The Role of Combination Therapy with α-Blockers and Hexanic Extract of Serenoa repens in the Treatment of LUTS/BPH
Abstract
1. Introduction
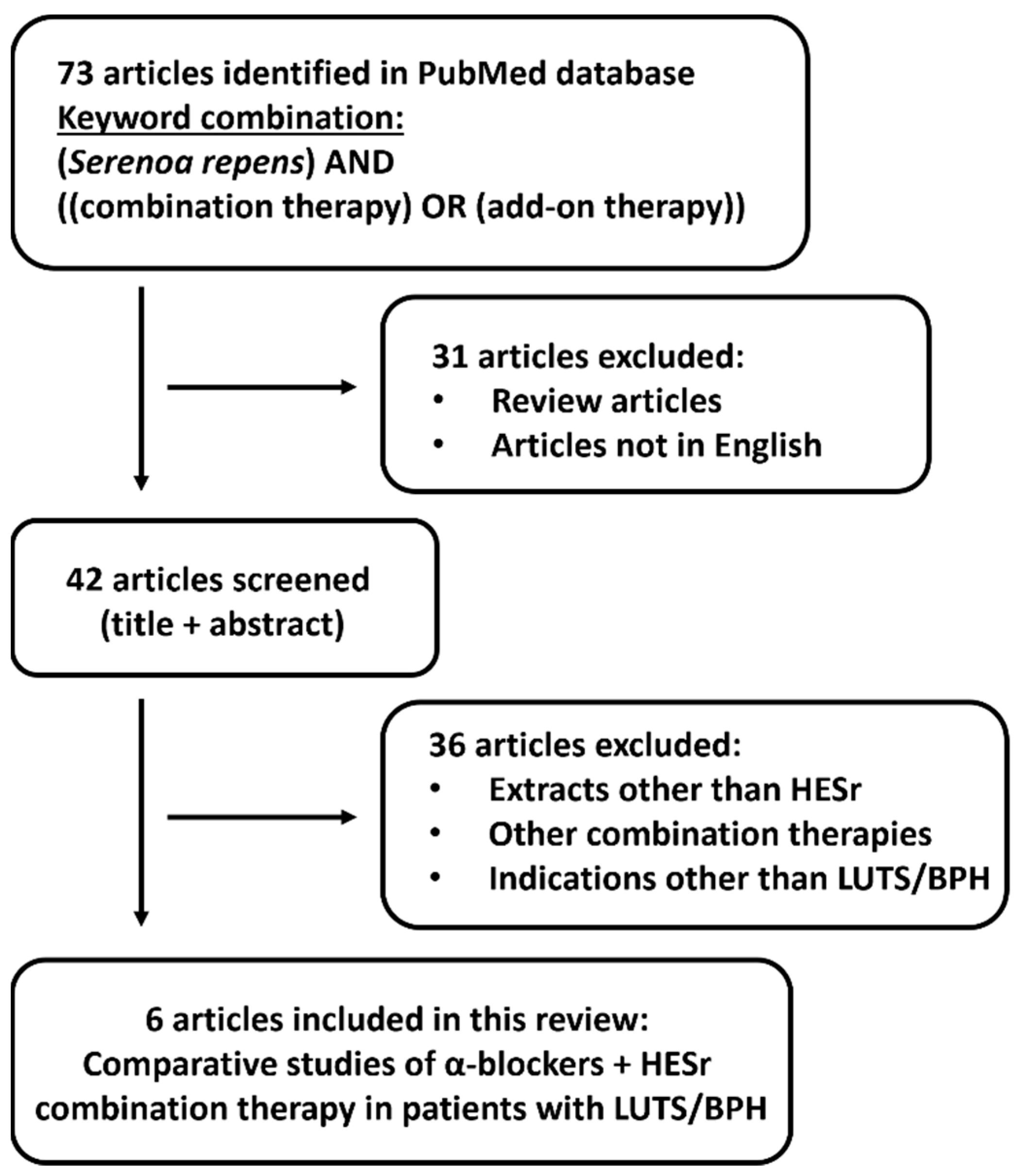
2. Materials and Methods
- (Serenoa repens) AND ((combination therapy) OR (add-on therapy))
3. Overview of HESr Monotherapy in the Management of LUTS/BPH
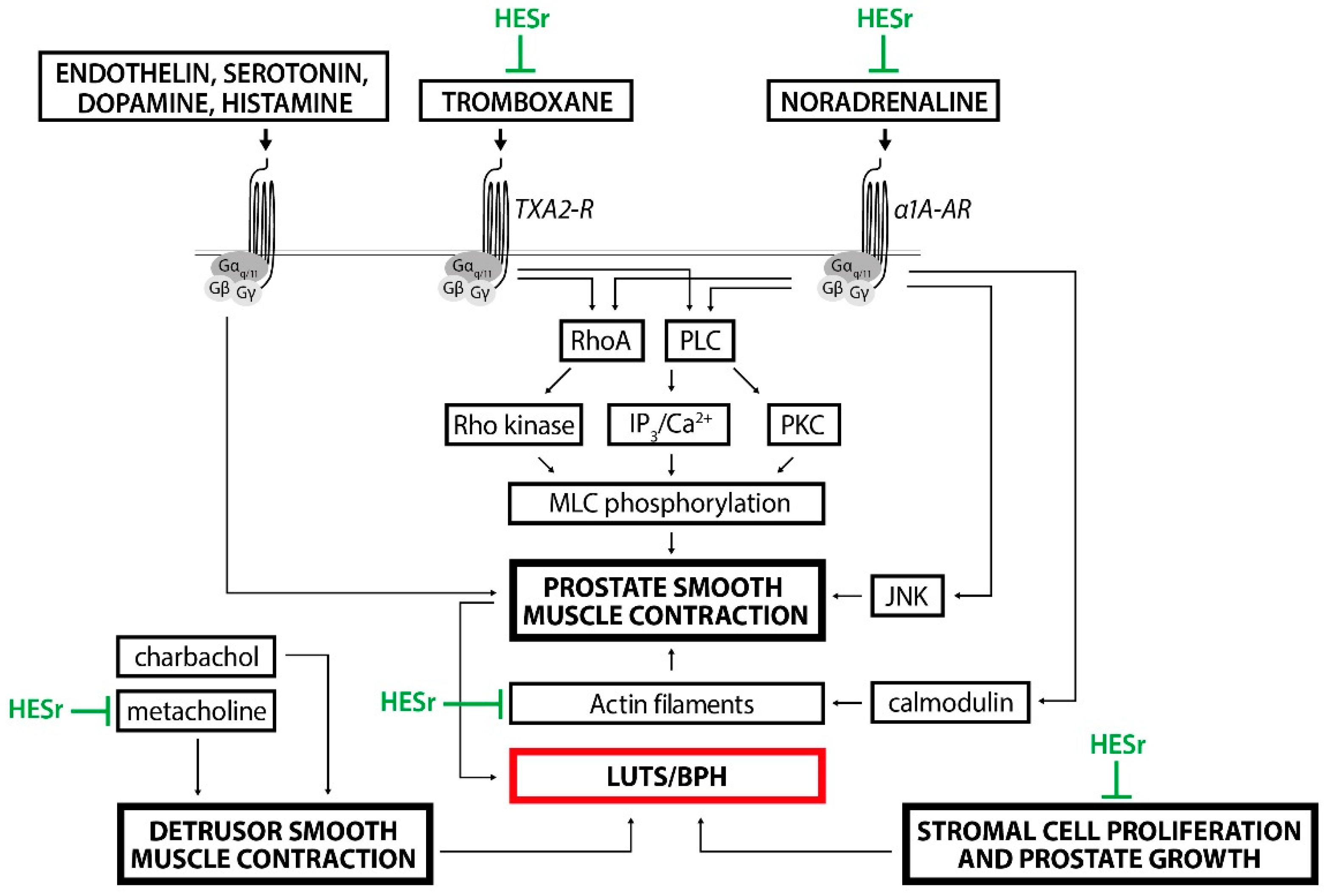
4. HESr as a Combination Therapy with α-Blockers in the Management of LUTS/BPH
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gravas, S.; Cornu, J.N.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; Speakman, M.J.; Tikkinen, K.A.O. EAU Guidelines on Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). Update March 2022. Available online: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts (accessed on 17 October 2022).
