Left Ventricular Diastolic Function in Subjects Conceived through Assisted Reproductive Technologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Medical History, Physical Examination, Pregnancy and Birth, and Level of Education
2.3. Echocardiographic Assessment of Left Ventricular Diastolic Function
2.3.1. Left Ventricular Dimensions
2.3.2. Mitral Inflow Velocities
2.3.3. Myocardial Peak Velocities
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Left Ventricular Diastolic Function
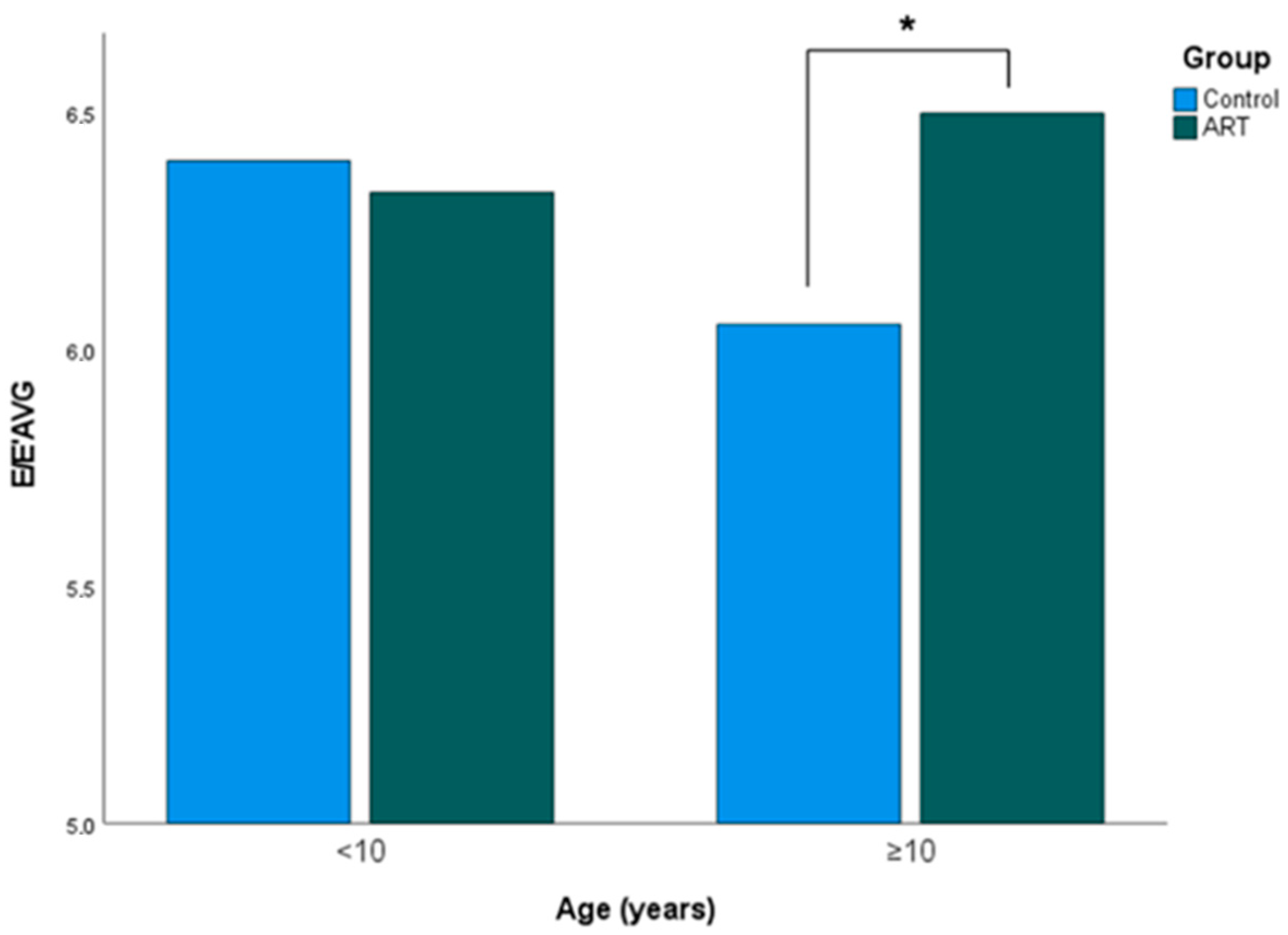
3.3. Influence of Age
4. Discussion
4.1. ART and Cardiovascular Morbidity: Pathophysiological Considerations and a Review of the Literature
4.1.1. Previous Cardiovascular Findings
4.1.2. Fetal Programming and Perinatal Risk Factors
4.1.3. ART and Left Ventricular Diastolic Function
4.2. Strengths and Limitations
4.2.1. Study Design
4.2.2. Methodology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; de Mouzon, J.; Ishihara, O.; Mansour, R.; Nygren, K.; Sullivan, E.; Vanderpoel, S.; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil. Steril. 2009, 92, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 2, 366. [Google Scholar] [CrossRef] [PubMed]
- Calhaz-Jorge, C.; De Geyter, C.H.; Kupka, M.S.; Wyns, C.; Mocanu, E.; Motrenko, T.; Scaravelli, G.; Smeenk, J.; Vidakovic, S.; Goossens, V. Survey on ART and IUI: Legislation, regulation, funding and registries in European countries: The European IVF-monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). Hum. Reprod. Open 2020, 2020, hoz044. [Google Scholar] [CrossRef]
- Scherrer, U.; Rexhaj, E.; Allemann, Y.; Sartori, C.; Rimoldi, S.F. Cardiovascular dysfunction in children conceived by assisted reproductive technologies. Eur. Heart J. 2015, 36, 1583–1589. [Google Scholar] [CrossRef]
- Johnson, M.H. The problematic in-vitro embryo in the age of epigenetics. Reprod. Biomed. Online 2005, 10 (Suppl. 1), 88–96. [Google Scholar] [CrossRef]
- Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Cerny, D.; Von Arx, R.; Soria, R.; Germond, M.; Allemann, Y.; Scherrer, U. Vascular dysfunction in children conceived by assisted reproductive technologies: Underlying mechanisms and future implications. Swiss Med. Wkly. 2014, 144, w13973. [Google Scholar] [CrossRef]
- Meister, T.A.; Rimoldi, S.F.; Soria, R.; von Arx, R.; Messerli, F.H.; Sartori, C.; Scherrer, U.; Rexhaj, E. Association of Assisted Reproductive Technologies with Arterial Hypertension during Adolescence. J. Am. Coll. Cardiol. 2018, 72, 1267–1274. [Google Scholar] [CrossRef]
