NT-pro-BNP as a Predictor for Recurrence of Atrial Fibrillation after Primary Cryoballoon Pulmonary Vein Isolation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Baseline Data Collection
2.2. Cryo-Pulmonary Vein Isolation
2.3. Definition of Treatment Failure
2.4. Follow-Up and Endpoint Data Collection
2.5. Statistical Methods
3. Results
3.1. Baseline Data of the Cohort under Investigation
3.2. Procedural Data
3.3. Follow-Up and Time to Event Analysis
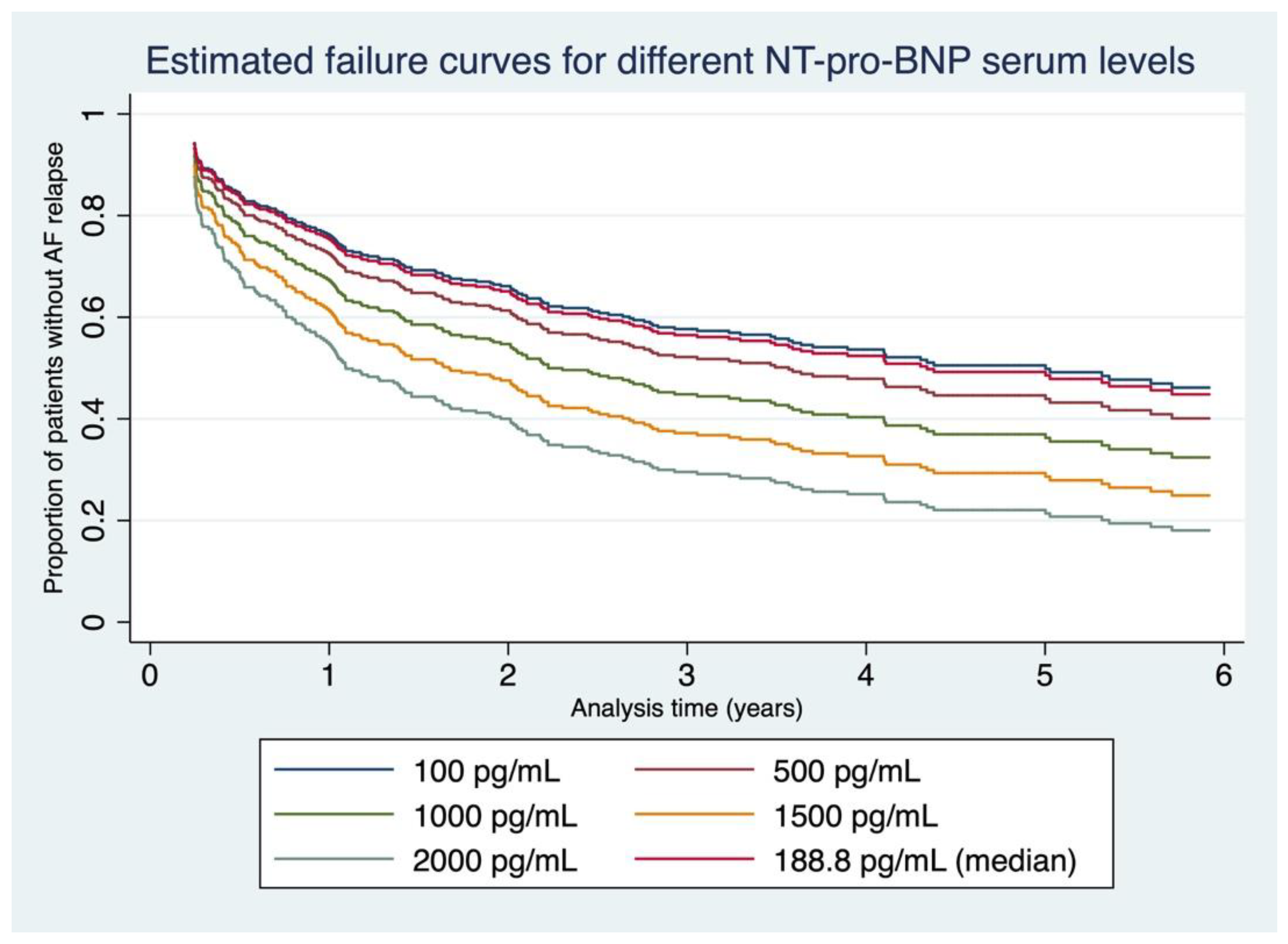
3.4. Failure Curves Derived from Our Data
3.5. Multivariate Cox Regression Models
3.6. Association between NT-pro-BNP Levels and LAP
4. Discussion
4.1. Procedural Variables and Long-Term Success Rate after the First-Ever Cryoballoon Ablation
4.2. NT-pro-BNP as a Predictor of Success after Cryo-PVI
4.3. Association between NT-pro-BNP and LAP
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Purerfellner, H.; Ouyang, F.; Kiuchi, M.G.; Meyer, C.; Martinek, M.; Futyma, P.; Zhu, L.; Schratter, A.; Wang, J.; et al. Catheter ablation vs. antiarrhythmic drugs as ’first-line’ initial therapy for atrial fibrillation: A pooled analysis of randomized data. Europace 2021, 23, 1950–1960. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Kheirkhahan, M.; Brachmann, J. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 379, 492. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Kuck, K.H.; Furnkranz, A.; Chun, K.R.; Metzner, A.; Ouyang, F.; Schluter, M.; Elvan, A.; Lim, H.W.; Kueffer, F.J.; Arentz, T.; et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: Reintervention, rehospitalization, and quality-of-life outcomes in the FIRE AND ICE trial. Eur. Heart J. 2016, 37, 2858–2865. [Google Scholar] [CrossRef]
- Kis, Z.; Muka, T.; Franco, O.H.; Bramer, W.M.; De Vries, L.J.; Kardos, A.; Szili-Torok, T. The Short and Long-Term Efficacy of Pulmonary Vein Isolation as a Sole Treatment Strategy for Paroxysmal Atrial Fibrillation: A Systematic Review and Meta-Analysis. Curr. Cardiol. Rev. 2017, 13, 199–208. [Google Scholar] [CrossRef]
- Kuck, K.H.; Brugada, J.; Furnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef]
- Cappato, R.; Calkins, H.; Chen, S.A.; Davies, W.; Iesaka, Y.; Kalman, J.; Kim, Y.H.; Klein, G.; Natale, A.; Packer, D.; et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 32–38. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef]
