Cardiac Troponins for the Clinical Management of Patients with Claudication and without Cardiac Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Cardiac Troponins
2.3. Peripheral Endovascular Procedures
2.4. Cardiac Testing and Catheterization
2.5. Endpoints of Our Study
2.6. Statistical Analysis
3. Results
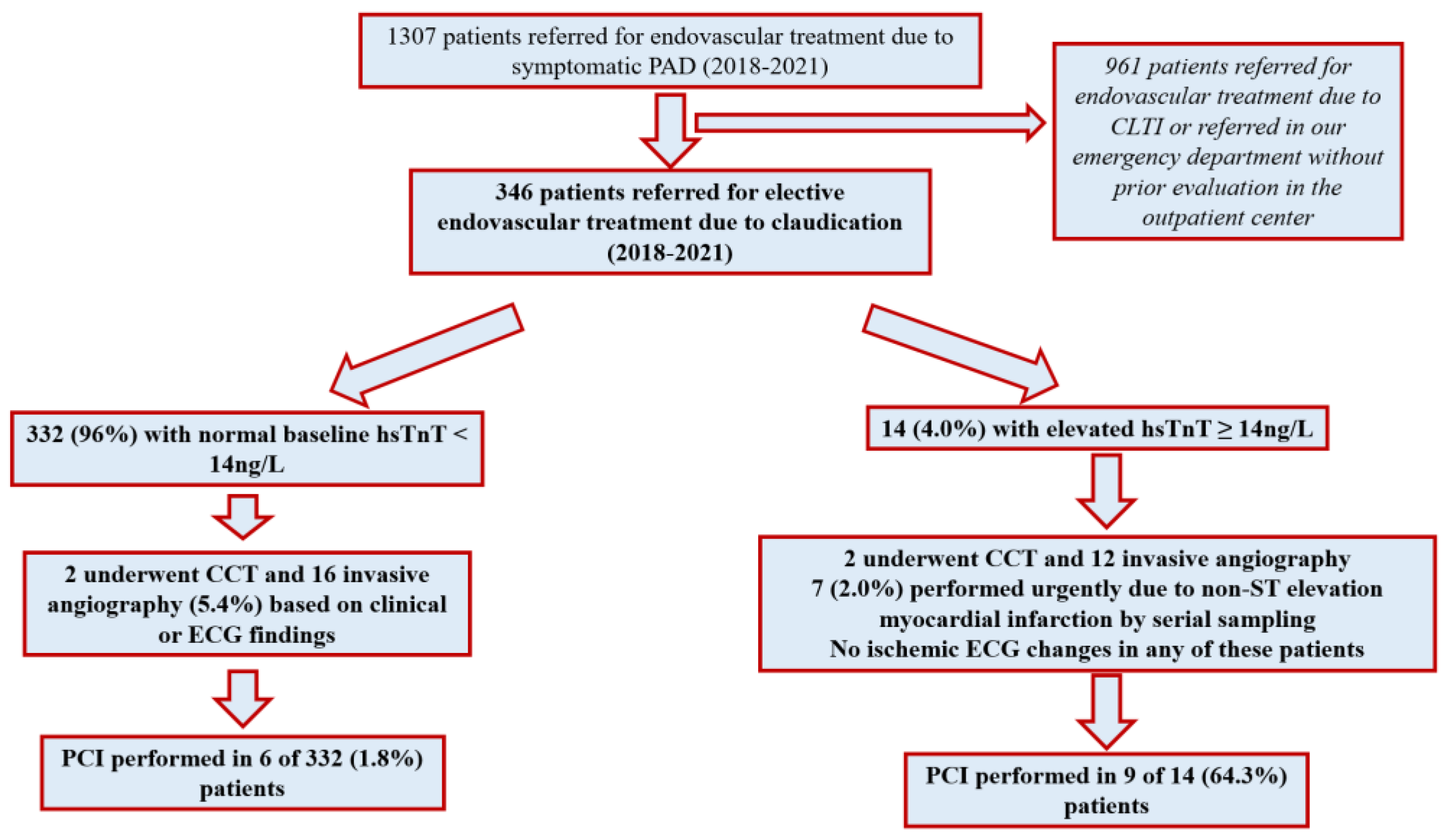
3.1. Demographic Data and Peripheral Endovascular Procedures
3.2. Peripheral Endovascular Procedures
3.3. Predictors of Elev#ated hsTnT
3.4. Cardiac Management by hsTnT and Subsequent Coronary Revascularization
3.5. Predictors of Cardiac Work-Up and PCI
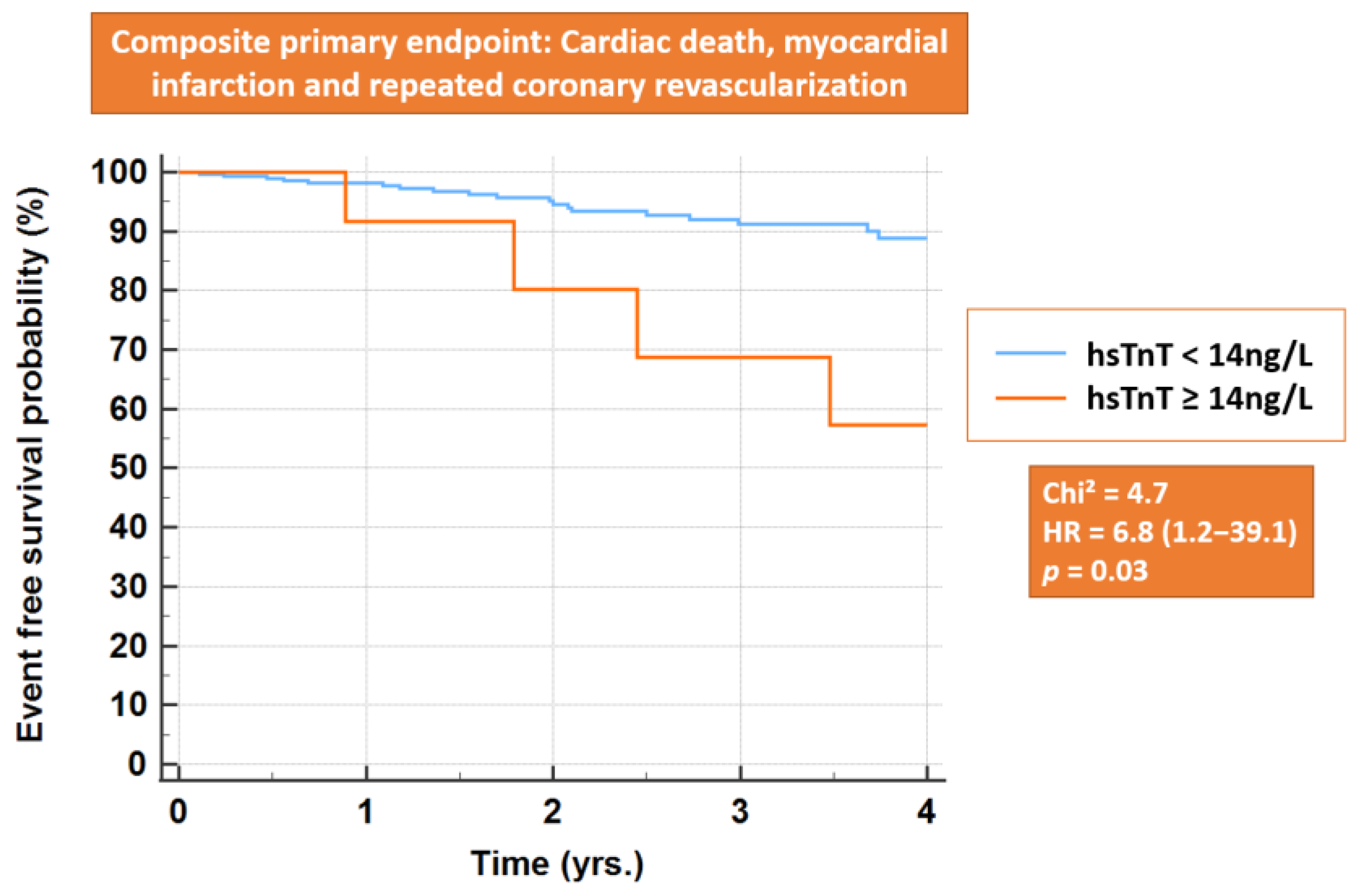
3.6. Primary Endpoint and All-Cause Mortality
4. Discussion
4.1. Previous Studies and Current Recommendations
4.2. Current Findings
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTK | Below-the-knee |
| CAD | Coronary artery disease |
| CCS | Chronic coronary syndromes |
| CCTA | Cardiac computed tomography angiography |
| CFA | Common femoral artery |
| CLTI | Chronic limb-threatening ischemia |
| DCB | Drug coated balloon |
| DM | Diabetes mellitus |
| FFR | Fractional flow reserve |
| GFR | Glomerular filtration rate |
| HsTnT | High-sensitive troponin T |
| PAD | Peripheral arterial disease |
| PCI | Percutaneous coronary intervention |
| POBA | Plain old balloon angioplasty |
| RC | Rutherford category |
References
- Gallino, A.; Aboyans, V.; Diehm, C.; Cosentino, F.; Stricker, H.; Falk, E.; Schouten, O.; Lekakis, J.; Amann-Vesti, B.; Siclari, F.; et al. Non-Coronary Atherosclerosis. Eur. Heart J. 2014, 35, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.W.; Adam, D.J.; Bell, J.; Forbes, J.F.; Fowkes, F.G.; Gillespie, I.; Ruckley, C.V.; Raab, G.M.; Participants, B. Trial Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) Trial: A Description of the Severity and Extent of Disease Using the Bollinger Angiogram Scoring Method and the TransAtlantic Inter-Society Consensus II Classification. J. Vasc. Surg. 2010, 51, 32S–42S. [Google Scholar] [CrossRef] [PubMed]