- Speakman, M.; Kirby, R.; Doyle, S.; Ioannou, C. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH)—Focus on the UK. BJU Int. 2015, 115, 508–519. [Google Scholar] [CrossRef]
- Mitropoulos, D.; Anastasiou, I.; Giannopoulou, C.; Nikolopoulos, P.; Alamanis, C.; Zervas, A.; Dimopoulos, C. Symptomatic benign prostate hyperplasia: Impact on partners’ quality of life. Eur. Urol. 2002, 41, 240–245. [Google Scholar] [CrossRef]
- Vuichoud, C.; Loughlin, K.R. Benign prostatic hyperplasia: Epidemiology, economics and evaluation. Can. J. Urol. 2015, 22, 1–6. [Google Scholar]
- Nickel, J.C.; Roehrborn, C.G.; O’Leary, M.P.; Bostwick, D.G.; Somerville, M.C.; Rittmaster, R.S. The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur. Urol. 2008, 54, 1379–1384. [Google Scholar] [CrossRef]
- Ficarra, V.; Rossanese, M.; Zazzara, M.; Giannarini, G.; Abbinante, M.; Bartoletti, R.; Mirone, V.; Scaglione, F. The role of inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr. Urol. Rep. 2014, 15, 463. [Google Scholar] [CrossRef]
- Nickel, J.C.; Roehrborn, C.G.; Castro-Santamaria, R.; Freedland, S.J.; Moreira, D.M. Chronic prostate inflammation is associated with severity and progression of benign prostatic hyperplasia, lower urinary tract symptoms and risk of acute urinary retention. J. Urol. 2016, 196, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Presicce, F.; Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 2016, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Kramer, G.; Marberger, M.; Montironi, R.; Nelson, W.; Schröder, F.; Sciarra, A.; Tubaro, A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: The role of inflammation. Eur. Urol. 2011, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Gacci, M.; Vignozzi, L.; Sebastianelli, A.; Salvi, M.; Giannessi, C.; De Nunzio, C.; Tubaro, A.; Corona, G.; Rastrelli, G.; Santi, R.; et al. Metabolic syndrome and lower urinary tract symptoms: The role of inflammation. Prostate Cancer Prostatic Dis. 2013, 16, 101–106. [Google Scholar] [CrossRef]
- Gandaglia, G.; Briganti, A.; Gontero, P.; Mondaini, N.; Novara, G.; Salonia, A.; Sciarra, A.; Montorsi, F. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. 2013, 112, 432–441. [Google Scholar] [CrossRef]
- De Nunzio, C.; Salonia, A.; Gacci, M.; Ficarra, V. Inflammation is a target of medical treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia. World J. Urol. 2020, 38, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Cornu, J.N.; Cussenot, O.; Haab, F.; Lukacs, B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. Eur. Urol. 2010, 58, 450–456. [Google Scholar] [CrossRef]
- Fourcade, R.O.; Lacoin, F.; Rouprêt, M.; Slama, A.; Le Fur, C.; Michel, E.; Sitbon, A.; Cotté, F.E. Outcomes and general health-related quality of life among patients medically treated in general daily practice for lower urinary tract symptoms due to benign prostatic hyperplasia. World J. Urol. 2012, 30, 419–426. [Google Scholar] [CrossRef]
- Scaglione, F.; Lucini, V.; Pannacci, M.; Caronno, A.; Leone, C. Comparison of the potency of different brands of Serenoa repens extract on 5α-reductase types I and II in prostatic co-cultured epithelial and fibroblast cells. Pharmacology 2008, 82, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Lucini, V.; Pannacci, M.; Dugnani, S.; Leone, C. Comparison of the potency of 10 different brands of Serenoa repens extracts. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 569–574. [Google Scholar]