- Scherrer, U.; Rimoldi, S.F.; Rexhaj, E.; Stuber, T.; Duplain, H.; Garcin, S.; de Marchi, S.F.; Nicod, P.; Germond, M.; Allemann, Y.; et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation 2012, 125, 1890–1896. [Google Scholar] [CrossRef]
- Ceelen, M.; van Weissenbruch, M.M.; Vermeiden, J.P.; van Leeuwen, F.E.; Delemarre-van de Waal, H.A. Cardiometabolic differences in children born after in vitro fertilization: Follow-up study. J. Clin. Endocrinol. Metab. 2008, 93, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Liu, X.M.; Jin, L.; Wang, T.T.; Ullah, K.; Sheng, J.Z.; Huang, H.F. Cardiovascular and metabolic profiles of offspring conceived by assisted reproductive technologies: A systematic review and meta-analysis. Fertil. Steril. 2017, 107, 622–631.e5. [Google Scholar] [CrossRef]
- Frohlich, E.D.; Apstein, C.; Chobanian, A.V.; Devereux, R.B.; Dustan, H.P.; Dzau, V.; Fauad-Tarazi, F.; Horan, M.J.; Marcus, M.; Massie, B.; et al. The heart in hypertension. N. Engl. J. Med. 1992, 327, 998–1008. [Google Scholar] [CrossRef]
- Mawad, W.; Friedberg, M.K. The continuing challenge of evaluating diastolic function by echocardiography in children: Developing concepts and newer modalities. Curr. Opin. Cardiol. 2017, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lalande, S.; Johnson, B.D. Diastolic dysfunction: A link between hypertension and heart failure. Drugs Today 2008, 44, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef]
- Voigt, M.D.; Fusch, C.; Olbertz, D.; Hartmann, K.; Rochow, N.; Renken, C.; Schneider, K.T.M. Analyse des Neugeborenenkollektivs der Bundesrepublik Deutschland. Geburtshilfe und Frauenheilkd. 2006, 66, 956–970. [Google Scholar] [CrossRef]
- Kampmann, C.; Wiethoff, C.M.; Wenzel, A.; Stolz, G.; Betancor, M.; Wippermann, C.F.; Huth, R.G.; Habermehl, P.; Knuf, M.; Emschermann, T.; et al. Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 2000, 83, 667–672. [Google Scholar] [CrossRef]
- Diedenhofen, B.; Musch, J. cocor: A comprehensive solution for the statistical comparison of correlations. PLoS ONE 2015, 10, e0121945. [Google Scholar] [CrossRef]
- Chen, M.; Wu, L.; Zhao, J.; Wu, F.; Davies, M.J.; Wittert, G.A.; Norman, R.J.; Robker, R.L.; Heilbronn, L.K. Altered glucose metabolism in mouse and humans conceived by IVF. Diabetes 2014, 63, 3189–3198. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Alcaraz, B.; Crispi, F.; Bijnens, B.; Cruz-Lemini, M.; Creus, M.; Sitges, M.; Bartrons, J.; Civico, S.; Balasch, J.; Gratacos, E. Assisted reproductive technologies are associated with cardiovascular remodeling in utero that persists postnatally. Circulation 2013, 128, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- Tararbit, K.; Houyel, L.; Bonnet, D.; De Vigan, C.; Lelong, N.; Goffinet, F.; Khoshnood, B. Risk of congenital heart defects associated with assisted reproductive technologies: A population-based evaluation. Eur. Heart J. 2011, 32, 500–508. [Google Scholar] [CrossRef]
