Leukocyte Count Predicts Carotid Artery Stenosis in Men with Ischemic Stroke: Sub Study of the Preventive Antibiotics in Stroke Study (PASS)
Abstract
1. Introduction
2. Materials and Methods
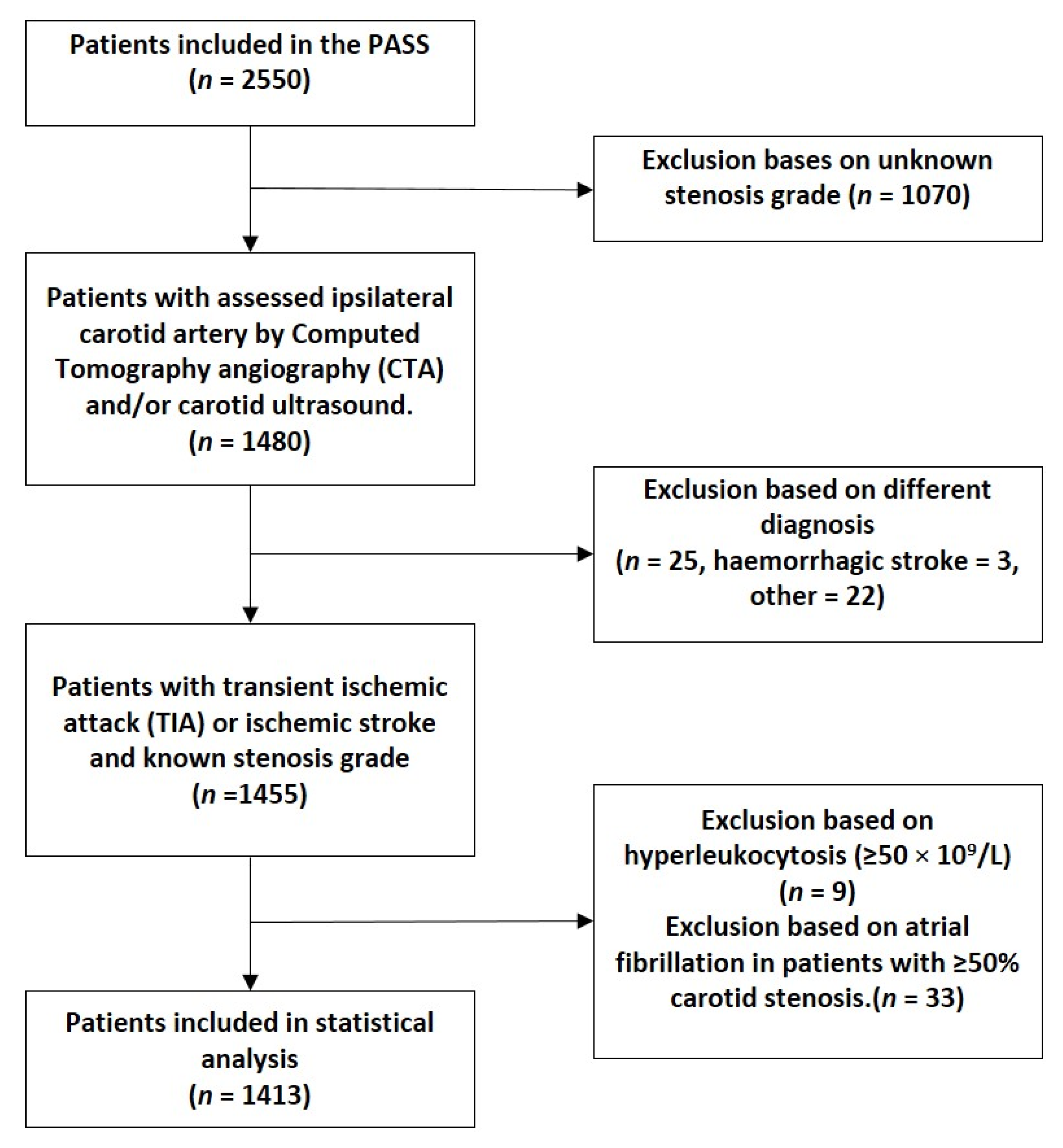
2.1. Study Population
2.2. Baseline Characteristics
2.3. Outcomes
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEA | carotid endarterectomy |
| CI | confidence interval |
| CONVINCE | Colchicine for Prevention of Vascular Inflammation in Non-cardio Embolic Stroke |
| CRP | C-reactive protein |
| CTA | computed tomography angiography |
| ICAS | internal carotid artery stenosis |
| IL | interleukin |
| IQR | inter-quartile range |
| MRI | magnetic resonance imaging |
| mRS | modified Rankin Scale |
| NIHSS | National Institutes of Health Stroke Scale |
| OR | odds ratio |
| PASS | Preventive antibiotics in stroke study |
| TIA | transient ischemic attack |
| TNF-α | tumor necrosis factor-alpha |
References
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef] [PubMed]
- European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998, 351, 1379–1387. [Google Scholar] [CrossRef]
- Howard, V.J.; Meschia, J.F.; Lal, B.K.; Turan, T.N.; Roubin, G.S.; Brown, R.D., Jr.; Voeks, J.H.; Barrett, K.M.; Demaerschalk, B.M.; Huston, J., 3rd; et al. Carotid revascularization and medical management for asymptomatic carotid stenosis: Protocol of the CREST-2 clinical trials. Int. J. Stroke Off. J. Int. Stroke Soc. 2017, 12, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, D.S.; Johnsen, S.H.; Mathiesen, E.B.; Njølstad, I. The association between inflammatory markers and carotid atherosclerosis is sex dependent: The Tromsø Study. Cerebrovasc. Dis. 2009, 27, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Westendorp, W.F.; Vermeij, J.D.; Zock, E.; Hooijenga, I.J.; Kruyt, N.D.; Bosboom, H.J.; Kwa, V.I.; Weisfelt, M.; Remmers, M.J.; ten Houten, R.; et al. The Preventive Antibiotics in Stroke Study (PASS): A pragmatic randomised open-label masked endpoint clinical trial. Lancet 2015, 385, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Nederkoorn, P.J.; Westendorp, W.F.; Hooijenga, I.J.; de Haan, R.J.; Dippel, D.W.J.; Vermeij, F.H.; Dijkgraaf, M.G.W.; Prins, J.M.; Spanjaard, L.; van de Beek, D. Preventive Antibiotics in Stroke Study: Rationale and Protocol for a Randomised Trial. Int. J. Stroke 2011, 6, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese, C.; Mascarucci, P.; Zoia, C.; Cavarretta, R.; Frigo, M.; Begni, B.; Sarinella, F.; Frattola, L.; De Simoni, M.G. Increased cytokine release from peripheral blood cells after acute stroke. J. Cereb. Blood Flow Metab. 1999, 19, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Basic Kes, V.; Simundic, A.M.; Nikolac, N.; Topic, E.; Demarin, V. Pro-inflammatory and anti-inflammatory cytokines in acute ischemic stroke and their relation to early neurological deficit and stroke outcome. Clin. Biochem. 