- Habib, F.K.; Wyllie, M.G. Not all brands are created equal: A comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer Prostatic Dis. 2004, 7, 195–200. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Granese, A.; Di Pierro, G.B.; Leonardo, C.; De Nunzio, C. Modern extraction techniques and their impact on the pharmacological profile of serenoa repens extracts for the treatment of lower urinary tract symptoms. BMC Urol. 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Tacklind, J.; Macdonald, R.; Rutks, I.; Stanke, J.U.; Wilt, T.J. Serenoa repens for benign prostatic hyperplasia (Review). Cochrane Database Syst. Rev. 2012, 12, CD001423. [Google Scholar] [CrossRef]
- MacDonald, R.; Tacklind, J.W.; Rutks, I.; Wilt, T.J. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): An updated Cochrane systematic review. BJU Int. 2012, 109, 1756–1761. [Google Scholar] [CrossRef]
- Trivisonno, L.F.; Sgarbossa, N.; Alvez, G.A.; Fieiras, C.; Escobar Liquitay, C.M.; Jung, J.H.; Franco, J.V.A. Serenoa repens for the treatment of lower urinary tract symptoms due to benign prostatic enlargement: A systematic review and meta-analysis. Investig. Clin. Urol. 2021, 62, 520–534. [Google Scholar] [CrossRef]
- Russo, G.I.; Scandura, C.; Di Mauro, M.; Cacciamani, G.; Albersen, M.; Hatzichristodoulou, G.; Fode, M.; Capogrosso, P.; Cimino, S.; Marcelissen, T.; et al. Clinical efficacy of Serenoa repens versus placebo versus alpha-blockers for the treatment of lower urinary tract symptoms/benign prostatic enlargement: A systematic review and network meta-analysis of randomized placebo-controlled clinical trials. Eur. Urol. Focus 2021, 7, 420–431. [Google Scholar] [CrossRef]
- De Nunzio, C.; Novara, G.; Damiano, R.; Bartoletti, R.; Tubaro, A.; Ficarra, V.; Research Urological Network. New evidence changing clinical practice or misunderstanding of statistical analyses? The case of serenoa repens and alpha-blockers. Reply to “Russo et al. Clinical efficacy of Serenoa repens versus placebo versus alpha-blockers for the treatment of lower urinary tract symptoms/benign prostatic enlargement: A systematic review and network meta-analysis of randomized placebo-controlled clinical trials. Eur. Urol. Focus. 2021, 7, 420–431. https://doi.org/10.1016/j.euf.2020.01.002”. Eur. Urol. Focus. 2021, 7, 894–896. [Google Scholar] [CrossRef]
- Alcaraz, A.; Carballido-Rodríguez, J.; Unda-Urzaiz, M.; Medina-López, R.; Ruiz-Cerdá, J.L.; Rodríguez-Rubio, F.; García-Rojo, D.; Brenes-Bermúdez, F.J.; Cózar-Olmo, J.M.; Baena-González, V.; et al. Quality of life in patients with lower urinary tract symptoms associated with BPH: Change over time in real-life practice according to treatment—The QUALIPROST study. Int. Urol. Nephrol. 2016, 48, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, A.; Rodríguez-Antolín, A.; Carballido-Rodríguez, J.; Castro-Díaz, D.; Medina-Polo, J.; Fernández-Gómez, J.M.; Ficarra, V.; Palou, J.; Ponce de León Roca, J.; Angulo, J.C.; et al. Efficacy and tolerability of the hexanic extract of Serenoa repens compared to tamsulosin in moderate-severe LUTS-BPH patients. Sci. Rep. 2021, 11, 19401. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, F. Comparison of a phytotherapeutic agent (permixon) with an alphablocker (tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomised international study. XVIIth Congress of the European Association of Urology, February 2002. Eur. Urol. Suppl. 2002, 1, 108. [Google Scholar] [CrossRef]