- Sakka, S.D.; Loutradis, D.; Kanaka-Gantenbein, C.; Margeli, A.; Papastamataki, M.; Papassotiriou, I.; Chrousos, G.P. Absence of insulin resistance and low-grade inflammation despite early metabolic syndrome manifestations in children born after in vitro fertilization. Fertil. Steril. 2010, 94, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Gkourogianni, A.; Kosteria, I.; Telonis, A.G.; Margeli, A.; Mantzou, E.; Konsta, M.; Loutradis, D.; Mastorakos, G.; Papassotiriou, I.; Klapa, M.I.; et al. Plasma metabolomic profiling suggests early indications for predisposition to latent insulin resistance in children conceived by ICSI. PLoS ONE 2014, 9, e94001. [Google Scholar] [CrossRef]
- von Arx, R.; Allemann, Y.; Sartori, C.; Rexhaj, E.; Cerny, D.; de Marchi, S.F.; Soria, R.; Germond, M.; Scherrer, U.; Rimoldi, S.F. Right ventricular dysfunction in children and adolescents conceived by assisted reproductive technologies. J. Appl. Physiol. 2015, 118, 1200–1206. [Google Scholar] [CrossRef]
- Norrman, E.; Petzold, M.; Gissler, M.; Spangmose, A.L.; Opdahl, S.; Henningsen, A.K.; Pinborg, A.; Tiitinen, A.; Rosengren, A.; Romundstad, L.B.; et al. Cardiovascular disease, obesity, and type 2 diabetes in children born after assisted reproductive technology: A population-based cohort study. PLoS Med. 2021, 18, e1003723. [Google Scholar] [CrossRef]
- Halliday, J.; Lewis, S.; Kennedy, J.; Burgner, D.P.; Juonala, M.; Hammarberg, K.; Amor, D.J.; Doyle, L.W.; Saffery, R.; Ranganathan, S.; et al. Health of adults aged 22 to 35 years conceived by assisted reproductive technology. Fertil. Steril. 2019, 112, 130–139. [Google Scholar] [CrossRef]
- Langer, M.; Li, P.; Vilsmaier, T.; Kramer, M.; Sciuk, F.; Kolbinger, B.; Jakob, A.; Rogenhofer, N.; Haas, N.A.; Dalla-Pozza, R.; et al. Subjects Conceived through Assisted Reproductive Technologies Display Normal Arterial Stiffness. Diagnostics 2022, 12, 2763. [Google Scholar] [CrossRef]
- Law, C.M.; de Swiet, M.; Osmond, C.; Fayers, P.M.; Barker, D.J.; Cruddas, A.M.; Fall, C.H. Initiation of hypertension in utero and its amplification throughout life. Br. Med. J. 1993, 306, 24–27. [Google Scholar] [CrossRef]
