Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score
Abstract
Highlights
- Pneumothorax/pneumomediastinum developed without positive pressure ventilation among COVID-19 patients had high fatality.
- Presence of comorbidity, bilateral pneumothorax, and fever were related with in-hospital mortality among COVID-19 associated spontaneous pneumothorax/pneumomediastinum patients
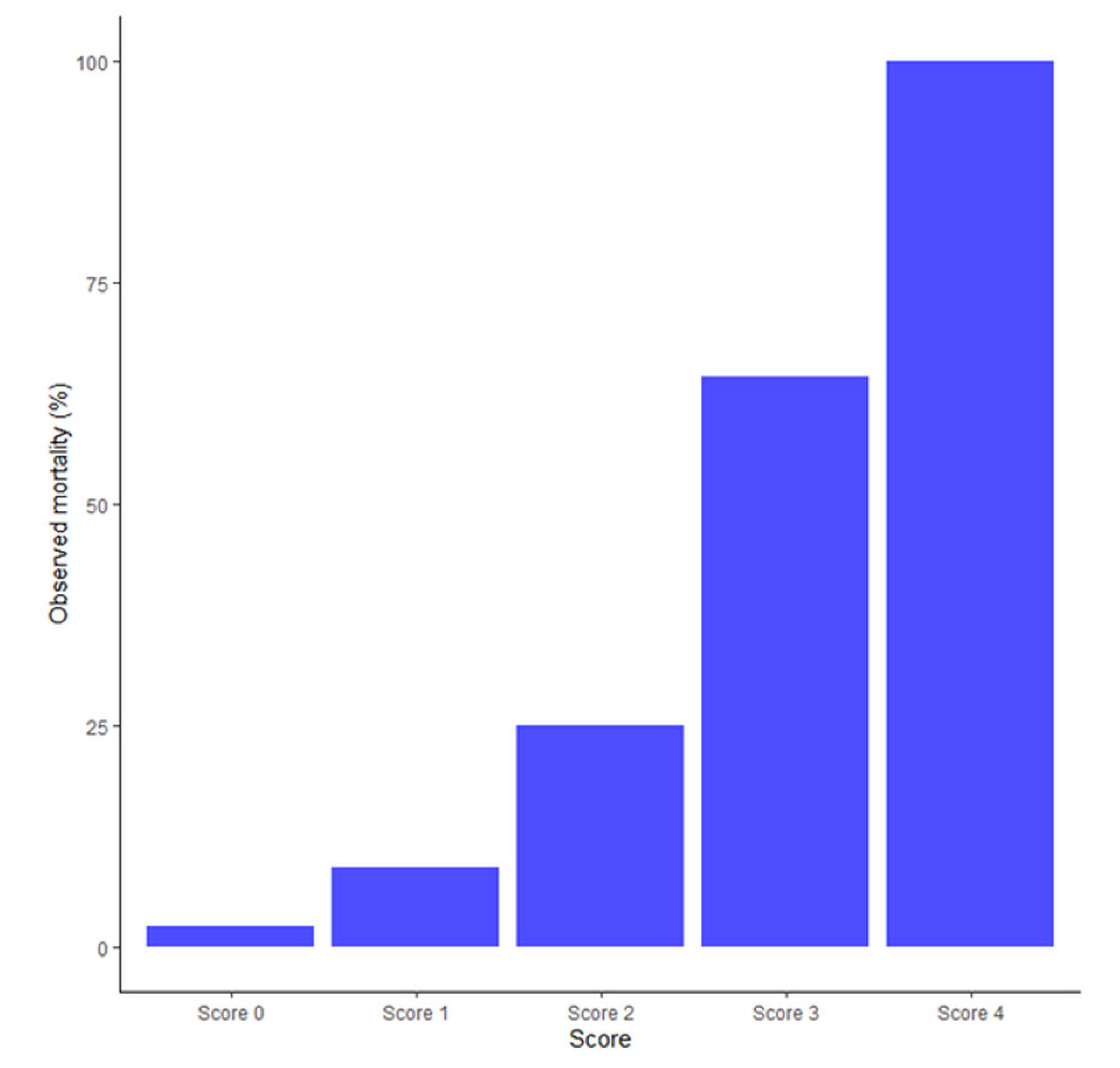
- The CoBiF score (Co = comorbidity, Bi = bilateral pneumothorax, F = fever) well-predicted the early mortality of these patients.
- The CoBiF score was validated in multinational cohorts, and it could improve early recognition and treatment of COVID-19 pneumothorax.
Abstract
1. Introduction
2. Patients and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Collection
3. Statistical Analysis
3.1. Step 1: Development and Internal Validation of the CoBiF Scoring System
3.2. Step 2: External Validation
4. Results
4.1. Demographics and Clinical Characteristics
4.2. Comparison between Pneumomediastinum and Pneumothorax ± Pneumomediastinum
4.3. Clinical Characteristics According to Clinical Scenarios
4.4. Patient Characteristics According to Mortality
4.5. Risk Factor Analysis for in-Hospital Mortality
4.6. The CoBiF Scoring System for in-Hospital Mortality
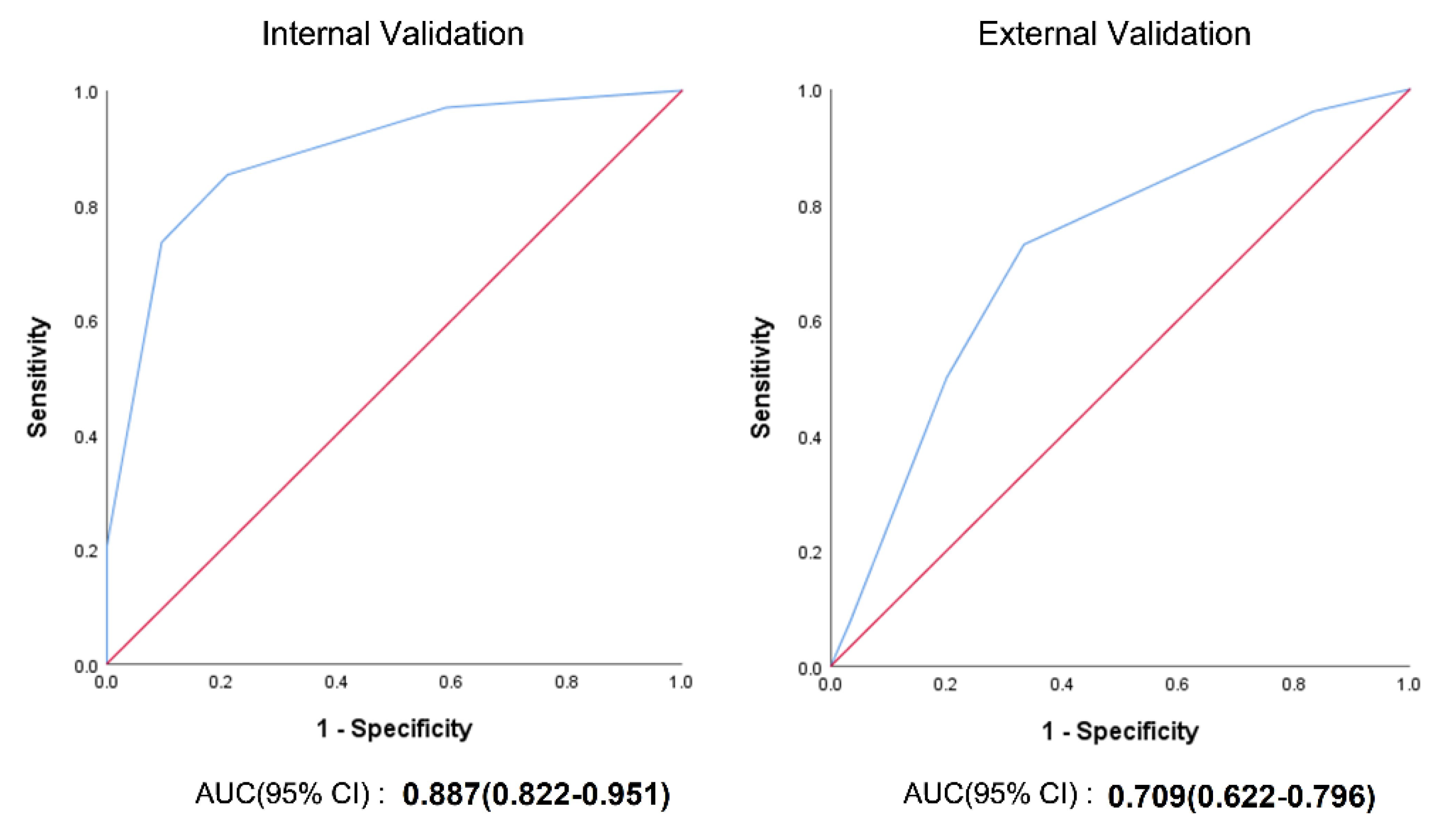
4.7. Internal and External Validation of the CoBiF Scoring System
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Coronavirus disease 2019 | COVID-19 |
| Pneumothorax | PNx |
| Pneumomediastinum | PMEx |
| invasive mechanical ventilation | IMV |
| International COVID-19 Pneumothorax Working Group | ICP-WG |
| Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols | PRISMA-P |
| computed tomography | CT |
| intensive care unit | ICU |
| odds ratio | OR |
| area under the curve | AUC |
| Statistical Package for the Social Sciences | SPSS |
| confidence interval | CI |
References
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Sihoe, A.D.; Wong, R.H.; Lee, A.T.; Lau, L.S.; Leung, N.Y.; Law, K.I.; Yim, A.P. Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest 2004, 125, 2345–2351. [Google Scholar] [CrossRef]
- Das, K.M.; Lee, E.Y.; Al Jawder, S.E.; Enani, M.A.; Singh, R.; Skakni, L.; Al-Nakshabandi, N.; AlDossari, K.; Larsson, S.G. Acute Middle East Respiratory Syndrome Coronavirus: Temporal Lung Changes Observed on the Chest Radiographs of 55 Patients. AJR Am. J. Roentgenol. 2015, 205, W267–W274. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, S.J.; Farrell, J.; Rostron, A.; Smith, I.; Openshaw, P.J.M.; Baillie, J.K.; Docherty, A.; Semple, M.G. COVID-19 pneumothorax in the UK: A prospective observational study using the ISARIC WHO clinical characterisation protocol. Eur. Respir. J. 2021, 58, 2100929. [Google Scholar] [CrossRef] [PubMed]
- Miró, Ò.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo-Putze, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberetchs, E.J.; Jacob, J.; Alquézar-Arbé, A.; et al. Frequency, Risk Factors, Clinical Characteristics, and Outcomes of Spontaneous Pneumothorax in Patients with Coronavirus Disease 2019: A Case-Control, Emergency Medicine-Based Multicenter Study. Chest 2021, 159, 1241–1255. [Google Scholar] [CrossRef]
- López Vega, J.M.; Parra Gordo, M.L.; Diez Tascón, A.; Ossaba Vélez, S. Pneumomediastinum and spontaneous pneumothorax as an extrapulmonary complication of COVID-19 disease. Emerg. Radiol. 2020, 27, 727–730. [Google Scholar] [CrossRef]
- McGuinness, G.; Zhan, C.; Rosenberg, N.; Azour, L.; Wickstrom, M.; Mason, D.M.; Thomas, K.M.; Moore, W.H. High Incidence of Barotrauma in Patients with COVID-19 Infection on Invasive Mechanical Ventilation. Radiology 2020, 202352. [Google Scholar] [CrossRef]
- Palumbo, D.; Campochiaro, C.; Belletti, A.; Marinosci, A.; Dagna, L.; Zangrillo, A.; De Cobelli, F. Pneumothorax/pneumomediastinum in non-intubated COVID-19 patients: Differences between first and second Italian pandemic wave. Eur. J. Intern. Med. 2021, 88, 144–146. [Google Scholar] [CrossRef]
- Lee, S.W. Methods for testing statistical differences between groups in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, e1. [Google Scholar] [CrossRef]
- Udwadia, Z.F.; Toraskar, K.K.; Pinto, L.; Mullerpatan, J.; Wagh, H.D.; Mascarenhas, J.M.; Gandhi, B.M.; Tripathi, A.; Sunavala, A.; Agrawal, U.; et al. Increased frequency of pneumothorax and pneumomediastinum in COVID-19 patients admitted in the ICU: A multicentre study from Mumbai, India. Clin. Med. 2021, 21, e615–e619. [Google Scholar] [CrossRef]