- Sigvant, B.; Hasvold, P.; Thuresson, M.; Jernberg, T.; Janzon, M.; Nordanstig, J. Myocardial Infarction and Peripheral Arterial Disease: Treatment Patterns and Long-Term Outcome in Men and Women Results from a Swedish Nationwide Study. Eur. J. Prev. Cardiol. 2021, 28, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Gutierrez, J.A.; Creager, M.A.; Scirica, B.M.; Olin, J.; Murphy, S.A.; Braunwald, E.; Morrow, D.A. Acute Limb Ischemia and Outcomes With Vorapaxar in Patients With Peripheral Artery Disease: Results From the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in Patients With Atherosclerosis-Thrombolysis in Myocardial Infarct. Circulation 2016, 133, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Laspas, F.; Pipikos, T.; Karatzis, E.; Georgakopoulos, N.; Prassopoulos, V.; Andreou, J.; Moulopoulos, L.A.; Chatziioannou, A.; Danias, P.G. Cardiac Magnetic Resonance Versus Single-Photon Emission Computed Tomography for Detecting Coronary Artery Disease and Myocardial Ischemia: Comparison with Coronary Angiography. Diagnostics 2020, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Hromadka, M.; Baxa, J.; Seidlerova, J.; Suchy, D.; Sedivy, J.; Stepankova, L.; Rajdl, D.; Rokyta, R. Incidence of Severe Coronary Stenosis in Asymptomatic Patients with Peripheral Arterial Disease Scheduled for Major Vascular Surgery. Int. Angiol. 2016, 35, 411–417. [Google Scholar]
- Roos, A.; Bandstein, N.; Lundback, M.; Hammarsten, O.; Ljung, R.; Holzmann, M.J. Stable High-Sensitivity Cardiac Troponin T Levels and Outcomes in Patients With Chest Pain. J. Am. Coll. Cardiol. 2017, 70, 2226–2236. [Google Scholar] [CrossRef]
- Willeit, P.; Welsh, P.; Evans, J.D.W.; Tschiderer, L.; Boachie, C.; Jukema, J.W.; Ford, I.; Trompet, S.; Stott, D.J.; Kearney, P.M.; et al. High-Sensitivity Cardiac Troponin Concentration and Risk of First-Ever Cardiovascular Outcomes in 154,052 Participants. J. Am. Coll. Cardiol. 2017, 70, 558–568. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Hamburg, N.M.; Creager, M.A. Contemporary Medical Management of Peripheral Artery Disease. Circ. Res. 2021, 128, 1868–1884. [Google Scholar] [CrossRef]
- Vrsalovic, M.; Vrsalovic Presecki, A.; Aboyans, V. Cardiac Troponins Predict Mortality and Cardiovascular Outcomes in Patients with Peripheral Artery Disease: A Systematic Review and Meta-Analysis of Adjusted Observational Studies. Clin. Cardiol. 2022, 45, 198–204. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in Collaboration with the European Society for Vascular Surgery (ESVS): Document Covering Atherosclerotic Disease of Extracranial Carotid and Vertebral, Mesenteric, Renal. Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Arshed, S.; Luo, H.X.; Zafar, S.; Regeti, K.; Malik, N.; Alam, M.; Yousif, A. Elevated Troponin I in the Absence of Coronary Artery Disease: A Case Report With Review of Literature. J. Clin. Med. Res. 2015, 7, 820–824. [Google Scholar] [CrossRef]
- Chaulin, A.M. Cardiac Troponins Metabolism: From Biochemical Mechanisms to Clinical Practice (Literature Review). Int. J. Mol. Sci. 2021, 22, 10928. [Google Scholar] [CrossRef]
- Alushi, B.; Jost-Brinkmann, F.; Kastrati, A.; Cassese, S.; Fusaro, M.; Stangl, K.; Landmesser, U.; Thiele, H.; Lauten, A. High-Sensitivity Cardiac Troponin T in Patients with Severe Chronic Kidney Disease and Suspected Acute Coronary Syndrome. J. Clin. Med. 2021, 10, 4216. [Google Scholar] [CrossRef]
- Gitsioudis, G.; Schussler, A.; Nagy, E.; Maurovich-Horvat, P.; Buss, S.J.; Voss, A.; Hosch, W.; Hofmann, N.; Kauczor, H.U.; Giannitsis, E.; et al. Combined Assessment of High-Sensitivity Troponin T and Noninvasive Coronary Plaque Composition for the Prediction of Cardiac Outcomes. Radiology 2015, 276, 73–81. [Google Scholar] [CrossRef]
- Giannitsis, E.; Kurz, K.; Hallermayer, K.; Jarausch, J.; Jaffe, A.S.; Katus, H.A. Analytical Validation of a High-Sensitivity Cardiac Troponin T Assay. Clin. Chem. 2010, 56, 254–261. [Google Scholar] [CrossRef]
- Stengl, H.; Ganeshan, R.; Hellwig, S.; Blaszczyk, E.; Fiebach, J.B.; Nolte, C.H.; Bauer, A.; Schulz-Menger, J.; Endres, M.; Scheitz, J.F. Cardiomyocyte Injury Following Acute Ischemic Stroke: Protocol for a Prospective Observational Cohort Study. JMIR Res. Protoc. 2021, 10, e24186. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Giusca, S.; Hagstotz, S.; Lichtenberg, M.; Heinrich, U.; Eisenbach, C.; Andrassy, M.; Korosoglou, G. Phoenix Atherectomy for Patients with Peripheral Artery Disease. EuroIntervention 2022, 18, e432–e442. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.P.; Hardt, S.; Erbacher, M.; Dickhaus, H.; Katus, H.A.; Korosoglou, G. Quantitative Myocardial Blush Grade Reserve during Pharmacologic Hyperaemia: A Way to Perform a Real Wireless Fractional Flow Reserve Measurement in Patients with Coronary Artery Disease and Intermediate Coronary Lesions. EuroIntervention 2017, 12, e2219–e2227. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Multiple Comparison Analysis Testing in ANOVA. Biochem. Med. 2011, 21, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Raggi, P.; Korosoglou, G. Carotid Ultrasound and Coronary Calcium for the Prediction of Incident Cardiac Disease in Asymptomatic Individuals: A Further Step towards Precision Medicine Especially in Women? Atherosclerosis 2022, 346, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Steg, P.G.; Bhatt, D.L.; Wilson, P.W.; D’Agostino, R., Sr.; Ohman, E.M.; Rother, J.; Liau, C.S.; Hirsch, A.T.; Mas, J.L.; Ikeda, Y.; et al. One-Year Cardiovascular Event Rates in Outpatients with Atherothrombosis. JAMA 2007, 297, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Szarek, M.; Hess, C.; Patel, M.R.; Jones, W.S.; Berger, J.S.; Baumgartner, I.; Katona, B.; Mahaffey, K.W.; Norgren, L.; Blomster, J.; et al. Total Cardiovascular and Limb Events and the Impact of Polyvascular Disease in Chronic Symptomatic Peripheral Artery Disease. J. Am. Heart Assoc. 2022, 11, e025504. [Google Scholar] [CrossRef] [PubMed]
- Portas, L.; Bauersachs, R.; Bowrin, K.; Briere, J.B.; Cohen, A.; Huelsebeck, M.; Jones, S.; Quint, J.K. Assessment of the Burden of Disease for Patients with Peripheral Artery Disease Undergoing Revascularization in England. Vasc. Med. 2022, 27, 440–449. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Howard, D.P.J.; Nichol, K.G.; Dobell, E.; Rothwell, P.M.; Oxford Vascular, S. Hospital and Institutionalisation Care Costs after Limb and Visceral Ischaemia Benchmarked Against Stroke: Long-Term Results of a Population Based Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2018, 56, 271–281. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; Lopez-Sendon, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Pinxterhuis, T.H.; Ploumen, E.H.; Zocca, P.; Doggen, C.J.M.; Schotborgh, C.E.; Anthonio, R.L.; Roguin, A.; Danse, P.W.; Benit, E.; Aminian, A.; et al. Outcome after Percutaneous Coronary Intervention with Contemporary Stents in Patients with Concomitant Peripheral Arterial Disease: A Patient-Level Pooled Analysis of Four Randomized Trials. Atherosclerosis 2022, 355, 52–59. [Google Scholar] [CrossRef]