- Chen, M.; Heilbronn, L.K. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). J. Dev. Orig. Health Dis. 2017, 8, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Alcaraz, B.; Cruz-Lemini, M.; Rodriguez-Lopez, M.; Gonce, A.; Garcia-Otero, L.; Ayuso, H.; Sitges, M.; Bijnens, B.; Balasch, J.; Gratacos, E.; et al. Fetal cardiac remodeling in twin pregnancy conceived by assisted reproductive technology. Ultrasound Obstet. Gynecol. 2018, 51, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhao, M.; Zhang, Z.; Zhou, W.; Lv, J.; Hu, J.; Ma, J.; Fang, M.; Yang, L.; Magnussen, C.G.; et al. Assessment of Cardiovascular Health of Children Ages 6 to 10 Years Conceived by Assisted Reproductive Technology. JAMA Netw. Open 2021, 4, e2132602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, H.; Gu, H.T.; Cui, Y.G.; Zhao, N.N.; Chen, J.; Gao, L.; Zhang, Y.; Liu, J.Y. Association of cardiac development with assisted reproductive technology in childhood: A prospective single-blind pilot study. Cell. Physiol. Biochem. 2014, 34, 988–1000. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Gu, H.T.; Feng, Q.L.; Liu, J.Y.; Zhou, J.; Yan, F. Association between assisted reproductive technology and cardiac alteration at age 5 years. JAMA Pediatr. 2015, 169, 603–605. [Google Scholar] [CrossRef]
- Bi, W.; Xiao, Y.; Wang, X.; Cui, L.; Song, G.; Yang, Z.; Zhang, Y.; Ren, W. The association between assisted reproductive technology and cardiac remodeling in fetuses and early infants: A prospective cohort study. BMC Med. 2022, 20, 104. [Google Scholar] [CrossRef]
- Valenzuela-Alcaraz, B.; Serafini, A.; Sepulveda-Martinez, A.; Casals, G.; Rodriguez-Lopez, M.; Garcia-Otero, L.; Cruz-Lemini, M.; Bijnens, B.; Sitges, M.; Balasch, J.; et al. Postnatal persistence of fetal cardiovascular remodelling associated with assisted reproductive technologies: A cohort study. BJOG 2019, 126, 291–298. [Google Scholar] [CrossRef]
- Crispi, F.; Bijnens, B.; Figueras, F.; Bartrons, J.; Eixarch, E.; Le Noble, F.; Ahmed, A.; Gratacos, E. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 2010, 121, 2427–2436. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of well-being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1359–1366. [Google Scholar] [CrossRef]
- Lane, M.; Robker, R.L.; Robertson, S.A. Parenting from before conception. Science 2014, 345, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Kuijk, E.W.; Macklon, N.S. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction 2010, 139, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Market-Velker, B.A.; Fernandes, A.D.; Mann, M.R. Side-by-side comparison of five commercial media systems in a mouse model: Suboptimal in vitro culture interferes with imprint maintenance. Biol. Reprod. 2010, 83, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Rexhaj, E.; Paoloni-Giacobino, A.; Rimoldi, S.F.; Fuster, D.G.; Anderegg, M.; Somm, E.; Bouillet, E.; Allemann, Y.; Sartori, C.; Scherrer, U. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. J. Clin. Investig. 2013, 123, 5052–5060. [Google Scholar] [CrossRef]