- Marza, A.M.; Petrica, A.; Lungeanu, D.; Sutoi, D.; Mocanu, A.; Petrache, I.; Mederle, O.A. Risk Factors, Characteristics, and Outcome in Non-Ventilated Patients with Spontaneous Pneumothorax or Pneumomediastinum Associated with SARS-CoV-2 Infection. Int. J. Gen. Med. 2022, 15, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Hamouri, S.; AlQudah, M.; Albawaih, O.; Al-zoubi, N.; Syaj, S. Spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema in non-ventilated COVID-19 patients. Future Sci OA 2022, 8, FSO771. [Google Scholar] [CrossRef] [PubMed]
- Akram, J.; Yousaf, Z.; Alabbas, Y.; Almoyaaf, M.I.A.; Ibrahim, A.S.S.; Kharma, N. Epidemiological and outcome analysis of COVID-19-associated pneumothorax: Multicentre retrospective critical care experience from Qatar. BMJ Open 2022, 12, e053398. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef]
- Estenssoro, E.; Loudet, C.I.; Ríos, F.G.; Kanoore Edul, V.S.; Plotnikow, G.; Andrian, M.; Romero, I.; Piezny, D.; Bezzi, M.; Mandich, V.; et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): A prospective, multicentre cohort study. Lancet Respir. Med. 2021, 9, 989–998. [Google Scholar] [CrossRef]
- Han, Y.J.; Lee, K.H.; Lee, J.-Y.; Kim, O.Y.; Moon, S.; Kim, S.; Ryu, S.; Lee, D.; Kim, J.Y.; Kim, T.; et al. Extrapulmonary clinical manifestations of COVID-19: An umbrella review of meta-analysis. Life Cycle 2022, 2, e6. [Google Scholar] [CrossRef]
- Sohrabi, M.-R.; Amin, R.; Maher, A.; Bahadorimonfared, A.; Janbazi, S.; Hannani, K.; Kolahi, A.-A.; Zali, A.-R. Sociodemographic determinants and clinical risk factors associated with COVID-19 severity: A cross-sectional analysis of over 200,000 patients in Tehran, Iran. BMC Infect. Dis. 2021, 21, 474. [Google Scholar] [CrossRef]
- Graf-Deuel, E.; Knoblauch, A. Simultaneous bilateral spontaneous pneumothorax. Chest 1994, 105, 1142–1146. [Google Scholar] [CrossRef]
- Sayar, A.; Turna, A.; Metin, M.; Küçükyağci, N.; Solak, O.; Gürses, A. Simultaneous bilateral spontaneous pneumothorax report of 12 cases and review of the literature. Acta Chir. Belg. 2004, 104, 572–576. [Google Scholar] [CrossRef]
- Lee, S.-C.; Cheng, Y.-L.; Huang, C.-W.; Tzao, C.; Hsu, H.-H.; Chang, H. Simultaneous bilateral primary spontaneous pneumothorax. Respirology 2008, 13, 145–148. [Google Scholar] [CrossRef]
- Huang, T.-W.; Cheng, Y.-L.; Tzao, C.; Hung, C.; Hsu, H.-H.; Chen, J.-C.; Lee, S.-C. Factors related to primary bilateral spontaneous pneumothorax. Thorac. Cardiovasc. Surg. 2007, 55, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Liu, X.; Zhang, H.; Li, Y.; Liu, J. Coronavirus Disease 2019 (COVID-19) CT Findings: A Systematic Review and Meta-analysis. J. Am. Coll. Radiol. 2020, 17, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Schalekamp, S.; Huisman, M.; Dijk RA van Boomsma, M.F.; Freire Jorge, P.J.; de Boer, W.S.; Herder, G.J.M.; Bonarius, M.; Groot, O.A.; Jong, E. Model-based Prediction of Critical Illness in Hospitalized Patients with COVID-19. Radiol. Radiol. Soc. North Am. 2021, 298, E46–E54. [Google Scholar] [CrossRef] [PubMed]
- Kanne, J.P.; Bai, H.; Bernheim, A.; Chung, M.; Haramati, L.B.; Kallmes, D.F.; Little, B.P.; Rubin, G.D.; Sverzellati, N. COVID-19 Imaging: What We Know Now and What Remains Unknown. Radiology 2021, 299, 204522. [Google Scholar] [CrossRef] [PubMed]
- Toussie, D.; Voutsinas, N.; Finkelstein, M.; Cedillo, M.A.; Manna, S.; Maron, S.Z.; Jacobi, A.; Chung, M.; Bernheim, A.; Eber, C.; et al. Clinical and Chest Radiography Features Determine Patient Outcomes in Young and Middle-aged Adults with COVID-19. Radiology 2020, 297, E197–E206. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.-M.M.; Elmahrouk, A.F.; Abdulatty, O.A. Alveolar air leakage in COVID-19 patients: Pneumomediastinum and/or pneumopericardium. Heart Lung 2020, 49, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Everden, S.; Zaki, I.; Trevelyan, G.; Briggs, J. COVID-19 pneumonitis and cystic lung disease, pneumothorax and pneumomediastinum. Thorax 2022, 77, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Konopka, K.E.; Nguyen, T.; Jentzen, J.M.; Rayes, O.; Schmidt, C.J.; Wilson, A.M.; Farver, C.F.; Myers, J.L. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD. Histopathology 2020, 77, 570–578. [Google Scholar] [CrossRef]