- Inoue, S.; Murakami, Y.; Sano, K.; Katoh, H.; Shimada, T. Atrium as a source of brain natriuretic polypeptide in patients with atrial fibrillation. J. Card. Fail. 2000, 6, 92–96. [Google Scholar] [CrossRef]
- Sepehri Shamloo, A.; Bollmann, A.; Dagres, N.; Hindricks, G.; Arya, A. Natriuretic peptides: Biomarkers for atrial fibrillation management. Clin. Res. Cardiol. 2020, 109, 957–966. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Fradley, M.G.; Larson, M.G.; Cheng, S.; McCabe, E.; Coglianese, E.; Shah, R.V.; Levy, D.; Vasan, R.S.; Wang, T.J. Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am. J. Cardiol. 2011, 108, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jin, Z. Optimal survival time-related cut-point with censored data. Stat. Med. 2015, 34, 515–524. [Google Scholar] [CrossRef]
- Canpolat, U.; Kocyigit, D.; Aytemir, K. Complications of Atrial Fibrillation Cryoablation. J. Atr. Fibrillation 2017, 10, 1620. [Google Scholar] [CrossRef]
- Andrade, J.G.; Champagne, J.; Dubuc, M.; Deyell, M.W.; Verma, A.; Macle, L.; Leong-Sit, P.; Novak, P.; Badra-Verdu, M.; Sapp, J.; et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019, 140, 1779–1788. [Google Scholar] [CrossRef]
- Ang, R.; Hunter, R.J.; Lim, W.Y.; Opel, A.; Ullah, W.; Providencia, R.; Baker, V.; Finlay, M.C.; Dhinoja, M.B.; Earley, M.J.; et al. Long Term Outcome and Pulmonary Vein Reconnection of Patients Undergoing Cryoablation and/or Radiofrequency Ablation: Results from The Cryo Versus RF Trial. J. Atr. Fibrillation 2018, 11, 2072. [Google Scholar]
- Zhang, Y.; Chen, A.; Song, L.; Li, M.; Chen, Y.; He, B. Association Between Baseline Natriuretic Peptides and Atrial Fibrillation Recurrence After Catheter Ablation. Int. Heart J. 2016, 57, 183–189. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Wang, C.; Xie, X.; Hou, Y. Association of pre-ablation level of potential blood markers with atrial fibrillation recurrence after catheter ablation: A meta-analysis. Europace 2017, 19, 392–400. [Google Scholar] [CrossRef]
- Elmas, E.; Tulumen, E.; Liebe, V.; Rudic, B.; Lang, S.; Akin, I.; Schimpf, R.; Borggrefe, M. Mid-regional pro-adrenomedullin and N-terminal pro B-type natriuretic peptide predict the recurrence of atrial fibrillation after cryoballoon pulmonary vein isolation. Int. J. Cardiol. 2016, 203, 369–371. [Google Scholar] [CrossRef]