- Navarese, E.P.; Lansky, A.J.; Kereiakes, D.J.; Kubica, J.; Gurbel, P.A.; Gorog, D.A.; Valgimigli, M.; Curzen, N.; Kandzari, D.E.; Bonaca, M.P.; et al. Cardiac Mortality in Patients Randomised to Elective Coronary Revascularisation plus Medical Therapy or Medical Therapy Alone: A Systematic Review and Meta-Analysis. Eur. Heart J. 2021, 42, 4638–4651. [Google Scholar] [CrossRef] [PubMed]
- Hikita, H.; Shigeta, T.; Kimura, S.; Takahashi, A.; Isobe, M. Coronary Artery Disease Severity and Cardiovascular Biomarkers in Patients with Peripheral Artery Disease. Int. J. Angiol. 2015, 24, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Szczeklik, W.; Krzanowski, M.; Maga, P.; Partyka, Ł.; Kościelniak, J.; Kaczmarczyk, P.; Maga, M.; Pieczka, P.; Suska, A.; Wachsmann, A.; et al. Myocardial Injury after Endovascular Revascularization in Critical Limb Ischemia Predicts 1-Year Mortality: A Prospective Observational Cohort Study. Clin. Res. Cardiol. 2018, 107, 319–328. [Google Scholar] [CrossRef]
- Krievins, D.; Zellans, E.; Latkovskis, G.; Babuskina, I.; Kumsars, I.; Jegere, S.; Zvaigzne, L.; Krievina, A.K.; Erglis, A.; Zarins, C.K. Coronary Revascularization of Patients with Silent Coronary Ischemia May Reduce the Risk of Myocardial Infarction and Cardiovascular Death after Carotid Endarterectomy. J. Vasc. Surg. 2022, 76, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Krievins, D.; Zellans, E.; Latkovskis, G.; Kumsars, I.; Jegere, S.; Rumba, R.; Bruvere, M.; Zarins, C.K. Diagnosis of Silent Coronary Ischemia with Selective Coronary Revascularization Might Improve 2-Year Survival of Patients with Critical Limb-Threatening Ischemia. J. Vasc. Surg. 2021, 74, 1261–1271. [Google Scholar] [CrossRef] [PubMed]

| Baseline and Laboratory Data | All Patients (n = 346) | Patients with Normal hsTnT (n = 332) | Patients with Elevated hsTnT (n = 14) | p-Values |
|---|---|---|---|---|
| Demographic Data | ||||
| Age | 68.3 ± 9.5 | 68.2 ± 9.4 | 69.1 ± 12.0 | 0.72 |
| Female gender | 140(40.5%) | 138(41.6%) | 2(14.3%) | 0.04 |
| 1. Arterial hypertension | 318(91.9%) | 304(91.6%) | 14(100%) | 0.26 |
| 2. Hyperlipidemia | 274(79.2%) | 261(78.6%) | 13(92.9%) | 0.42 |
| 3. Diabetes mellitus | 98(28.3%) | 91(27.4%) | 7(50.0%) | 0.07 |
| 4. Active or former smoking | 232(67.1%) | 221(66.5%) | 11(84.6%) | 0.47 |
| Total number of risk factors (0–4) | 2.7 ± 0.9 | 2.6 ± 0.9 | 3.2 ± 0.7 | 0.03 |
