cardiorespiratory fitness in relation to late adolescent body fatness in boys: A 6-year follow-up study. Int. J. Environ. Res. Public Health 2021, 18, 4881. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P.; Tillmann, V. Serum sclerostin and cytokine responses to prolonged sculling exercise in highly-trained male rowers. J. Sports Sci. 2021, 39, 591–597. [Google Scholar] [CrossRef]
- Hopkins, W.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Corsi Romanelli, M.M. Irisin, a potential link between physical exercise and metabolism—An observational study in differently trained subjects, from elite athletes to sedentary people. J. Diabetes Res. 2017, 2017, 1039161. [Google Scholar] [CrossRef] [PubMed]
- De Meneck, F.; De Souza, L.V.; Brioschi, M.L.; Do Carmo Franco, M. Emerging evidence for the opposite role of circulating irisin levels and brown adipose tissue activity measured by infrared thermography in anthropometric and metabolic profile during childhood. J. Therm. Biol. 2021, 99, 103010. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Hofmann, T.; Goebel-Stengel, M.; Elbelt, U.; Kobelt, P.; Klapp, B.F. Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity—Correlation with body mass index. Peptides 2013, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, I.G. Is irisin the new player in exercise-induced adaptations or not? A 2017 update. Clin. Chem. Lab. Med. 2018, 56, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Venojärvi, M.; Wasenius, N.; Manderoos, S.; Deruisseau, K.C.; Gidlund, E.K.; Heinonen, O.J.; Lindholm, H.; Aunola, S.; Eriksson, J.G.; et al. Plasma irisin is increased following 12 weeks of Nordic walking and associates with glucose homeostasis in overweight/obese men with impaired glucose regulation. Eur. J. Sport Sci. 2019, 19, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Elbelt, U.; Ahnis, A.; Kobelt, P.; Rose, M.; Stengel, A. Irisin levels are not affected by physical activity in patients with anorexia nervosa. Front. Endocrinol. 2014, 4, 202. [Google Scholar] [CrossRef]
- Witek, K.; Zurek, P.; Zmijewski, P.; Jaworska, J.; Lipinska, P.; Dzedzej-Gmiat, A.; Antosiewicz, J.; Ziemann, E. Myokines in response to a tournament season among young tennis players. BioMed Res. Int. 2016, 2016, 1460892. [Google Scholar] [CrossRef]
- Grzebisz-Zatonska, N.; Poprzecki, S.; Pokora, I.; Mikolajec, K.; Kaminski, T. Effect of seasonal variation during annual cyclist training on somatic function, white blood cell composition, immunological system, selected hormones and their interaction with irisin. J. Clin. Med. 2021, 10, 3299. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P. Irisin and inflammatory cytokines in elite male rowers: Adaptation to volume-extended training period. J. Sports Med. Phys. Fit. 2021, 61, 102–108. [Google Scholar] [CrossRef]