- Weng, W.; Choudhury, R.; Sapp, J.; Tang, A.; Healey, J.S.; Nault, I.; Rivard, L.; Greiss, I.; Bernick, J.; Parkash, R. The role of brain natriuretic peptide in atrial fibrillation: A substudy of the Substrate Modification with Aggressive Blood Pressure Control for Atrial Fibrillation (SMAC-AF) trial. BMC Cardiovasc. Disord. 2021, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Son, J.W.; Nam, B.H.; Joung, B.; Lee, B.; Kim, J.B.; Lee, M.H.; Jang, Y.; Chung, N.; Shim, W.H.; et al. Incremental predictive value of pre-procedural N-terminal pro-B-type natriuretic peptide for short-term recurrence in atrial fibrillation ablation. Clin. Res. Cardiol. 2009, 98, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Braisch, U.; Koenig, W.; Rothenbacher, D.; Denkinger, M.; Friedrich, N.; Felix, S.B.; Ittermann, T.; Dorr, M.; Dallmeier, D. N-terminal pro brain natriuretic peptide reference values in community-dwelling older adults. ESC Heart Fail 2022, 9, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, S.M. Left Atrial Hypertension in Atrial Fibrillation: Dealing With the Pressure. JACC Clin. Electrophysiol. 2017, 3, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Guichard, J.B.; Nattel, S. Atrial Cardiomyopathy: A Useful Notion in Cardiac Disease Management or a Passing Fad? J. Am. Coll. Cardiol. 2017, 70, 756–765. [Google Scholar] [CrossRef]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]



| Parameter | Median (IQR) n (%) µ ± SD | Range | Missing n (%) | p-Value † | HR (95% CI) |
|---|---|---|---|---|---|
| Age at PVI a | 59 (52–68) | 21–81 | 0 (0.0) | 0.109 | 1.01 (1.00–1.02) |
| Male sex b | 267 (71.2) | n/a | 0 (0.0) | 0.191 | 0.81 (0.60–1.11) |
| BMI (kg/m2) a | 27.1 (24.8–30.1) | 16.1–50.9 | 0 (0.0) | 0.011 | 1.04 (1.01–1.08) |
| Paroxysmal AF b | 327 (87.2) | n/a | 0 (0.0) | n/a | |
| Persistent AF b (vs. paroxysm. AF) | 43 (11.5) | 0.005 | 1.81 (1.20–2.73) | ||
| Long-persistent AF b (vs. paroxysm. AF) | 5 (1.3) | 0.016 | 2.98 (1.22–7.28) | ||
| EF (%) a | 65 (60–65) | 35–70 | 36 (9.6) | 0.055 | 0.98 (0.95–1.00) |
| GFR (mL/min/1.73 m2) a | 78.3 (66.2–89.7) | 6.5–139.7 | 1 (0.3) | 0.659 | 1.00 (0.99–1.01) |
| TSH (µU/mL) a | 1.33 (0.96–2.11) | 0.03–28.39 | 126 (33.6) | 0.717 | 1.01 (0.94–1.09) |
| NT-pro-BNP (pg/mL) a | 188.8 (86–465) | 5–3309 | 0 (0.0) | <0.001 | 1.00 (1.00–1.00) |
| log NT-pro-BNP (pg/mL) c | 5.3 ± 1.2 | 1.61–8.10 | 0 (0.0) | <0.001 | 1.31 (1.16–1.49) |
| Arterial hypertension b | 196 (52.3) | n/a | 0 (0.0) | 0.275 | 1.17 (0.88–1.56) |
| Hypercholesterolemia b | 253 (67.5) | n/a | 0 (0.0) | 0.224 | 0.83 (0.62–1.12) |