2008, 41, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F. Systemic effects of smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.C.; Luebke, R.W. Immunological Aging. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Prass, K.; Meisel, C.; Höflich, C.; Braun, J.; Halle, E.; Wolf, T.; Ruscher, K.; Victorov, I.V.; Priller, J.; Dirnagl, U.; et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 2003, 198, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Puz, P.; Lasek-Bal, A.; Ziaja, D.; Kazibutowska, Z.; Ziaja, K. Inflammatory markers in patients with internal carotid artery stenosis. Arch. Med. Sci. 2013, 9, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Shindo, A.; Tanemura, H.; Yata, K.; Hamada, K.; Shibata, M.; Umeda, Y.; Asakura, F.; Toma, N.; Sakaida, H.; Fujisawa, T.; et al. Inflammatory biomarkers in atherosclerosis: Pentraxin 3 can become a novel marker of plaque vulnerability. PLoS ONE 2014, 9, e100045. [Google Scholar] [CrossRef] [PubMed]
- Pini, R.; Faggioli, G.; Fittipaldi, S.; Pasquinelli, G.; Tonon, C.; Beltrandi, E.; Mauro, R.; Stella, A. Inflammatory mediators and cerebral embolism in carotid stenting: New markers of risk. J. Endovasc. Ther. 2013, 20, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arter. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Murphy, S.; Coveney, S.; Purroy, F.; Lemmens, R.; Tsivgoulis, G.; Price, C. Anti-inflammatory approaches to ischaemic stroke prevention. J. Neurol. Neurosurg. Psychiatry 2018, 89, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef] [PubMed]
| Sex | |||
|---|---|---|---|
| Female (n = 577) | Male (n = 836) | p-Value | |
| Age, years | 75 (65–82) | 71 (62–79) | <0.001 |
| Caucasian | 540/577 (94%) | 782 (94%) | <0.972 |
| Systolic blood pressure (mmHg) | 165 (162–167) | 164 (162–166) | 0.670 |
| Diastolic blood pressure (mmHg) | 84 (83–86) | 89 (87–90) | <0.001 |
| Carotid artery stenosis (≥50%) | 73/577 (13%) | 166/836 (20%) | <0.001 |
| Serum markers | |||
| Leukocytes (×109/L) | 8.55 (8.29–8.75) | 8.29 (8.1–8.46) | 0.077 |
| Thrombocytes (×109/L) | 259 (252–265) | 224 (219–229) | <0.001 |
| Medical History | |||
| Atrial fibrillation/Flutter | 62/575 (11%) | 84/834 (10%) | 0.667 |
| Stroke | 177/575 (31%) | 272/836 (32%) | 0.487 |
| Hypercholesterolemia | 141/572 (25%) | 240/827 (29%) | 0.071 |
| Hypertension | 338/576 (59%) | 427/836 (51%) | 0.005 |
| Myocardial infarction | 39/575 (7%) | 137/836 (16%) | <0.001 |
| Cardiac valve disease a | 33/576 (6%) | 46/834 (6%) | 0.864 |
| Peripheral vascular disease | 39/574 (7%) | 71/834 (9%) | 0.237 |
| Diabetes Mellitus | 103/576 (18%) | 166/836 (20%) | 0.353 |
| Malignancy | 49/576 (9%) | 61/834 (7%) | 0.411 |
| Current smoker | 141/573 (25%) | 239/834 (29%) | 0.093 |
| Previous medication | |||
| Anticoagulants | 41/577 (7%) | 63/836 (8%) | 0.761 |
| Antiplatelet therapy | 216/577 (37%) | 365/835 (44%) | 0.018 |