- Belletti, A.; Palumbo, D.; Zangrillo, A.; Fominskiy, E.V.; Franchini, S.; Dell’Acqua, A.; Marinosci, A.; Monti, G.; Vitali, G.; Colombo, S.; et al. Predictors of Pneumothorax/Pneumomediastinum in Mechanically Ventilated COVID-19 Patients. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3642–3651. [Google Scholar] [CrossRef]
- Ferrando, C.; Suarez-Sipmann, F.; Mellado-Artigas, R.; Hernández, M.; Gea, A.; Arruti, E.; Aldecoa, C.; Martínez-Pallí, G.; Martínez-González, M.A.; Slutsky, A.S.; et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020, 46, 2200–2211. [Google Scholar] [CrossRef]
- Amoah, K.; Gunasekaran, K.; Rahi, M.S.; Buscher, M.G. A case of secondary tension pneumothorax in COVID-19 pneumonia in a patient with no prior history of lung disease. SAGE Open Med. Case Rep. 2020, 8, 2050313X20967504. [Google Scholar] [CrossRef] [PubMed]
- Caviezel, C.; Weiss, L.; Haessig, G.; Alfaré, C.; Haberecker, M.; Varga, Z.; Frauenfelder, T.; Opitz, I. Case report of sequential bilateral spontaneous pneumothorax in a never-ventilated, lung-healthy COVID-19-patient. Int. J. Surg. Case Rep. 2020, 75, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.G.; Rapparini, C.; Gomes, B.M.; Pinto, L.A.C.; Freire, M.S.D.S.E. Pneumothorax as a late complication of COVID-19. Rev. Inst. Med. Trop Sao Paulo 2020, 62, e61. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.; Dincher, N.; Fazio, P.; Okorji, O.; Finkle, J.; Can, A. Coronavirus disease 2019 (COVID-19) complicated by Spontaneous Pneumomediastinum and Pneumothorax. Respir. Med. Case Rep. 2020, 31, 101232. [Google Scholar] [CrossRef] [PubMed]
- Kolani, S.; Houari, N.; Haloua, M.; Lamrani, Y.A.; Boubbou, M.; Serraj, M.; Aamara, B.; Maaroufi, M.; Alami, B. Spontaneous pneumomediastinum occurring in the SARS-CoV-2 infection. IDCases 2020, 21, e00806. [Google Scholar] [CrossRef] [PubMed]
- Mimouni, H.; Diyas, S.; Ouachaou, J.; Laaribi, I.; Oujidi, Y.; Merbouh, M.; Bkiyer, H.; Housni, B. Spontaneous Pneumomediastinum Associated with COVID-19 Pneumonia. Case Rep. Med. 2020, 2020, 4969486. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Boynton, E.J.; Naureen, S. COVID-19 with bilateral pneumothoraces- case report. Respir. Med. Case Rep. 2020, 31, 101254. [Google Scholar] [CrossRef]
- Rehman, T.; Josephson, G.; Sunbuli, M.; Chadaga, A.R. Spontaneous Pneumothorax in an Elderly Patient with Coronavirus Disease (COVID-19) Pneumonia. Ochsner. J. 2020, 20, 343–345. [Google Scholar] [CrossRef]
- Shan, S.; Guangming, L.; Wei, L.; Xuedong, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19: Case report and literature review. Rev. Inst. Med. Trop Sao Paulo 2020, 62, e76. [Google Scholar] [CrossRef]
- Ahluwalia, A.S.; Qarni, T.; Narula, N.; Sadiq, W.; Chalhoub, M.N. Bilateral pneumothorax as possible atypical presentation of coronavirus disease 2019 (COVID-19). Respir. Med. Case Rep. 2020, 31, 101217. [Google Scholar] [CrossRef]
- Wang, W.; Gao, R.; Zheng, Y.; Jiang, L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J. Travel. Med. 2020, 27, taaa062. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zeng, Y.; Xie, P.; Ye, X.; Xu, G.; Liu, J.; Wang, H.; Qian, J. COVID-19 with cystic features on computed tomography: A case report. Medicine 2020, 99, e20175. [Google Scholar] [CrossRef]
- Rohailla, S.; Ahmed, N.; Gough, K. SARS-CoV-2 infection associated with spontaneous pneumothorax. CMAJ 2020, 192, E510. [Google Scholar] [CrossRef] [PubMed]
- Bellini, D.; Lichtner, M.; Vicini, S.; Rengo, M.; Ambrogi, C.; Carbone, I. Spontaneous pneumomediastinum as the only CT finding in an asymptomatic adolescent positive for COVID-19. BJR Case Rep. 2020, 6, 20200051. [Google Scholar] [CrossRef] [PubMed]
- Flower, L.; Carter, J.-P.L.; Rosales Lopez, J.; Henry, A.M. Tension pneumothorax in a patient with COVID-19. BMJ Case Rep. 2020, 13, e235861. [Google Scholar] [CrossRef] [PubMed]
- Ucpinar, B.A.; Sahin, C.; Yanc, U. Spontaneous pneumothorax and subcutaneous emphysema in COVID-19 patient: Case report. J. Infect. Public Health 2020, 13, 887–889. [Google Scholar] [CrossRef]
- Goldman, N.; Ketheeswaran, B.; Wilson, H. COVID-19-associated pneumomediastinum. Clin. Med. (Lond) 2020, 20, e91–e92. [Google Scholar] [CrossRef]
- Wegner, U.; Jeffery, G.; Abrajan, O.; Sampablo, I.; Singh, C. Spontaneous Pneumomediastinum Associated With SARS-CoV-2: Infrequent Complication of the Novel Disease. Cureus 2020, 12, e9189. [Google Scholar] [CrossRef]