| Diabetes b | 29 (7.7) | n/a | 0 (0.0) | 0.388 | 0.78 (0.44–1.37) |
| Coronary heart disease b | 36 (9.6) | n/a | 0 (0.0) | 0.189 | 1.35 (0.86–2.10) |
| HFrEF (EF < 40%) b | 5 (1.3) | n/a | 36 (9.6) | 0.638 | 0.72 (0.18–2.88) |
| History of stroke b | 27 (7.2) | n/a | 0 (0.0) | 0.329 | 0.74 0.40–1.36 |
| PAD b | 4 (1.1) | n/a | 0 (0.0) | 0.174 | 2.21 (0.71–6.93) |
| CHA2DS2VaSc score a | 1 (0–2) | 0–5 | 36 (9.6) | 0.193 | 1.08 (0.96–1.22) |
| RR systolic (mmHg) a | 130 (120–140) | 90–200 | 0 (0.0) | 0.689 | 1.00 (0.99–1.01) |
| RR diastolic (mmHg) c | 80 (70–80) | 50–110 | 0 (0.0) | 0.974 | 1.00 (0.99–1.01) |
| LAP maximum (mmHg) a | 18 (15–23) | 6–55 | 97 (25.9) | 0.004 | 1.04 (1.01–1.06) |
| LAP minimum (mmHg) a | 9 (6–12) | 0–30 | 97 (25.9) | 0.034 | 1.04 (1.00–1.08) |
| Mean LAP (mmHg) a | 13.5 (11.0–17.0) | 3.0–42.5 | 97 (25.9) | 0.006 | 1.04 (1.01–1.08) |
| Pulmonary vein anatomy b | n/a | 9 (2.5) | |||
| Four veins (two separate veins on the left and right side = standard) | 337 (92.1) | n/a | n/a | ||
| One additional accessory vein (vs. standard) | 23 (6.3) | 0.056 | 0.48 (0.22–1.02) | ||
| Common ostium (vs. standard) | 6 (1.6) | 0.529 | 0.64 (0.16–2.58) | ||
| AAR drug use at discharge (class I or III) b | 171 (45.6) | n/a | 0 (0.0) | 0.462 | 1.11 (0.84–1.48) |
| Beta-blocker use at discharge b | 196 (52.3) | n/a | 0 (0.0) | 0.043 | 1.34 (1.01–1.79) |
| Left atrial volume (mL) a as obtained by cardiac CT | 109 (90–130) | 47–333 | 40 (10.7) | <0.001 | 1.01 (1.01–1.01) |
| Model 1—100 pg/mL NT-pro-BNP Increase vs. Composite Endpoint | |||
| Variable | HR | 95% CI | p-Value |
| 100 pg/mL increase in NT-pro-BNP | 1.04 | 1.01–1.07 | 0.005 |
| Non-paroxysmal AF | 1.83 | 1.16–2.87 | 0.009 |
| EF (%) | 1.00 | 0.97–1.03 | 0.990 |
| BMI | 1.03 | 0.99–1.07 | 0.149 |
| GFR | 1.01 | 1.00–1.02 | 0.276 |
| Coronary artery disease | 1.48 | 0.91–2.43 | 0.115 |
| Age | 0.99 | 0.97–1.01 | 0.365 |
| Male sex | 0.68 | 0.46–1.01 | 0.056 |
| Cryoballoon generation: | |||
| 2nd vs. 1st | 1.12 | 0.74–1.69 | 0.593 |
| 3rd vs. 1st | 0.97 | 0.50–1.88 | 0.928 |
| Left atrial volume | 1.01 | 1.00–1.01 | <0.001 |
| Model 2—NT-pro-BNP Tertile vs. Composite Endpoint | |||
| Variable | HR | 95% CI | p-Value |
| NT-pro-BNP tertile: | |||
| 2nd vs. 1st | 1.21 | 0.76–1.91 | 0.417 |
| 3rd vs. 1st | 1.78 | 1.10–2.87 | 0.018 |
| Non-paroxysmal AF | 1.65 | 1.03–2.63 | 0.036 |
| EF (%) | 1.00 | 0.97–1.03 | 0.910 |
| BMI | 1.03 | 0.99–1.07 | 0.150 |