- Jayet, P.Y.; Rimoldi, S.F.; Stuber, T.; Salmon, C.S.; Hutter, D.; Rexhaj, E.; Thalmann, S.; Schwab, M.; Turini, P.; Sartori-Cucchia, C.; et al. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 2010, 122, 488–494. [Google Scholar] [CrossRef]
- Yang, H.; Kuhn, C.; Kolben, T.; Ma, Z.; Lin, P.; Mahner, S.; Jeschke, U.; von Schonfeldt, V. Early Life Oxidative Stress and Long-Lasting Cardiovascular Effects on Offspring Conceived by Assisted Reproductive Technologies: A Review. Int. J. Mol. Sci. 2020, 21, 5175. [Google Scholar] [CrossRef]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Gaasch, W.H. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N. Engl. J. Med. 2004, 350, 1953–1959. [Google Scholar] [CrossRef]
- Agabiti-Rosei, E.; Muiesan, M.L. Hypertension and diastolic function. Drugs 1993, 46, 61–67. [Google Scholar] [CrossRef]
- Harrison, D.G.; Treasure, C.B.; Mugge, A.; Dellsperger, K.C.; Lamping, K.G. Hypertension and the coronary circulation. With special attention to endothelial regulation. Am. J. Hypertens. 1991, 4, 454S–459S. [Google Scholar] [CrossRef]
- Ohara, T.; Little, W.C. Evolving focus on diastolic dysfunction in patients with coronary artery disease. Curr. Opin. Cardiol. 2010, 25, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.D.; Carroll, E.P. Diastolic function in coronary artery disease. Herz 1991, 16, 1–12. [Google Scholar] [PubMed]
- Schubert, U.; Muller, M.; Abdul-Khaliq, H.; Norman, M. Preterm Birth Is Associated with Altered Myocardial Function in Infancy. J. Am. Soc. Echocardiogr. 2016, 29, 670–678. [Google Scholar] [CrossRef]
- Selamet Tierney, E.S.; Newburger, J.W.; Graham, D.; Baker, A.; Fulton, D.R.; Colan, S.D. Diastolic function in children with Kawasaki disease. Int. J. Cardiol. 2011, 148, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, N.; Breatnach, C.; Levy, P.T.; McCallion, N.; Franklin, O.; El-Khuffash, A. Early diastolic dysfunction and respiratory morbidity in premature infants: An observational study. J. Perinatol. 2018, 38, 1205–1211. [Google Scholar] [CrossRef]