- Giné, C.; Laín, A.; García, L.; López, M. Thoracoscopic Bullectomy for Persistent Air Leak in a 14-Year-Old Child with COVID-19 Bilateral Pulmonary Disease. J. Laparoendosc. Adv. Surg. Tech. A 2020, 30, 935–938. [Google Scholar] [CrossRef]
- Khurram, R.; Johnson, F.T.F.; Naran, R.; Hare, S. Spontaneous tension pneumothorax and acute pulmonary emboli in a patient with COVID-19 infection. BMJ Case Rep. 2020, 13, e237475. [Google Scholar] [CrossRef]
- Kong, N.; Gao, C.; Xu, M.-S.; Xie, Y.-L.; Zhou, C.-Y. Spontaneous pneumomediastinum in an elderly COVID-19 patient: A case report. World J. Clin. Cases 2020, 8, 3573–3577. [Google Scholar] [CrossRef] [PubMed]
- Sonia, F.; Kumar, M. A Complication of Pneumothorax and Pneumomediastinum in a Non-Intubated Patient With COVID-19: A Case Report. Cureus 2020, 12, e10044. [Google Scholar] [CrossRef] [PubMed]
- Alhakeem, A.; Khan, M.M.; Al Soub, H.; Yousaf, Z. Case Report: COVID-19-Associated Bilateral Spontaneous Pneumothorax-A Literature Review. Am. J. Trop. Med. Hyg. 2020, 103, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Quincho-Lopez, A.; Quincho-Lopez, D.L.; Hurtado-Medina, F.D. Case Report: Pneumothorax and Pneumomediastinum as Uncommon Complications of COVID-19 Pneumonia-Literature Review. Am. J. Trop. Med. Hyg. 2020, 103, 1170–1176. [Google Scholar] [CrossRef]
- Yasukawa, K.; Vamadevan, A.; Rollins, R. Bulla Formation and Tension Pneumothorax in a Patient with COVID-19. Am. J. Trop. Med. Hyg. 2020, 103, 943–944. [Google Scholar] [CrossRef]
- Salah, O.; Faisal, M.; Alshahwani, I.; Elhiday, A. Bilateral Hemopneumothorax in COVID-19. Cureus 2020, 12, e10314. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, G.; Tang, Y.; Peng, Z.; Pan, H. The coronavirus diseases 2019 (COVID-19) pneumonia with spontaneous pneumothorax: A case report. BMC Infect. Dis. 2020, 20, 662. [Google Scholar] [CrossRef]
- Fahad, A.M.; Mohammad, A.A.; Al-Khalidi, H.A.; Alshewered, A.S. Spontaneous pneumothorax as a complication in COVID-19 male patient: A case report. Clin. Case Rep. 2020, 8, 3116–3119. [Google Scholar] [CrossRef]
- Fan, Q.; Pan, F.; Yang, L. Spontaneous pneumothorax and subpleural bullae in a patient with COVID-19: A 92-day observation. Eur. J. Cardiothorac. Surg. 2020, 58, 858–860. [Google Scholar] [CrossRef]
- Bellini, R.; Salandini, M.C.; Cuttin, S.; Mauro, S.; Scarpazza, P.; Cotsoglou, C. Spontaneous pneumothorax as unusual presenting symptom of COVID-19 pneumonia: Surgical management and pathological findings. J. Cardiothorac. Surg. 2020, 15, 310. [Google Scholar] [CrossRef]
- Hameed, M.; Jamal, W.; Yousaf, M.; Thomas, M.; Haq, I.U.; Ahmed, S.; Ahmad, M.; Khatib, M. Pneumothorax in COVID-19 Pneumonia: A case series. Respir. Med. Case Rep. 2020, 31, 101265. [Google Scholar] [CrossRef] [PubMed]
- Berhane, S.; Tabor, A.; Sahu, A.; Singh, A. Development of bullous lung disease in a patient with severe COVID-19 pneumonitis. BMJ Case Rep. 2020, 13, e237455. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Maron, S.; Cedillo, M.A.; Voutsinas, N.; Toussie, D.; Finkelstein, M.; Steinberger, S.; Chung, M.; Bernheim, A.; Eber, C.; et al. Spontaneous subcutaneous emphysema and pneumomediastinum in non-intubated patients with COVID-19. Clin. Imaging 2020, 67, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Perice, L.; Roit, Z.; Llovera, I.; Flanagan-Kundle, M.G. Spontaneous Pneumothorax as a Complication of COVID-19 Pneumonia: A Case Report. Clin. Pract. Cases Emerg. Med. 2020, 4, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Zayet, S.; Klopfenstein, T.; Mezher, C.; Gendrin, V.; Conrozier, T.; Ben Abdallah, Y. Coronavirus disease 2019 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema, France. New Microbes New Infect. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Singh, A.; Bass, J.; Lindner, D.H. Rare Complication of Pneumomediastinum and Pneumopericardium in a Patient with COVID-19 Pneumonia. Case Rep. Pulmonol. 2020, 2020, 8845256. [Google Scholar] [CrossRef]
- Yamaya, T.; Baba, T.; Hagiwara, E.; Ikeda, S.; Niwa, T.; Kitayama, T.; Murohashi, K.; Higa, K.; Sato, Y.; Ogura, T. Pneumothorax in a COVID-19 Pneumonia Patient without Underlying Risk Factors. Intern. Med. 2020, 59, 2921–2925. [Google Scholar] [CrossRef]