- Debruyne, F.; Boyle, P.; Calais Da Silva, F.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P.; Schulman, C.C. Evaluation of the clinical benefit of permixon and tamsulosin in severe BPH patients-PERMAL study subset analysis. Eur. Urol. 2004, 45, 773–779. [Google Scholar] [CrossRef]
- Vela-Navarrete, R.; Alcaraz, A.; Rodríguez-Antolín, A.; Miñana López, B.; Fernández-Gómez, J.M.; Angulo, J.C.; Castro Díaz, D.; Romero-Otero, J.; Brenes, F.J.; Carballido, J.; et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon((R)) ) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018, 122, 1049–1065. [Google Scholar] [CrossRef]
- Novara, G.; Giannarini, G.; Alcaraz, A.; Cózar-Olmo, J.M.; Descazeaud, A.; Montorsi, F.; Ficarra, V. Efficacy and safety of hexanic lipidosterolic extract of Serenoa repens (Permixon) in the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: Systematic review and meta-analysis of randomized controlled trials. Eur. Urol. Focus 2016, 2, 553–561. [Google Scholar] [CrossRef]
- Alcaraz, A.; Gacci, M.; Ficarra, V.; Medina-Polo, J.; Salonia, A.; Fernández-Gómez, J.M.; Ciudin, A.; Castro-Díaz, D.; Rodríguez-Antoln, A.; Carballido-Rodríguez, J.; et al. Efficacy and safety of the hexanic extract of Serenoa repens vs. watchful waiting in men with moderate to severe LUTS-BPH: Results of a paired matched clinical study. J. Clin. Med. 2022, 11, 967. [Google Scholar] [CrossRef]
- European Medicines Agency: Assessment Report on Serenoa Repens (W. Bartram) Small, Fructus. Final 2015. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-serenoa-repens-w-bartram-small-fructus_en.pdf (accessed on 17 October 2022).
- Blair, H.A. Hexanic extract of serenoa repens (permixon): A review in symptomatic benign prostatic hyperplasia. Drugs Aging 2022, 39, 235–243. [Google Scholar] [CrossRef]
- Giulianelli, R.; Pecoraro, S.; Sepe, G.; Leonardi, R.; Gentile, B.C.; Albanesi, L.; Brunori, S.; Mavilla, L.; Pisanti, F.; Giannella, R.; et al. Multicentre study on the efficacy and tolerability of an extract of Serenoa repens in patients with chronic benign prostate conditions associated with inflammation. Arch. Ital. Urol. Androl. 2012, 84, 94–98. [Google Scholar] [PubMed]
- Latil, A.; Pétrissans, M.T.; Rouquet, J.; Robert, G.; de la Taille, A. Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 2015, 75, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Gravas, S.; Samarinas, M.; Zacharouli, K.; Karatzas, A.; Tzortzis, V.; Koukoulis, G.; Melekos, M. The effect of hexanic extract of Serenoa repens on prostatic inflammation: Results from a randomized biopsy study. World J. Urol. 2019, 37, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Samarinas, M.; Karatzas, A.; Tzortzis, V.; Gravas, S. The clinical impact of hexanic extract of serenoa repens in men with prostatic inflammation: A post hoc analysis of a randomized biopsy study. J. Clin. Med. 2020, 9, 957. [Google Scholar] [CrossRef]
- Hizli, F.; Uygur, M.C. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int. Urol. Nephrol. 2007, 39, 879–886. [Google Scholar] [CrossRef]