| History of CAD | 108(31.2%) | 98(29.5%) | 10(71.4%) | 0.001 |
| History of myocardial infarction | 36(10.4%) | 27(8.1%) | 9(64.3%) | <0.0001 |
| History of PCI | 33(9.5%) | 30(9.0%) | 3(21.4%) | 0.12 |
| History of CABG | 28(8.1%) | 24(7.2%) | 4(28.6%) | 0.0001 |
| Cardiac Medications | ||||
| Aspirin | 339(98.0%) | 328(98.8%) | 11(78.6%) | 0.43 |
| Dual platelet therapy after treatment | 285(82.4%) | 274(82.5%) | 11(78.6%) | 0.70 |
| Oral anticoagulants | 86(24.9%) | 83(25.0%) | 3(21.4%) | 0.73 |
| Diuretics | 112(32.3%) | 105(31.6%) | 7(50.0%) | 0.15 |
| ACE inhibitors or AT2 blockers | 127(36.7%) | 123(37.0%) | 4(28.6%) | 0.51 |
| ß-Blockers | 156(45.1%) | 147(44.2%) | 9(64.3%) | 0.14 |
| Statins | 320(92.5%) | 308(92.8%) | 12(85.7%) | 0.32 |
| Laboratory Data | ||||
| Estimated GFR(mL/min/1.73 m2) | 76.2 ± 24.0 | 76.2 ± 23.7 | 74.1 ± 31.0 | 0.74 |
| Creatinine(mg/dL) | 0.93 ± 0.24 | 0.93 ± 0.23 | 1.08 ± 0.38 | 0.02 |
| Urea(mg/dL) | 34.3 ± 12.8 | 33.9 ± 12.2 | 44.2 ± 21.1 | 0.003 |
| Hemoglobin(mg/dL) | 14.2 ± 1.5 | 14.2 ± 1.5 | 13.9 ± 1.6 | 0.53 |
| High-sensitive troponin T(ng/L) | 11.1 ± 23.8 | 8.2 ± 3.1 | 80.1 ± 97.1 | <0.001 |
| High-sensitive troponin T(ng/L) (median and interquartile range) | 8.4(5.8–11.2) | 8.2(5.7–11.0) | 45.3 (32.1–63.5) | <0.001 |
| Rutherford Categories (RC) | ||||
| RC 2 | 6(1.7%) | 6(1.8%) | 0(0%) | 0.61 |
| RC 3 | 340(98.2%) | 326(98.2%) | 14(100%) | 0.61 |
| Covariates | Coefficient | SE | Wald | Odds Ratios | 95%Cl | p-Values |
|---|---|---|---|---|---|---|
| Predictors of elevated hsTnT including myocardial infarction as covariate | ||||||
| Age | 0.0289 | 0.040 | 0.51 | 1.02 | 0.95 to 1.11 | 0.47 |
| Female gender | −2.079 | 0.84 | 6.01 | 0.12 | 0.02 to 0.65 | 0.01 |
| Estimated GFR (ml/min/1.73 m2) | 0.012 | 0.015 | 0.58 | 1.01 | 0.98 to 1.04 | 0.44 |
| History of myocardial infarction | 3.50 | 0.66 | 27.62 | 33.19 | 8.99 to 122.53 | <0.0001 |
| Predictors of elevated hsTnT including history of coronary artery disease as covariate | ||||||
| Age | −0.0021 | 0.039 | 0.002 | 0.99 | 0.92 to 1.07 | 0.95 |
| Female gender | −1.42 | 0.78 | 3.25 | 0.24 | 0.05 to 1.12 | 0.07 |
| Estimated GFR (ml/min/1.73 m2) | −0.0033 | 0.014 | 0.052 | 0.99 | 0.96 to 1.02 | 0.81 |
| History of coronary artery disease | 1.75 | 0.62 | 7.95 | 5.8 | 1.71 to 19.74 | 0.005 |