- Tucker, L.; Patel, S.; Vatsis, C.; Poma, A.; Ammar, A.; Nasser, W.; Mukkera, S.; Vo, M.; Khan, R.; Carlan, S. Pneumothorax and Pneumomediastinum Secondary to COVID-19 Disease Unrelated to Mechanical Ventilation. Case Rep. Crit. Care 2020, 2020, 6655428. [Google Scholar] [CrossRef]
- Ayazi, S.; Zebarjadi, J.; Grubic, A.D.; Tahmasbi, H.; Ayazi, K.; Jobe, B.A. Pneumothorax as the presenting manifestation of COVID-19. J. Thorac. Dis. 2020, 12, 7488–7493. [Google Scholar] [CrossRef]
- Brogna, B.; Bignardi, E.; Salvatore, P.; Alberigo, M.; Brogna, C.; Megliola, A.; Fontanella, G.; Mazza, E.M.; Musto, L. Unusual presentations of COVID-19 pneumonia on CT scans with spontaneous pneumomediastinum and loculated pneumothorax: A report of two cases and a review of the literature. Heart Lung 2020, 49, 864–868. [Google Scholar] [CrossRef]
- Spiro, J.E.; Sisovic, S.; Ockert, B.; Böcker, W.; Siebenbürger, G. Secondary tension pneumothorax in a COVID-19 pneumonia patient: A case report. Infection 2020, 48, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Urigo, C.; Soin, S.; Sahu, A. Spontaneous pneumomediastinum as a complication of a COVID-19 related pneumonia: Case report and review of literature. Radiol. Case Rep. 2020, 15, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- Sanivarapu, R.R.; Farraj, K.; Sayedy, N.; Anjum, F. Rapidly developing large pneumatocele and spontaneous pneumothorax in SARS-CoV-2 infection. Respir. Med. Case Rep. 2020, 31, 101303. [Google Scholar] [CrossRef] [PubMed]
- Vahidirad, A.; Jangjoo, A.; Ghelichli, M.; Arian Nia, A.; Zandbaf, T. Tension pneumothorax in patient with COVID-19 infection. Radiol. Case Rep. 2020, 16, 358–360. [Google Scholar] [CrossRef]
- Elhakim, T.S.; Abdul, H.S.; Pelaez Romero, C.; Rodriguez-Fuentes, Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19 pneumonia: A rare case and literature review. BMJ Case Rep. 2020, 13, e239489. [Google Scholar] [CrossRef]
- Afrazi, A.; Garcia-Rodriguez, S.; Maloney, J.D.; Morgan, C.T. Cavitary lung lesions and pneumothorax in a healthy patient with active coronavirus-19 (COVID-19) viral pneumonia. Interact. Cardiovasc. Thorac. Surg. 2020, 32, 150–152. [Google Scholar] [CrossRef]
- Capleton, P.; Ricketts, W.; Lau, K.; Ellis, S.; Sheaff, M.; Giaslakiotis, K.; Uys, S.; Tchrakian, N. Pneumothorax and Pneumatocoele Formation in a Patient with COVID-19: A Case Report. SN Compr. Clin. Med. 2021, 3, 269–272. [Google Scholar] [CrossRef]
- Tamaskani, N.; Khandashpour, M.; Livani, S. Can spontaneous pneumothorax be resolved in COVID-19 without hospital care? A case report. Caspian J. Intern. Med. 2021, 12, S368–S370. [Google Scholar]
- González-Pacheco, H.; Gopar-Nieto, R.; Jiménez-Rodríguez, G.-M.; Manzur-Sandoval, D.; Sandoval, J.; Arias-Mendoza, A. Bilateral spontaneous pneumothorax in SARS-CoV-2 infection: A very rare, life-threatening complication. Am. J. Emerg. Med. 2021, 39, 258.e1–258.e3. [Google Scholar] [CrossRef]
- Nobre Pereira, M.; Blanco, R.; Areias, V. Pneumomediastinum: An Uncommon Complication of COVID-19 Pneumonia. Arch. Bronconeumol. 2021, 57, 68. [Google Scholar] [CrossRef]
- Szewczyk, J.; Adkinson, B.C.; Akkineni, S.; Nguyen, D.M.; Arias, S.A.; Villamizar, N.R. Endobronchial valves: A bridge to definitive surgical management in COVID-19 recurrent pneumothorax. J. Thorac. Dis. 2021, 13, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, T.A.; Khan, A.A.; Mohiuddin, O.; Choudhry, M.S.; Yasmin, F.; Jalees, S. Spontaneous pneumomediastinum and subcutaneous emphysema in a non-intubated COVID-19 patient: A case report. Pan. Afr. Med. J. 2021, 38, 37. [Google Scholar] [CrossRef] [PubMed]
- Nunna, K.; Braun, A.B. Development of a large spontaneous pneumothorax after recovery from mild COVID-19 infection. BMJ Case Rep. 2021, 14, e238863. [Google Scholar] [CrossRef] [PubMed]
- Marza, A.M.; Petrica, A.; Buleu, F.N.; Mederle, O.A. Case Report: Massive Spontaneous Pneumothorax—A Rare Form of Presentation for Severe COVID-19 Pneumonia. Medicina 2021, 57, 82. [Google Scholar] [CrossRef]
- Janssen, J.; Kamps, M.J.A.; Joosten, T.M.B.; Barten, D.G. Spontaneous pneumomediastinum in a male adult with COVID-19 pneumonia. Am. J. Emerg. Med. 2021, 40, 228.e3–228.e5. [Google Scholar] [CrossRef]
- Nalewajska, M.; Feret, W.; Wojczyński, Ł.; Witkiewicz, W.; Wiśniewska, M.; Kotfis, K. Spontaneous Pneumothorax in COVID-19 Patients Treated with High-Flow Nasal Cannula outside the ICU: A Case Series. Int. J. Environ. Res. Public Health 2021, 18, 2191. [Google Scholar] [CrossRef]