- Dünwald, T.; Melmer, A.; Gatterer, H.; Salzmann, K.; Ebenbichler, C.; Burtscher, M.; Schobersberger, W.; Grander, W. Supervised short-term high-intensity training on plasma irisin concentrations in type 2 diabetic patients. Int. J. Sports Med. 2019, 40, 158–164. [Google Scholar] [CrossRef]
- Jürimäe, J.; Purge, P.; Remmel, L.; Ereline, J.; Kums, T.; Kamandulis, S.; Brazaitis, M.; Venckunas, T.; Pääsuke, M. Changes in irisin, inflammatory cytokines and aerobic capacity in response to three weeks of supervised sprint interval training in older men. J. Sports Med. Phys. Fit. 2023, 63, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Morelli, C.; Avolio, E.; Galluccio, A.; Caparello, G.; Manes, E.; Ferraro, S.; De Rose, D.; Santoro, M.; Basone, I.; Catalano, S.; et al. Impact of vigorous-intensity physical activity on body composition parameters, lipid profile markers, and irisin levels in adolescents: A cross-sectional study. Nutrients 2020, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Gannon, N.P.; Mermier, C.M.; Conn, C.A. Irisin, a unique non-inflammatory myokine in stimulating skeletal muscle metabolism. J. Physiol. Biochem. 2015, 71, 679–689. [Google Scholar] [CrossRef]
- Berti, L.; Imler, M.; Zdichavsky, M.; Meile, T.; Böhm, A.; Stefan, N.; Fritsche, A.; Beckers, J.; Köningsrainer, A.; Häring, H.U. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Mol. Metab. 2015, 4, 519–527. [Google Scholar] [CrossRef]
- Dostalova, I.; Kavalkova, P.; Haluzikova, D.; Lacinova, Z.; Mraz, M.; Papezova, H.; Haluzik, M. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 2008, 93, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Kosaki, K.; Myoenzono, K.; Yoshikawa, T.; Park, J.; Kuro-O, M.; Maeda, S. Effect of aerobic exercise training on circulating fibroplast growth factor-21 response to glucose challenge in overweight and obese men: A pilot study. Exp. Clin. Endocrinol. Diabetes 2022, 130, 723–729. [Google Scholar] [PubMed]
- Cuevas-Ramos, D.; Paloma, A.V.; Meza-Arana, C.E.; Brito-Cordova, G.; Gomez-Perez, F.J.; Mehta, R.; Oseguera-Moguel, J.; Aguilar-Salinas, C.A. Exercise increases serum Fibroblast Growth Factor 21 (FGF21) levels. PLoS ONE 2012, 7, e38022. [Google Scholar] [CrossRef]
- Taniguchi, H.; Tanisawa, K.; Sun, X.; Kubo, T.; Higuchi, M. Endurance exercise reduces hepatic fat content and serum fibroblast growth factor 21 levels in elderly men. J. Clin. Endocrinol. Metab. 2016, 101, 191–198. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853–10858. [Google Scholar] [CrossRef]
| Variable | RG (n = 33) | UC (n = 20) | p Value | ES |
|---|---|---|---|---|
| Age (yrs) | 16.0 ± 1.2 | 16.5 ± 1.6 | 0.202 | 0.03 |
| Age at menarche (yrs) | 13.6 ± 1.2 | 12.5 ± 0.7 | <0.0001 | 0.26 |
| Body height (cm) | 166.8 ± 5.3 | 166.8 ± 5.0 | 0.976 | 0.01 |
| Body mass (kg) | 55.7 ± 7.0 | 58.4 ± 7.4 | 0.180 | 0.04 |
| BMI (kg/m2) | 20.0 ± 2.0 | 21.0 ± 2.2 | 0.100 | 0.05 |
| Body fat % | 19.5 ± 5.7 | 30.4 ± 6.2 | <0.0001 | 0.45 |
| Body fat mass (kg) | 11.2 ± 4.3 | 17.8 ± 4.8 | <0.0001 | 0.35 |