- Ryu, Y.W.; Lim, S.W.; Kim, J.H.; Ahn, S.H.; Choi, J.D. Comparison of tamsulosin plus serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia in Korean men: 1-year randomized open label study. Urol. Int. 2015, 94, 187–193. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Cazzaniga, W.; Pederzoli, F.; Moretti, D.; Dehò, F.; Montanari, E.; Montorsi, F.; Salonia, A. Clinically meaningful improvements in LUTS/BPH severity in men treated with silodosin plus hexanic extract of Serenoa repens or silodosin alone. Sci. Rep. 2017, 7, 15179. [Google Scholar] [CrossRef]
- Alcaraz, A.; Rodríguez-Antolín, A.; Carballido-Rodríguez, J.; Castro-Díaz, D.; Esteban-Fuertes, M.; Cózar-Olmo, J.M.; Ficarra, V.; Medina-López, R.; Fernández-Gómez, J.M.; Angulo, J.C.; et al. Clinical benefit of tamsulosin and the hexanic extract of serenoa repens, in combination or as monotherapy, in patients with moderate/severe LUTS-BPH: A subset analysis of the QUALIPROST study. J. Clin. Med. 2020, 9, 2909. [Google Scholar] [CrossRef]
- Alcaraz, A.; Gacci, M.; Ficarra, V.; Medina-Polo, J.; Salonia, A.; Fernandez-Gomez, J.M.; Ciudin, A.; Castro-Diaz, D.; Rodriguez-Antolin, A.; Carballido-Rodriguez, J.; et al. Efficacy and tolerability of 6-month treatment with tamsulosin plus the hexanic extract of serenoa repens versus tamsulosin plus 5-alpha-reductase inhibitors for moderate-to-severe LUTS-BPH patients: Results of a paired matched clinical study. J. Clin. Med. 2022, 11, 3615. [Google Scholar] [CrossRef]
- Gacci, M.; Ficarra, V.; Sebastianelli, A.; Corona, G.; Serni, S.; Shariat, S.F.; Maggi, M.; Zattoni, F.; Carini, M.; Novara, G. Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex. Med. 2014, 11, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Tirabassi, G.; Santi, D.; Maseroli, E.; Gacci, M.; Dicuio, M.; Sforza, A.; Mannucci, E.; Maggi, M. Sexual dysfunction in subjects treated with inhibitors of 5alpha-reductase for benign prostatic hyperplasia: A comprehensive review and meta-analysis. Andrology 2017, 5, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Favilla, V.; Russo, G.I.; Privitera, S.; Castelli, T.; Giardina, R.; Calogero, A.E.; Condorelli, R.A.; La Vignera, S.; Cimino, S.; Morgia, G. Impact of combination therapy 5-alpha reductase inhibitors (5-ARI) plus alpha-blockers (AB) on erectile dysfunction and decrease of libido in patients with LUTS/BPH: A systematic review with meta-analysis. Aging Male 2016, 19, 175–181. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Siami, P.; Barkin, J.; Damião, R.; Major-Walker, K.; Nandy, I.; Morrill, B.B.; Gagnier, R.P.; Montorsi, F. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT Study. Eur. Urol. 2010, 57, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hennenberg, M.; Stief, C.G.; Gratzke, C. Prostatic alpha1-adrenoceptors: New concepts of function, regulation, and intracellular signaling. Neurourol. Urodyn. 2014, 33, 1074–1085. [Google Scholar] [CrossRef]
- Tamalunas, A.; Wendt, A.; Springer, F.; Vigodski, V.; Ciotkowska, A.; Rutz, B.; Wang, R.; Huang, R.; Liu, Y.; Schulz, H.; et al. Permixon®, hexane-extracted Serenoa repens, inhibits human prostate and bladder smooth muscle contraction and exerts growth-related functions in human prostate stromal cells. Life Sci. 2022, 308, 120931. [Google Scholar] [CrossRef] [PubMed]
- Malde, S.; Umbach, R.; Wheeler, J.R.; Lytvyn, L.; Cornu, J.N.; Gacci, M.; Gratzke, C.; Herrmann, T.R.W.; Mamoulakis, C.; Rieken, M.; et al. A systematic review of patients’ values, preferences, and expectations for the diagnosis and treatment of male lower urinary tract symptoms. Eur. Urol. 2021, 79, 796–809. [Google Scholar] [CrossRef]