| GFR | 1.01 | 1.00–1.02 | 0.218 |
| Coronary artery disease | 1.39 | 0.85–2.27 | 0.186 |
| Age | 0.99 | 0.97–1.01 | 0.317 |
| Male sex | 0.65 | 0.44–0.94 | 0.023 |
| Cryoballoon generation: | |||
| 2nd vs. 1st | 1.07 | 0.71–1.61 | 0.736 |
| 3rd vs. 1st | 0.99 | 0.51–1.89 | 0.968 |
| Left atrial volume | 1.01 | 1.00–1.01 | <0.001 |
| Model 3—Log NT-pro-BNP vs. Composite Endpoint | |||
| Variable | HR | 95% CI | p-Value |
| NT-pro-BNP increase of one natural log unit | 1.32 | 1.10–1.58 | 0.002 |
| Non-paroxysmal AF | 1.68 | 1.06–2.66 | 0.027 |
| EF (%) | 1.00 | 0.97–1.03 | 0.911 |
| BMI | 1.03 | 0.99–1.07 | 0.164 |
| GFR | 1.01 | 1.00–1.02 | 0.153 |
| Coronary artery disease | 1.44 | 0.88–2.35 | 0.145 |
| Age | 0.99 | 0.97–1.01 | 0.175 |
| Male sex | 0.68 | 0.47–1.01 | 0.053 |
| Cryoballoon generation: | |||
| 2nd vs. 1st | 1.14 | 0.75–1.72 | 0.539 |
| 3rd vs. 1st | 1.01 | 0.53–1.95 | 0.972 |
| Left atrial volume | 1.01 | 1.00–1.01 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blessberger, H.; Lambert, T.; Nahler, A.; Hrncic, D.; Hönig, S.; Maier, J.; Rechberger, S.; Windhager, A.; Reiter, C.; Kellermair, J.; et al. NT-pro-BNP as a Predictor for Recurrence of Atrial Fibrillation after Primary Cryoballoon Pulmonary Vein Isolation. J. Clin. Med. 2022, 11, 7400. https://doi.org/10.3390/jcm11247400
Blessberger H, Lambert T, Nahler A, Hrncic D, Hönig S, Maier J, Rechberger S, Windhager A, Reiter C, Kellermair J, et al. NT-pro-BNP as a Predictor for Recurrence of Atrial Fibrillation after Primary Cryoballoon Pulmonary Vein Isolation. Journal of Clinical Medicine. 2022; 11(24):7400. https://doi.org/10.3390/jcm11247400
Chicago/Turabian StyleBlessberger, Hermann, Thomas Lambert, Alexander Nahler, Denis Hrncic, Simon Hönig, Julian Maier, Stefan Rechberger, Armin Windhager, Christian Reiter, Joerg Kellermair, and et al. 2022. "NT-pro-BNP as a Predictor for Recurrence of Atrial Fibrillation after Primary Cryoballoon Pulmonary Vein Isolation" Journal of Clinical Medicine 11, no. 24: 7400. https://doi.org/10.3390/jcm11247400
APA StyleBlessberger, H., Lambert, T., Nahler, A., Hrncic, D., Hönig, S., Maier, J., Rechberger, S., Windhager, A., Reiter, C., Kellermair, J., Kammler, J., Wagner, H., & Steinwender, C. (2022). NT-pro-BNP as a Predictor for Recurrence of Atrial Fibrillation after Primary Cryoballoon Pulmonary Vein Isolation. Journal of Clinical Medicine, 11(24), 7400. https://doi.org/10.3390/jcm11247400