- Nagueh, S.F. Left Ventricular Diastolic Function: Understanding Pathophysiology, Diagnosis, and Prognosis with Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 228–244. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Middleton, K.J.; Kopelen, H.A.; Zoghbi, W.A.; Quinones, M.A. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J. Am. Coll. Cardiol. 1997, 30, 1527–1533. [Google Scholar] [CrossRef]
- Mitsnefes, M.M.; Xu, Y.; Ng, D.K.; Hill, G.; Kimball, T.; Furth, S.L.; Warady, B.A. Diastolic Function and Ambulatory Hypertension in Children With Chronic Kidney Disease. Hypertension 2021, 78, 1347–1354. [Google Scholar] [CrossRef]
- Telles, F.; McNamara, N.; Nanayakkara, S.; Doyle, M.P.; Williams, M.; Yaeger, L.; Marwick, T.H.; Leeson, P.; Levy, P.T.; Lewandowski, A.J. Changes in the Preterm Heart From Birth to Young Adulthood: A Meta-analysis. Pediatrics 2020, 146, e20200146. [Google Scholar] [CrossRef] [PubMed]
- Azegami, T.; Uchida, K.; Tokumura, M.; Mori, M. Blood Pressure Tracking From Childhood to Adulthood. Front. Pediatr. 2021, 9, 785356. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.; Fovargue, L.; Tondel, K.; Porter, B.; Sieniewicz, B.; Gould, J.; Rinaldi, C.A.; Ismail, T.; Chiribiri, A.; Carr-White, G. The Emerging Role of Cardiac Magnetic Resonance Imaging in the Evaluation of Patients with HFpEF. Curr. Heart Fail. Rep. 2018, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
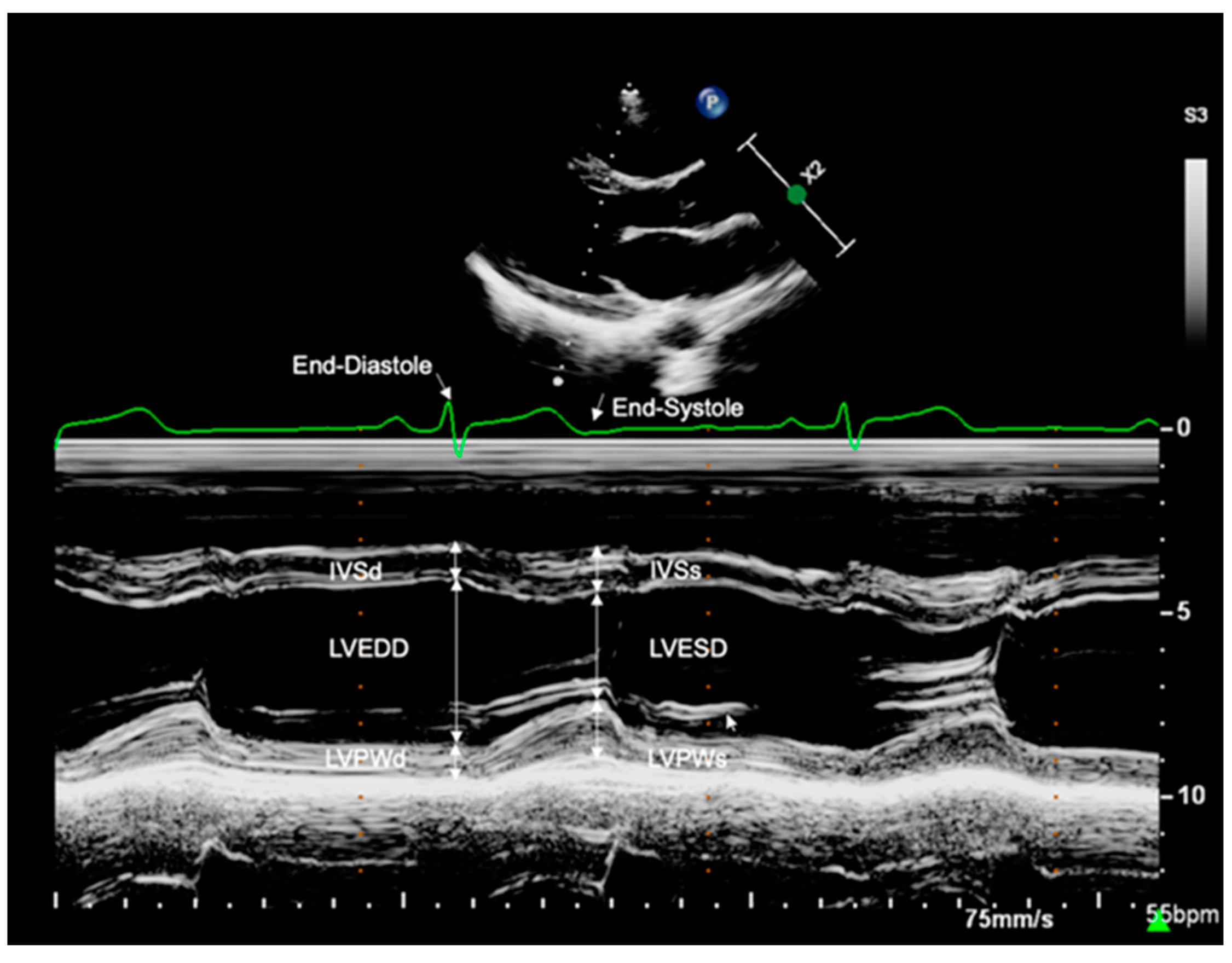
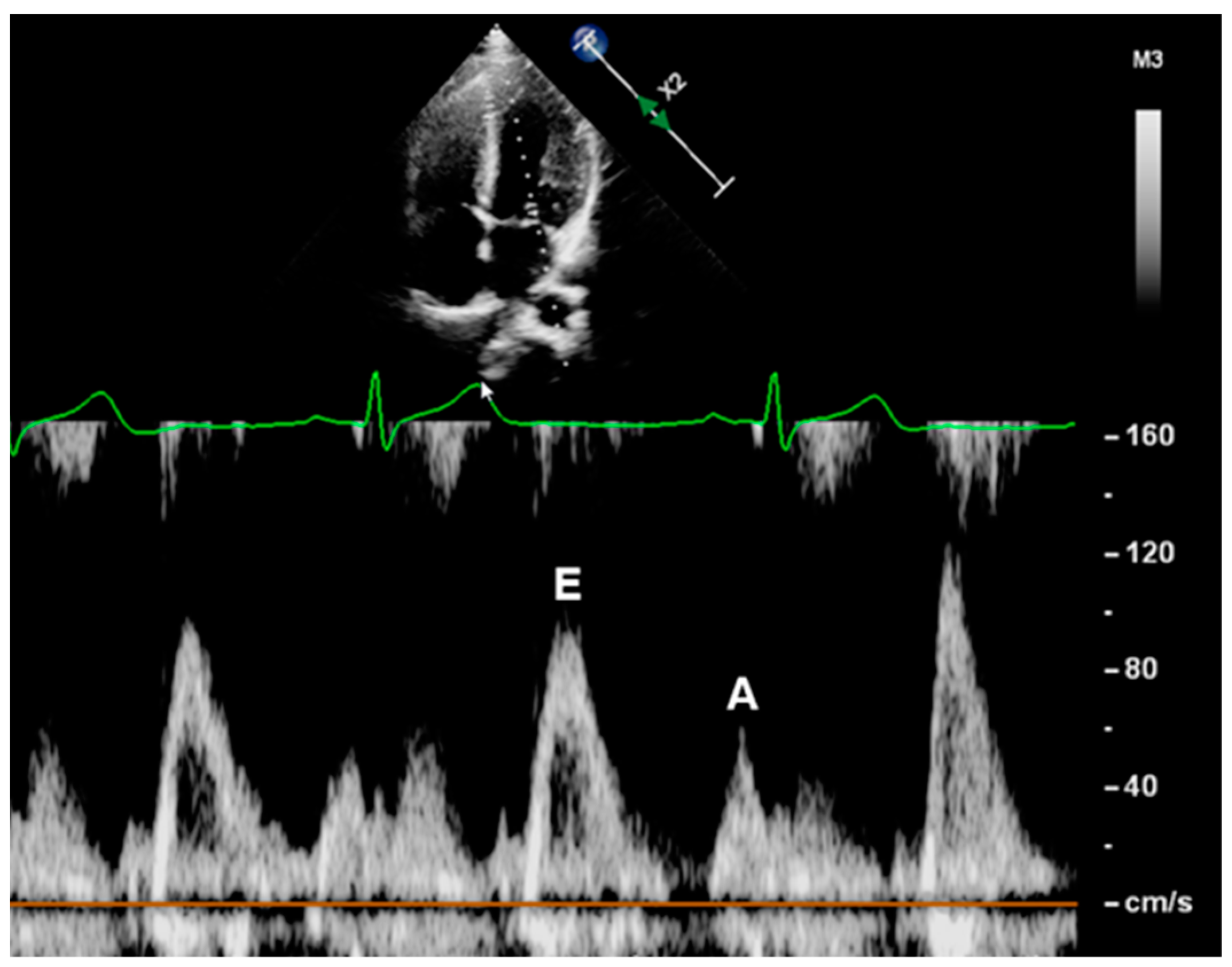
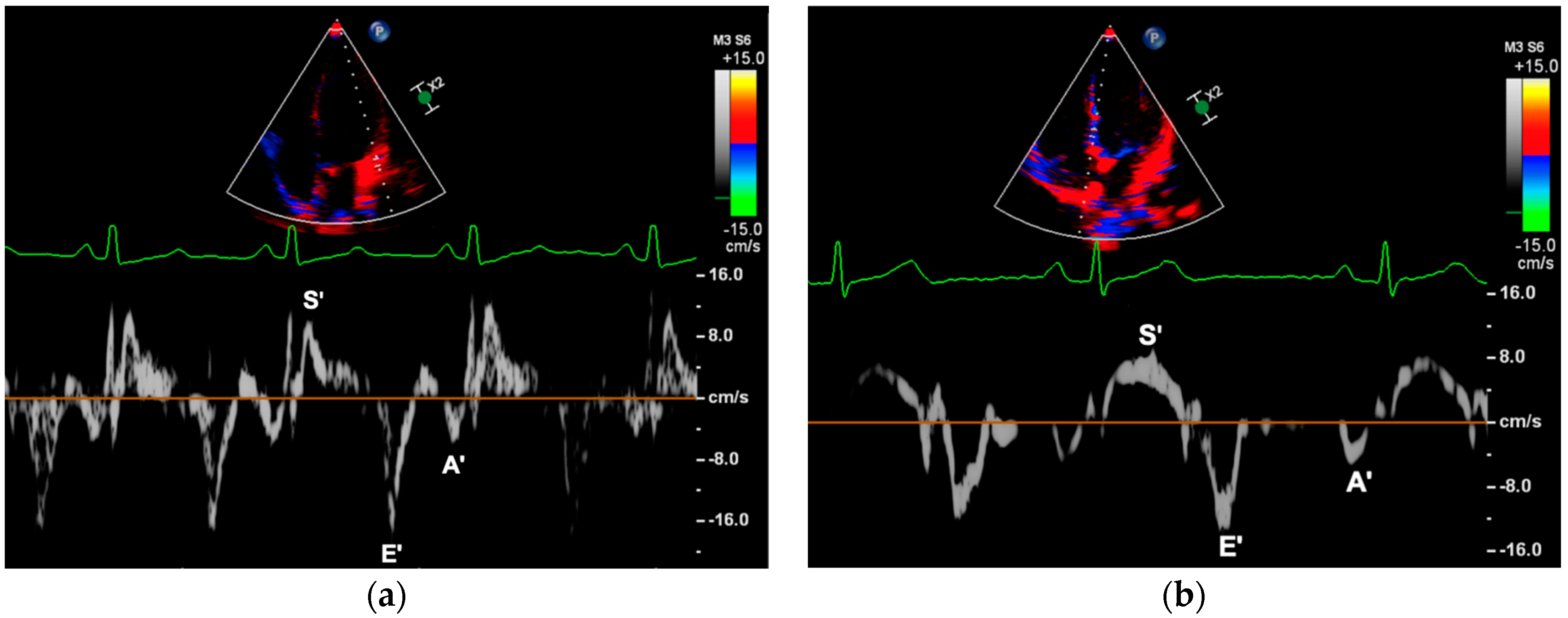
| Variable | Control (n = 83) | ART (n = 66) | p-Value |
|---|---|---|---|
| Age (years) | 13.25 ± 5.89 | 12.85 ± 5.80 | 0.677 |
| Female (n (%)) | 43 (51.81) | 38 (57.58) | 0.483 |
| Body weight (kg) | 44.68 ± 18.22 | 42.37 ± 19.29 | 0.454 |
| Body height (cm) | 151.92 ± 22.67 | 147.47 ± 21.49 | 0.225 |
| BMI (kg/m2) | 18.34 ± 3.26 | 18.27 ± 3.64 | 0.898 |
| Weight classification | 1.000 | ||
| Underweight (n (%)) | 6 (7.23) | 4 (6.06) | |
| Normal weight (n (%)) | 71 (85.54) | 57 (86.36) | |
| Overweight (n (%)) | 6 (7.23) | 5 (7.58) | |
| Obese (n (%)) | 0 (0) | 0 (0) | |
| BSA (m2) | 1.36 ± 0.38 | 1.30 ± 0.39 | 0.357 |
| SBP (mmHg) | 111.04 ± 11.33 | 110.92 ± 11.38 | 0.952 |
| DPB (mmHg) | 67.55 ± 7.73 | 67.82 ± 8.45 | 0.843 |
| Heart rate (bpm) | 72.60 ± 13.13 | 71.77 ± 12.34 | 0.695 |
| Smoking (n (%)) | 2 (2.41) | 3 (4.55) | 0.655 |
| Birth weight (g) 1 | 3393 ± 511 | 2786 ± 795 | <0.001 *** |
| Birth weight percentile 2 | 52.40 ± 23.80 | 38.75 ± 24.46 | 0.001 *** |
| Gestational age (weeks) 3 | 38.89 ± 1.81 | 36.84 ± 3.89 | <0.001 *** |
| Multiple pregnancy (n (%)) 4 | 3 (4.23) | 25 (43.86) | <0.001 *** |
| Maternal age at birth (years) 5 | 33.15 ± 4.10 | 35.37 ± 3.75 | <0.001 *** |