| Body lean mass (kg) | 42.2 ± 4.1 | 37.7 ± 3.7 | <0.0001 | 0.25 |
| REE (kcal/day) | 1495 ± 208 | 1520 ± 208 | 0.669 | 0.01 |
| REE/kg (kcal/day/kg LBM) | 33.4 ± 4.8 | 38.6 ± 5.0 | <0.0001 | 0.22 |
| Training volume (h/week) | 17.6 ± 5.3 | 2.1 ± 1.3 | <0.0001 | 0.76 |
| VO2peak/kg (mL/min/kg LBM) | 53.6 ± 7.7 | 48.4 ± 5.6 | 0.012 | 0.12 |
| Wmax/kg (W/kg) | 3.2 ± 0.6 | 2.3 ± 0.4 | <0.0001 | 0.41 |
| Variable | RG (n = 33) | UC (n = 20) | p Value | ES |
|---|---|---|---|---|
| Irisin (ng/mL) | 272.7 ± 140.0 | 207.3 ± 113.7 | 0.084 | 0.06 |
| FGF-21 (pg/mL) | 169.6 ± 56.4 | 188.1 ± 54.3 | 0.249 | 0.03 |
| Leptin (ng/mL) | 1.2 ± 0.6 | 3.7 ± 2.6 | <0.0001 | 0.34 |
| Resistin (ng/mL) | 4.6 ± 2.0 | 5.7 ± 2.4 | 0.077 | 0.06 |
| Variables | Irisin (ng/mL) | FGF-21 (ng/mL) | ||
|---|---|---|---|---|
| r | p Value | r | p Value | |
| Fat mass (kg) | ||||
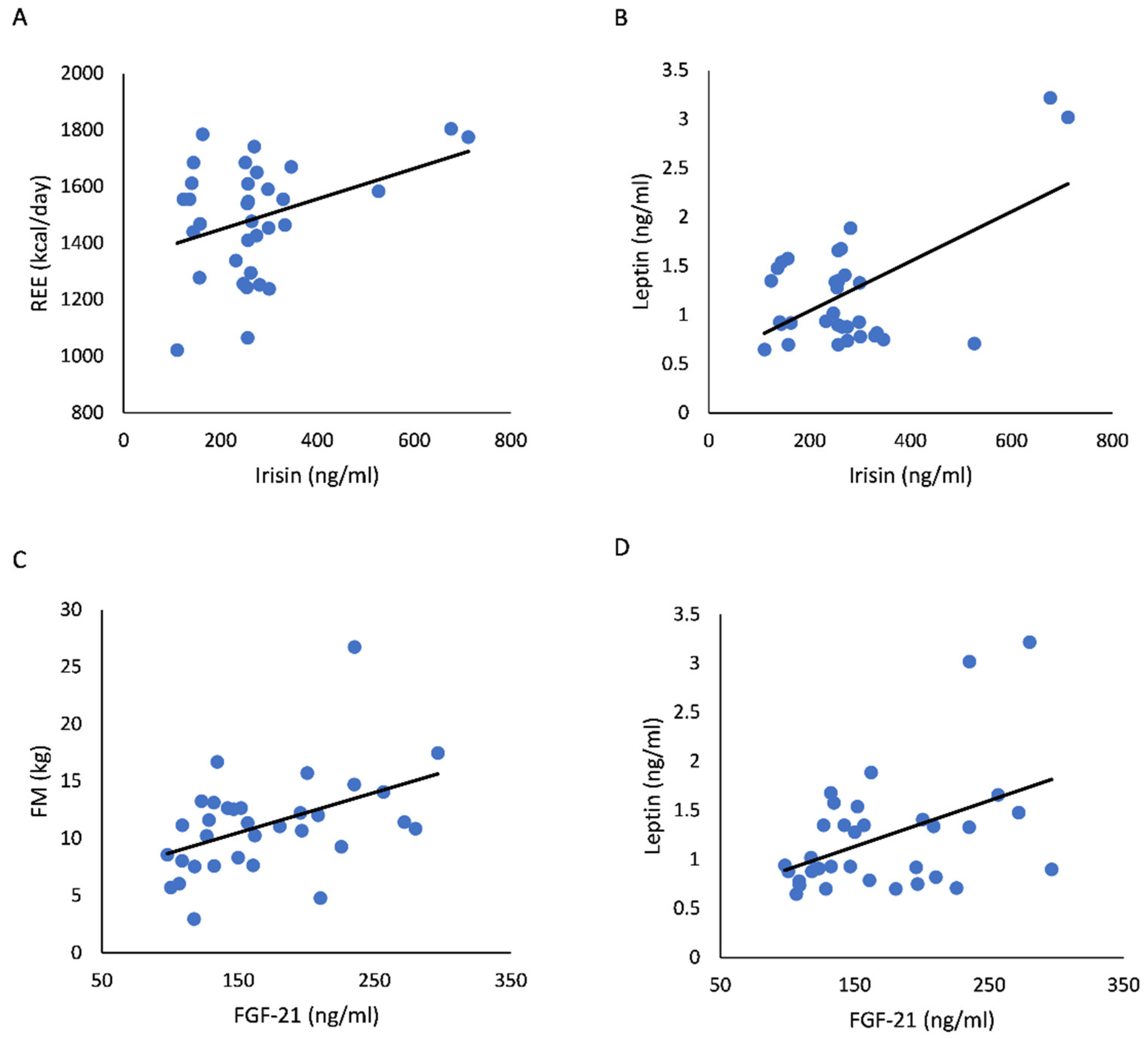
| RG | 0.25 | 0.154 | 0.46 | 0.007 |
| UC | −0.07 | 0.764 | 0.44 | 0.054 |
| Lean mass (kg) | ||||
| RG | 0.29 | 0.101 | 0.28 | 0.144 |
| UC | −0.20 | 0.398 | −0.31 | 0.187 |
| REE (kcal/day) | ||||
| RG | 0.4 | 0.021 | 0.26 | 0.143 |
| UC | −0.05 | 0.838 | 0.26 | 0.27 |
| Training volume (h/week) | ||||
| RG | 0.14 | 0.426 | −0.34 | 0.056 |
| UC | 0.03 | 0.886 | −0.21 | 0.365 |
| VO2peak/kg (mL/min/kg) | ||||
| RG | 0.04 | 0.826 | 0.19 | 0.299 |
| UC | 0.12 | 0.618 | 0.23 | 0.316 |
| Leptin (ng/mL) | ||||
| RG | 0.6 | <0.0001 | 0.45 | 0.009 |
| UC | 0.07 | 0.777 | 0.54 | 0.014 |
| Resistin (ng/mL) | ||||
| RG | 0.31 | 0.076 | 0.04 | 0.808 |
| UC | 0.47 | 0.036 | 0.21 | 0.376 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jürimäe, J.; Remmel, L.; Tamm, A.-L.; Purge, P.; Maasalu, K.; Tillmann, V. Associations of Circulating Irisin and Fibroblast Growth Factor-21 Levels with Measures of Energy Homeostasis in Highly Trained Adolescent Rhythmic Gymnasts. J. Clin. Med. 2022, 11, 7450. https://doi.org/10.3390/jcm11247450
Jürimäe J, Remmel L, Tamm A-L, Purge P, Maasalu K, Tillmann V. Associations of Circulating Irisin and Fibroblast Growth Factor-21 Levels with Measures of Energy Homeostasis in Highly Trained Adolescent Rhythmic Gymnasts. Journal of Clinical Medicine. 2022; 11(24):7450. https://doi.org/10.3390/jcm11247450
Chicago/Turabian StyleJürimäe, Jaak, Liina Remmel, Anna-Liisa Tamm, Priit Purge, Katre Maasalu, and Vallo Tillmann. 2022. "Associations of Circulating Irisin and Fibroblast Growth Factor-21 Levels with Measures of Energy Homeostasis in Highly Trained Adolescent Rhythmic Gymnasts" Journal of Clinical Medicine 11, no. 24: 7450. https://doi.org/10.3390/jcm11247450
APA StyleJürimäe, J., Remmel, L., Tamm, A.-L., Purge, P., Maasalu, K., & Tillmann, V. (2022). Associations of Circulating Irisin and Fibroblast Growth Factor-21 Levels with Measures of Energy Homeostasis in Highly Trained Adolescent Rhythmic Gymnasts. Journal of Clinical Medicine, 11(24), 7450. https://doi.org/10.3390/jcm11247450