- Doğan, B.İ.; Mahleç Anar, C.; Sertoğullarından, B.; Turan, M.O. Pne.eumomediastinum as a complication of COVID-19 disease: A case report. Tuberk Toraks 2021, 69, 94–97. [Google Scholar] [CrossRef]
- Rafiee, M.J.; Babaki Fard, F.; Samimi, K.; Rasti, H.; Pressacco, J. Spontaneous pneumothorax and pneumomediastinum as a rare complication of COVID-19 pneumonia: Report of 6 cases. Radiol. Case Rep. 2021, 16, 687–692. [Google Scholar] [CrossRef]
- Rashedi, S.; Mardani, M.; Fooladgar, M.; Aliannejad, R. Spontaneous pneumomediastinum, pneumopericardium, pneumothorax, and subcutaneous emphysema in a patient with COVID-19. Radiol. Case Rep. 2021, 16, 1158–1161. [Google Scholar] [CrossRef]
- Hamad, A.-M.M.; El-Saka, H.A. Post COVID-19 large pneumatocele: Clinical and pathological perspectives. Interact. Cardiovasc. Thorac. Surg. 2021, 33, ivab072. [Google Scholar] [CrossRef]
- Cancelliere, A.; Procopio, G.; Mazzitelli, M.; Lio, E.; Petullà, M.; Serapide, F.; Pelle, M.C.; Davoli, C.; Trecarichi, E.M.; Torti, C.; et al. A case report of pneumomediastinum in a COVID-19 patient treated with high-flow nasal cannula and review of the literature: Is this a “spontaneous” complication? Clin. Case Rep. 2021, 9, e04007. [Google Scholar] [CrossRef] [PubMed]
- Bosher, O.; Syed, M.A.; Bikmalla, S. Favourable outcome after a delayed complication secondary to COVID-19. BMJ Case Rep. 2021, 14, e241049. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.T.; Shah, F.; Perez-Corral, C. A case of spontaneous pneumomediastinum in a patient with severe SARS-CoV-2 and a review of the literature. SAGE Open Med. Case Rep. 2021, 9, 2050313X211010021. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, Z.; Brem, F.L.; Nasri, S.; Imane, S.; Noha, E.O. An uncommon presentation of COVID-19: Concomitant acute pulmonary embolism, spontaneous tension pneumothorax, pneumomediastinum and subcutaneous emphysema (a case report). Pan. Afr. Med. J. 2021, 39, 26. [Google Scholar] [CrossRef]
- Heijboer, F.; Oswald, L.; Cretier, S.; Braunstahl, G.-J. Pneumomediastinum in a patient with COVID-19 due to diffuse alveolar damage. BMJ Case Rep. 2021, 14, e242527. [Google Scholar] [CrossRef]
- Pimenta, I.; Varudo, R.; Lança, S.; Gonzalez, F.A. Exuberant spontaneous pneumothorax, pneumomediastinum, pneumopericardium and subcutaneous emphysema in COVID-19 pneumonia. BMJ Case Rep. 2021, 14, e243861. [Google Scholar] [CrossRef]
- Marzocchi, G.; Vassallo, A.; Monteduro, F. Spontaneous pneumothorax as a delayed complication after recovery from COVID-19. BMJ Case Rep. 2021, 14, e243578. [Google Scholar] [CrossRef]
- Buonsenso, D.; Gatto, A.; Graglia, B.; Rivetti, S.; Ferretti, S.; Paradiso, F.V.; Chiaretti, A. Early spontaneous pneumothorax, pneumomediastinum and pneumorrhachis in an adolescent with SARS-CoV-2 infection. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4413–4417. [Google Scholar]
- Cherian, A.; Jha, A.K.; Padala, S.R.A.N.; Senthilnathan, M. Unusual complications of spontaneous pneumomediastinum and subcutaneous emphysema in patients with SARS-CoV-2 infection: A case report. Indian J. Anaesth 2021, 65, 483–486. [Google Scholar]
- Montgomery, A.B.; Finck, C. Spontaneous hemopneumothorax in an adolescent with COVID-19. J. Pediatr. Surg. Case Rep. 2021, 69, 101852. [Google Scholar] [CrossRef]
- Essa, R.A.; Ahmed, S.K.; Bapir, D.H.; Abubakr, C.P. Subcutaneous emphysema and spontaneous pneumomediastinum in non-intubated COVID-19 patient: Presenting unusual case report. Int. J. Surg. Case Rep. 2021, 84, 106071. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Cegolon, L.; Masghsoudi, H.; Zhao, S.; Fathi, S.; Khedmat, L.; Javanbakht, M. Simultaneous Giant cavity pulmonary lesion and pneumothorax following COVID-19 pneumonia. Radiol. Case Rep. 2021, 16, 2534–2536. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.H.; Witkowski, A.; Clark, J.A.; Mata, A. A 17-Year-Old Girl with a Recent History of Marijuana Use Presented with Pneumomediastinum and Pneumopericardium and Tested Positive for SARS-CoV-2 Infection on Hospital Admission. Am. J. Case Rep. 2021, 22, e931800-1–e931800-5. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A. Tension pneumothorax complicating COVID-19 pneumonia. Clin. Case Rep. 2021, 9, e04342. [Google Scholar] [CrossRef]