| Maternal BMI at conception (kg/m2) 6 | 22.03 ± 2.99 | 23.16 ± 4.26 | 0.130 |
| Gestational diabetes (n (%)) 7 | 3 (4.29) | 3 (5.36) | 1.000 |
| Maternal blood pressure duringpregnancy ≥ 140/90 mmHg (n (%)) 8 | 3 (6.67) | 0 (0) | 0.281 |
| Maternal educational level 9 | 5 (0–6) | 4 (1–5) | 0.207 |
| Study participants < 18 years of age | ||||
| Variable | Control (n = 63) | ART (n = 49) | Unadjusted p-value | Adjusted p-value a,b |
| IVSd z-score | 0.21 ± 1.02 | 0.24 ± 1.02 | 0.857 | >0.999 |
| IVSs z-score | 0.32 ± 0.78 | 0.10 ± 0.94 | 0.179 | 0.774 |
| LVEDD z-score | −0.43 ± 1.01 | −0.34 ± 1.05 | 0.628 | >0.999 |
| LVESD z-score | −0.24 ± 0.90 | 0.03 ± 0.91 | 0.130 | >0.999 |
| LVPWd z-score | 0.27 ± 0.82 | 0.46 ± 0.82 | 0.226 | 0.327 |
| LVPWs z-score | −0.21 ± 0.95 | −0.49 ± 0.92 | 0.118 | 0.771 |
| Study participants ≥ 18 years of age | ||||
| Variable | Control (n = 20) | ART (n = 17) | Unadjusted p-value | Adjusted p-value a,b |
| IVSd (mm) | 9.45 (6.70–12.00) | 9.00 (7.30–10.00) | 0.218 | 0.761 |
| IVSs (mm) | 12.22 ± 1.80 | 12.04 ± 1.39 | 0.739 | 0.921 |
| LVEDD (mm) | 45.70 ± 4.11 | 44.41 ± 2.96 | 0.289 | 0.714 |
| LVESD (mm) | 30.00 (25.00–35.00) | 29.00 (26.00–34.00) | 0.469 | 0.500 |
| LVPWd (mm) | 8.70 (6.90–13.00) | 8.90 (7.40–11.00) | 0.551 | 0.824 |
| LVPWs (mm) | 12.50 (9.80–17.00) | 12.00 (9.80–16.00) | 0.468 | >0.999 |
| Variable | Control (n = 83) | ART (n = 66) | Unadjusted p-Value | Adjusted p-Value a,b |
|---|---|---|---|---|
| Mitral Inflow Velocities | ||||
| E (cm/s) | 99.82 ± 13.86 | 98.91 ± 15.46 | 0.703 | >0.999 |
| A (cm/s) | 50.44 ± 10.48 | 47.87 ± 10.83 | 0.145 | 0.225 |
| E/A | 2.05 ± 0.48 | 2.16 ± 0.54 | 0.220 | 0.297 |
| Tissue Doppler Imaging | ||||
| S’RV (cm/s) 1 | 12.27 ± 1.78 | 12.67 ± 2.09 | 0.222 | 0.261 |
| E’RV (cm/s) 2 | 14.51 ± 2.92 | 14.62 ± 2.77 | 0.833 | >0.999 |
| A’RV (cm/s) 3 | 8.31 ± 2.09 | 8.63 ± 3.20 | 0.511 | 0.774 |
| S’LV (cm/s) 4 | 11.48 ± 2.33 | 10.99 ± 2.84 | 0.253 | >0.999 |
| E’LV (cm/s) | 20.67 ± 3.78 | 19.29 ± 3.29 | 0.020 * | 0.552 |
| A’LV (cm/s) | 7.68 ± 2.47 | 7.05 ± 2.00 | 0.095 | 0.894 |
| S’IVS (cm/s) | 7.62 ± 1.10 | 7.61 ± 1.17 | 0.972 | >0.999 |
| E’IVS (cm/s) | 13.68 ± 1.77 | 13.14 ± 1.93 | 0.076 | >0.999 |
| A’IVS (cm/s) 5 | 5.45 ± 1.25 | 5.03 ± 1.32 | 0.057 | 0.312 |
| E/E’LV | 4.96 ± 1.01 | 5.22 ± 0.95 | 0.099 | >0.999 |
| E/E’IVS | 7.40 ± 1.30 | 7.63 ± 1.28 | 0.279 | >0.999 |
| E/E’AVG | 6.18 ± 0.96 | 6.43 ± 0.89 | 0.105 | >0.999 |
| Blood work | ||||
| Log (NT-proBNP) 6 | 1.56 ± 0.38 | 1.59 ± 0.34 | 0.655 | >0.999 |
| Variable | Control (n = 53) | ART (n = 38) | Unadjusted p-Value | Adjusted p-Value a,b |