| Statins | 188/577 (33%) | 347/835 (42%) | <0.001 |
| Modified Rankin Scale before stroke symptoms (pre-mRS) b | 1 (1–2) | 1 (1–2) | 0.001 |
| Baseline National Institutes of Health Stroke Scale (NIHSS) b | 5 (3–9) | 4 (3–7) | 0.006 |
| Treatment | |||
| Intravenous thrombolysis | 220/577 (38%) | 325/836 (39%) | 0.777 |
| Post-stroke Ceftriaxone | 289/577 (50%) | 421/836 (50%) | 0.920 |
| Internal Carotid Artery Stenosis (<50%) | Internal Carotid Artery Stenosis (≥50%) | p-Value | |
|---|---|---|---|
| Female patients | n = 507/577 (87%) | n = 70/577 (13%) | |
| Leukocytes (×109/L) | 8.44 (8.21–8.67) | 9.31 (8.51–10.1) | 0.041 |
| Thrombocytes (×109/L) | 256 (250–263) | 273 (252–295) | 0.128 |
| CRP (×mg/L) | 8.25 (6.51–9.99) | 11.68 (3.26–20.10) | |
| Male patients | n = 670/836 (80%) | n = 166/836 (20%) | |
| Leukocytes (×109/L) | 8.1 (7.91–8.3) | 9 (8.58–9.17) | <0.001 |
| Thrombocytes (×109/L) | 222 (216–227) | 234 (223–244) | 0.064 |
| CRP (×mg/L) | 8.09 (6.63–9.54) | 8.78 (6.22–11.35) |
| Unadjusted Model | Adjusted Model | |||
|---|---|---|---|---|
| Common Odds Ratio | p-Value | Common Odds Ratio | p-Value | |
| Female patients (n = 577) | ||||
| Leukocytes (×109/L) | 1.098 (1.017–1.186) | 0.017 | 1.041 (0.995–1.136) | 0.360 |
| Thrombocytes (×109/L) | 1.002 (0.999–1.005) | 0.130 | 1.002 (0.999–1.005) | 0.145 |
| Male patients (n = 836) | ||||
| Leukocytes (×109/L) | 1.131 (1.064–1.202) | <0.001 | 1.094 (1.024–1.169) | 0.008 |
| Thrombocytes (×109/L) | 1.002 (1.000–1.404) | 0.065 | 1.002 (0.999–1.004) | 0.160 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Velzen, T.J.; Stolp, J.; Westendorp, W.F.; Roos, Y.B.W.E.M.; van de Beek, D.; Nederkoorn, P.J. Leukocyte Count Predicts Carotid Artery Stenosis in Men with Ischemic Stroke: Sub Study of the Preventive Antibiotics in Stroke Study (PASS). J. Clin. Med. 2022, 11, 7286. https://doi.org/10.3390/jcm11247286
van Velzen TJ, Stolp J, Westendorp WF, Roos YBWEM, van de Beek D, Nederkoorn PJ. Leukocyte Count Predicts Carotid Artery Stenosis in Men with Ischemic Stroke: Sub Study of the Preventive Antibiotics in Stroke Study (PASS). Journal of Clinical Medicine. 2022; 11(24):7286. https://doi.org/10.3390/jcm11247286
Chicago/Turabian Stylevan Velzen, Twan J., Jeffrey Stolp, Willeke F. Westendorp, Yvo B. W. E. M. Roos, Diederik van de Beek, and Paul J. Nederkoorn. 2022. "Leukocyte Count Predicts Carotid Artery Stenosis in Men with Ischemic Stroke: Sub Study of the Preventive Antibiotics in Stroke Study (PASS)" Journal of Clinical Medicine 11, no. 24: 7286. https://doi.org/10.3390/jcm11247286
APA Stylevan Velzen, T. J., Stolp, J., Westendorp, W. F., Roos, Y. B. W. E. M., van de Beek, D., & Nederkoorn, P. J. (2022). Leukocyte Count Predicts Carotid Artery Stenosis in Men with Ischemic Stroke: Sub Study of the Preventive Antibiotics in Stroke Study (PASS). Journal of Clinical Medicine, 11(24), 7286. https://doi.org/10.3390/jcm11247286