- Jafari, R.; Cegolon, L.; Dehghanpoor, F.; Javanbakht, M.; Tabatabaei, S.M.H. Typical COVID-19 case with primary pneumomediastinum in a 37 year old male. Radiol. Case Rep. 2021, 16, 2286–2288. [Google Scholar] [CrossRef]
- Ramezani, R.; Jafari, F.; Fahami, Y.; Pakniyat, A.; Rad, M.G. A case report of pneumomediastinum and subcutaneous emphysema associated with pandemic COVID-19 in a 43-year-old man. Clin. Imaging 2021, 76, 74–76. [Google Scholar] [CrossRef]
- Protrka, M.R.; Ivanac, G.; Đudarić, L.; Vujević, F.; Brkljačić, B. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema: Radiological aspects of rare COVID-19 complications in 3 patients. Radiol. Case Rep. 2021, 16, 3237–3243. [Google Scholar] [CrossRef]
- Alavian, N.; Stephens, J.R.; DeWalt, D.A. Spontaneous Pneumomediastinum in a Patient with COVID-19 Pneumonia. J. Gen. Intern. Med. 2021, 36, 2845–2846. [Google Scholar] [CrossRef]
- Bozan, Ö.; Atiş, Ş.E.; Çekmen, B. A rare complication of Covid-19: Spontaneous pneumothorax following pneumomediastinum; case report. Am. J. Emerg. Med. 2021, 47, 342.e1–342.e2. [Google Scholar] [CrossRef]
- Hogan, G. COVID-19-Associated Pneumomediastinum: An Emerging Clinical Presentation. Cureus 2021, 13, e18287. [Google Scholar] [CrossRef]
- Kasturi, S.; Muthirevula, A.; Chinthareddy, R.R.; Lingaraju, V.C. Delayed recurrent spontaneous pneumothorax post-recovery from COVID-19 infection. Indian J. Thorac. Cardiovasc. Surg. 2021, 37, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Iuorio, A.; Nagar, F.; Attianese, L.; Grasso, A.; Torretta, G.; Fusco, P.; Ferrara, M.; Ferraro, F. Spontaneous Pneumomediastinum and Pneumothorax in Nonintubated COVID-19 Patients: A Multicenter Case Series. Am. J. Case Rep. 2021, 22, e933405. [Google Scholar] [CrossRef] [PubMed]
- S. Rashid Ali, M.R. The first reported use of autologous blood pleurodesis for treatment of prolonged air leak in COVID-19-related spontaneous pneumomediastinum and pneumothorax: A case report. Respirol. Case Rep. 2021, 9, e0840. [Google Scholar] [CrossRef] [PubMed]
- Komiya, K.; Hamanaka, R.; Shuto, H.; Yoshikawa, H.; Yokoyama, A.; Hiramatsu, K.; Kadota, J.-I. Re-expansion pulmonary edema following a pneumothorax drainage in a patient with COVID-19. BMC Pulm. Med. 2021, 21, 293. [Google Scholar] [CrossRef]
- Fantin, A.; Castaldo, N.; Vailati, P.; Morana, G.; Patruno, V. Full medical treatment of COVID-19 associated large pneumothorax—A case report. Monaldi Arch. Chest. Dis. 2021, 92. [Google Scholar] [CrossRef]
- Gutierrez-Ariza, J.C.; Rodriguez Yanez, T.; Martinez-Ávila, M.C.; Almanza Hurtado, A.; Dueñas-Castell, C. Pneumomediastinum and Pneumothorax Following Non-invasive Respiratory Support in Patients with Severe COVID-19 Disease. Cureus 2021, 13, e18796. [Google Scholar] [CrossRef]
- Shah, S.; Pokhrel, A.; Chamlagain, R.; Adhikari, Y.R.; Kandel, B.; Dhital, R.; Paudel, B.S.; Yadav, S. Case report of a spontaneous pneumothorax after the recovery from COVID-19 pneumonia: A delayed complication. Clin. Case Rep. 2021, 9, e04971. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, Y.; Tanaka, S.; Ike, A.; Tanaka, J.; Fujimi, S. Refractory pneumothorax secondary to COVID-19 treated by autologous blood patch pleurodesis. QJM 2021, hcab254. [Google Scholar] [CrossRef]
- Polistina, G.E.; Lanza, M.; Di Somma, C.; Annunziata, A.; Fiorentino, G. A Rare Evolution to Pneumopericardium in Patient with COVID-19 Pneumonia Treated with High Flow Nasal Cannula. Medicina (Kaunas) 2021, 57, 1122. [Google Scholar] [CrossRef]
- Ulutas, H.; Celik, M.R.; Gulcek, I.; Kalkan, M.; Agar, M.; Kilic, T.; Gulcek, E. Management of spontaneous pneumothorax in patients with COVID-19. Interact. CardioVascular Thorac. Surg. 2021, 34, ivab280. [Google Scholar] [CrossRef]
- Habib, M.B.; Mohammad Obeidat, I.; Ali, K.; Abdelrazek, M.; Mohamed, M.F.H. Bronchopleural fistula causing persistent pneumothorax in COVID-19 pneumonia patient with no risk factors. Clin. Case Rep. 2021, 9, e05128. [Google Scholar] [CrossRef] [PubMed]
- Panico, R.; Cai, J.; Butts, C.A.; To, J.Q. Understanding the course of COVID-19-induced pneumomediastinum. JAAPA 2021, 34, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Ufuk, F.; Yavas, H.G.; Kis, A. An unusual cause of spontaneous pneumothorax: Post-COVID-19 pulmonary fibrosis. Am. J. Emerg. Med. 2021, 49, 440.e5–440.e6. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.K.; Bonnier, A.; Chong, W.H.; Chenna, P. Successful use of endobronchial valve for persistent air leak in a patient with COVID-19 and bullous emphysema. BMJ Case Rep. 2021, 14, e246671. [Google Scholar] [CrossRef]