|---|---|---|---|---|
| E (cm/s) | 99.49 ± 14.32 | 100.69 ± 17.43 | 0.719 | >0.999 |
| A (cm/s) | 49.84 ± 11.18 | 51.16 ± 10.68 | 0.575 | 0.782 |
| E/A | 2.08 ± 0.50 | 2.03 ± 0.44 | 0.601 | 0.879 |
| E’LV (cm/s) | 21.27 ± 4.00 | 19.80 ± 3.39 | 0.068 | 0.632 |
| E’IVS (cm/s) | 13.88 ± 1.98 | 13.10 ± 2.27 | 0.084 | >0.999 |
| E/E’LV | 4.82 ± 1.10 | 5.18 ± 0.99 | 0.114 | >0.999 |
| E/E’IVS | 7.28 ± 1.33 | 7.82 ± 1.39 | 0.068 | >0.999 |
| E/E’AVG | 6.05 ± 0.99 | 6.50 ± 0.97 | 0.035 * | >0.999 |
| Variable | Control (n = 83) | ART (n = 66) | Zobs | ||
|---|---|---|---|---|---|
| r | p-Value | r | p-Value | ||
| LVEDD (mm) | 0.701 | <0.001 *** | 0.740 | <0.001 *** | −0.4822 |
| E (cm/s) | −0.132 | 0.235 | −0.006 | 0.961 | −0.7526 |
| A (cm/s) | −0.079 | 0.479 | 0.456 | <0.001 *** | −3.3923 |
| E/A | 0.030 | 0.786 | −0.427 | <0.001 *** | 2.8866 |
| E’LV (cm/s) | 0.248 | 0.024 * | 0.123 | 0.327 | 0.7697 |
| E’IVS (cm/s) | 0.067 | 0.546 | −0.071 | 0.574 | 0.8206 |
| E/E’LV | −0.346 | 0.001 *** | −0.164 | 0.188 | −1.1600 |
| E/E’IVS | −0.128 | 0.248 | 0.059 | 0.640 | −1.1148 |
| E/E’AVG | −0.236 | 0.032 * | −0.016 | 0.899 | −1.3330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciuk, F.; Vilsmaier, T.; Kramer, M.; Langer, M.; Kolbinger, B.; Li, P.; Jakob, A.; Rogenhofer, N.; Dalla-Pozza, R.; Thaler, C.; et al. Left Ventricular Diastolic Function in Subjects Conceived through Assisted Reproductive Technologies. J. Clin. Med. 2022, 11, 7128. https://doi.org/10.3390/jcm11237128
Sciuk F, Vilsmaier T, Kramer M, Langer M, Kolbinger B, Li P, Jakob A, Rogenhofer N, Dalla-Pozza R, Thaler C, et al. Left Ventricular Diastolic Function in Subjects Conceived through Assisted Reproductive Technologies. Journal of Clinical Medicine. 2022; 11(23):7128. https://doi.org/10.3390/jcm11237128
Chicago/Turabian StyleSciuk, Franziska, Theresa Vilsmaier, Marie Kramer, Magdalena Langer, Brenda Kolbinger, Pengzhu Li, André Jakob, Nina Rogenhofer, Robert Dalla-Pozza, Christian Thaler, and et al. 2022. "Left Ventricular Diastolic Function in Subjects Conceived through Assisted Reproductive Technologies" Journal of Clinical Medicine 11, no. 23: 7128. https://doi.org/10.3390/jcm11237128
APA StyleSciuk, F., Vilsmaier, T., Kramer, M., Langer, M., Kolbinger, B., Li, P., Jakob, A., Rogenhofer, N., Dalla-Pozza, R., Thaler, C., Haas, N. A., & Oberhoffer, F. S. (2022). Left Ventricular Diastolic Function in Subjects Conceived through Assisted Reproductive Technologies. Journal of Clinical Medicine, 11(23), 7128. https://doi.org/10.3390/jcm11237128