| Predictors of PCI including High-sensitive troponin T quartiles as a covariate | ||||||
| Age | 0.00119 | 0.032 | 0.001 | 1.00 | 0.93 to 1.06 | 0.97 |
| Total number of atherogenic risk factors | 0.688 | 0.32 | 4.4 | 1.98 | 1.05 to 3.76 | 0.03 |
| Estimated GFR (ml/min/1.73 m2) | −0.00133 | 0.012 | 0.01 | 0.99 | 0.97 to 1.02 | 0.91 |
| HsTnT (ng/L) quartiles | 1.555 | 0.42 | 13.1 | 4.73 | 2.04 to 10.96 | 0.0003 |
| Predictors of PCI including High-sensitive troponin T(ng/L) levels quartiles as a covariate | ||||||
| Age | 0.0417 | 0.04 | 1.06 | 1.04 | 0.96 to 1.12 | 0.30 |
| Total number of atherogenic risk factors | 0.748 | 0.36 | 4.31 | 2.11 | 1.04 to 4.27 | 0.03 |
| Estimated GFR (ml/min/1.73 m2) | 0.0021 | 0.014 | 0.021 | 1.00 | 0.97 to 1.03 | 0.88 |
| HsTnT (ng/L) | 0.064 | 0.016 | 14.58 | 1.06 | 1.03 to 1.1 | 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouselimis, D.; Hagstotz, S.; Lichtenberg, M.; Donas, K.P.; Heinrich, U.; Avranas, K.; Dimitriadis, Z.; Blessing, E.; Langhoff, R.; Frey, N.; et al. Cardiac Troponins for the Clinical Management of Patients with Claudication and without Cardiac Symptoms. J. Clin. Med. 2022, 11, 7287. https://doi.org/10.3390/jcm11247287
Mouselimis D, Hagstotz S, Lichtenberg M, Donas KP, Heinrich U, Avranas K, Dimitriadis Z, Blessing E, Langhoff R, Frey N, et al. Cardiac Troponins for the Clinical Management of Patients with Claudication and without Cardiac Symptoms. Journal of Clinical Medicine. 2022; 11(24):7287. https://doi.org/10.3390/jcm11247287
Chicago/Turabian StyleMouselimis, Dimitrios, Saskia Hagstotz, Michael Lichtenberg, Konstantinos P. Donas, Ulrike Heinrich, Konstantinos Avranas, Zisis Dimitriadis, Erwin Blessing, Ralf Langhoff, Norbert Frey, and et al. 2022. "Cardiac Troponins for the Clinical Management of Patients with Claudication and without Cardiac Symptoms" Journal of Clinical Medicine 11, no. 24: 7287. https://doi.org/10.3390/jcm11247287
APA StyleMouselimis, D., Hagstotz, S., Lichtenberg, M., Donas, K. P., Heinrich, U., Avranas, K., Dimitriadis, Z., Blessing, E., Langhoff, R., Frey, N., Katus, H. A., & Korosoglou, G. (2022). Cardiac Troponins for the Clinical Management of Patients with Claudication and without Cardiac Symptoms. Journal of Clinical Medicine, 11(24), 7287. https://doi.org/10.3390/jcm11247287