- Perry, R.; Milligan, G.; Anderson, P.; Gillon, A.; White, M. Real-world use of Permixon® in benign prostatic hyperplasia-determining appropriate monotherapy and combination treatment. Adv. Ther. 2012, 29, 538–550. [Google Scholar] [CrossRef]



| Study | Study Design | Arms (n) | Mean Age at Baseline (SD) | Follow Up | Change in IPSS from Baseline, Mean (SD) | Change in QoL Score, Mean (SD) | Change in Qmax mL/s, Mean (SD) |
|---|---|---|---|---|---|---|---|
| Hizli and Uygur (2007) [38] | Prospective, randomised | nHESr (20) | 56.8 (7.8) | 6 mo | 6.1 (2.7) | 2.6 (0.9) | 3.2 (2.2) |
| Tam (20) | 58.9 (5.7) | 6 mo | 4.6 (3.3) | 2.1 (0.8) | 3.7 (2.6) | ||
| Tam + nHESr (20) | 60.2 (6.3) | 6 mo | 4.9 (2.3) | 2.2 (1.0) | 4.2 (2.5) | ||
| Ryu et al. (2015) [39] | Prospective, randomised, open-label | Tam (53) | 63.4 (1.4) | 6 mo | 4.4 (0.4) | 2.0 (0.3) | 1.8 (0.2) |
| 12 mo | 5.5 (0.5) | 2.5 (0.4) | 2.0 (0.3) | ||||
| Tam + HESr (50) | 62.5 (1.2) | 6 mo | 4.7 (0.3) | 1.9 (0.2) | 2.0 (0.3) | ||
| 12 mo | 5.8 (0.4) | 2.4 (0.4) | 2.1 (0.3) | ||||
| Boeri et al. (2017) [40] | Retrospective, non-randomised, cross-sectional | Sil (93) | 57.9 (11.3) | 13.5 mo | 3.2 (0.6) | 0.2 (0.2) | 2.3 (0.4) |
| Sil + HESr (93) | 55.3 (12.2) | 13.5 mo | 6.4 (0.6) | 1.0 (0.2) | 4.3 (0.5) | ||
| Alcaraz et al. (2020) [41] | Retrospective, non-randomised, open-label | HESr (262) | 64.6 (8.9) | 6 mo | 5.4 (4.6) | 1.3 (1.3) | 3.1 (4.2) |
| Tam (263) | 65.4 (8.0) | 6 mo | 5.7 (4.3) | 1.3 (1.2) | 2.9 (3.8) | ||
| Tam + HESr (184) | 65.1 (8.0) | 6 mo | 7.2 (5.0) | 1.8 (1.2) | 2.0 (2.8) | ||
| Samarinas et al. (2020) [37] | Post hoc, randomised, blinded | Control (25) | 68.7 (NR) | 6 mo | 1.1 (NR) | NR | 1.5 (NR) |
| HESr (25) | 71.4 (NR) | 6 mo | 3.4 (NR) | NR | 0.3 (NR) | ||
| α-blocker (23) | 68.7 (NR) | 6 mo | 0.2 (NR) | NR | 0.2 (NR) | ||
| α-blocker +HESr (24) | 71.4 (NR) | 6 mo | 2.0 (NR) | NR | 0.3 (NR) | ||
| Alcaraz et al. (2022) [42] | Retrospective, paired matched | Tam + HESr (68) | 67.9 (7.9) | 6 mo | 6.7 (5.0) | 1.7 (1.2) | 1.6 (1.7) * |
| Tam + 5-ARI (68) | 68.3 (7.3) | 6 mo | 7.7 (6.3) | 1.7 (1.3) | 2.2 (5.6) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nunzio, C.; Salonia, A.; Gacci, M.; Ficarra, V. The Role of Combination Therapy with α-Blockers and Hexanic Extract of Serenoa repens in the Treatment of LUTS/BPH. J. Clin. Med. 2022, 11, 7169. https://doi.org/10.3390/jcm11237169
De Nunzio C, Salonia A, Gacci M, Ficarra V. The Role of Combination Therapy with α-Blockers and Hexanic Extract of Serenoa repens in the Treatment of LUTS/BPH. Journal of Clinical Medicine. 2022; 11(23):7169. https://doi.org/10.3390/jcm11237169
Chicago/Turabian StyleDe Nunzio, Cosimo, Andrea Salonia, Mauro Gacci, and Vincenzo Ficarra. 2022. "The Role of Combination Therapy with α-Blockers and Hexanic Extract of Serenoa repens in the Treatment of LUTS/BPH" Journal of Clinical Medicine 11, no. 23: 7169. https://doi.org/10.3390/jcm11237169
APA StyleDe Nunzio, C., Salonia, A., Gacci, M., & Ficarra, V. (2022). The Role of Combination Therapy with α-Blockers and Hexanic Extract of Serenoa repens in the Treatment of LUTS/BPH. Journal of Clinical Medicine, 11(23), 7169. https://doi.org/10.3390/jcm11237169