- Abbas, M.; Alibrahim, H.; Hasan, M.; Khouri, A.; Sawaf, B.; Swed, S. Subcutaneous emphysema and spontaneous pneumomediastinum in non-intubated COVID-19 patient: The first case report in Syria. Ann. Med. Surg. 2021, 72, 103074. [Google Scholar] [CrossRef]
- Al Armashi, A.R.; Somoza-Cano, F.J.; Patell, K.; Homeida, M.; Desai, O.; Al Zubaidi, A.; Altaqi, B.; Ravakhah, K. Spontaneous pneumomediastinum: A collaborative sequelae between COVID-19 and self-inflicted lung injury—A case report and literature review. Radiol. Case Rep. 2021, 16, 3655–3658. [Google Scholar] [CrossRef]
- Endres, F.; Spiro, J.E.; Bolt, T.A.; Tufman, A.; Ockert, B.; Helfen, T.; Gilbert, F.; Holzapfel, B.M.; Böcker, W.; Siebenbürger, G. One-year follow-up—Case report of secondary tension pneumothorax in a COVID-19 pneumonia patient. Infection 2022, 50, 525–529. [Google Scholar] [CrossRef]
- De Albuquerque, J.H.C.; da Silva, A.M.H.P.; de Almeida, T.Í.F.; Farias, L.A.B.G. COVID-19 and spontaneous pneumomediastinum: A rare complication. Rev. Soc. Bras. Med. Trop. 2022, 54, 525–529. [Google Scholar] [CrossRef]
- Mallick, T.; Dinesh, A.; Engdahl, R.; Sabado, M. COVID-19 Complicated by Spontaneous Pneumothorax. Cureus 2020, 12, e9104. [Google Scholar] [CrossRef]
- Natarajan, P.; Skidmore, J.; Aduroja, O.; Kunam, V.; Schuller, D. Bilateral pneumatoceles resulting in spontaneous bilateral pneumothoraces and secondary infection in a previously healthy man with COVID-19. Bayl. Univ. Med. Cent. Proc. 2021, 34, 590–592. [Google Scholar] [CrossRef]
- Sahagun, J.; Chopra, A.; David, A.G.; Dao, D.; Chittivelu, S. Secondary Spontaneous Pneumothorax in a COVID-19 Recovered Patient. Cureus 2021, 13, e16415. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Mohammadian, M.; Elkattawy, S.; Singh, Z.; Brescia, M.L. SARS-CoV-2 Associated with Pneumothorax: A Case Report and Literature Review. Cureus 2020, 12, e12191. [Google Scholar] [CrossRef] [PubMed]
| Factor | Total | PMEx | PNx ± PMEx | p-Value |
|---|---|---|---|---|
| n = 151 | n = 43 | n = 108 | ||
| Age, years | 56.0 [40.5, 67.5] | 54.0 [38.0, 64.5] | 57.5 [41.0, 68.0] | 0.340 |
| Sex | 0.462 | |||
| Female | 23 (15.2) | 8/43 (18.6) | 15/108 (13.9) | |
| Male | 128 (84.8) | 35/43 (81.4) | 93/108 (86.1) | |
| Comorbidity | ||||
| Comorbidity present | 80/147 (54.4) | 26/41 (63.4) | 54/106 (50.9) | 0.199 |
| Obesity | 17/143 (11.9) | 9/40 (22.5) | 8/103 (7.8) | 0.021 |
| Diabetes mellitus | 17/143 (11.9) | 5/40 (12.5) | 12/103 (11.7) | 1 |
| Hypertension | 39/143 (27.3) | 10/40 (25.0) | 29/103 (28.2) | 0.835 |
| Respiratory disease | 16/143 (11.2) | 3/40 (7.5) | 13/103 (12.6) | 0.557 |
| COVID-19-targeted treatments | ||||
| Steroid | 68/116 (58.6) | 25/35 (71.4) | 43/81 (53.1) | 0.100 |
| Convalescent plasma | 7/87 (8.0) | 0/29 (0.0) | 7/58 (12.1) | 0.090 |
| Intravenous immunoglobulin | 4/90 (4.4) | 2/30 (6.7) | 2/60 (3.3) | 0.598 |
| Lopinavir/ritonavir | 10/90 (11.1) | 4/30 (13.3) | 6/60 (10.0) | 0.726 |
| Remdesivir | 19/92 (20.7) | 7/30 (23.3) | 12/62 (19.4) | 0.784 |
| Tocilizumab | 13/90 (14.4) | 3/30 (10.0) | 10/60 (16.7) | 0.532 |
| Clinical manifestations | ||||
| Chest pain | 55/123 (44.7) | 11/34 (32.4) | 44/89 (49.4) | 0.107 |
| Cough | 45/134 (33.6) | 13/40 (32.5) | 32/94 (34.0) | 1 |
| Fever | 50/142 (35.2) | 1740 (42.5) | 33/102 (32.4) | 0.329 |
| Dyspnea | 114/136 (83.8) | 33/40 (82.5) | 81/96 (84.4) | 0.801 |
| Oxygen saturation (room air), % | 85.0 [80.0, 91.0] | 85.5 [81.8, 88.8] | 84.5 [80.0, 91.0] | 0.752 |
| Subcutaneous emphysema | 40/143 (28.0) | 13/42 (31.0) | 27/101 (26.7) | 0.683 |
| Chest CT findings | ||||
| Emphysema | 11/137 (8.0) | 5/36 (13.9) | 6/101 (5.9) | 0.157 |
| Ground-glass opacity | 106/130 (81.5) | 27/31 (87.1) | 79/99 (79.8) | 0.436 |
| Pleural effusion | 11/136 (8.1) | 2/35 (5.7) | 9/101 (8.9) | 0.728 |
| Treatments | ||||
| Conservative care | 73/135 (54.1) | 33/35 (94.3) | 40/100 (40.0) | <0.001 |
| Chest tube insertion | 82/131 (62.6) | 0/31 (0.0) | 82/100 (82.0) | <0.001 |
| Surgery | 8/139 (5.8) | 0/37 (0.0) | 8/102 (7.8) | 0.109 |
| Non-invasive ventilation | 9/134 (6.7) | 5/34 (14.7) | 4/100 (4.0) | 0.046 |
| Invasive mechanical ventilation | 30/145 (20.7) | 9/40 (22.5) | 21/105 (20.0) | 0.819 |
| Outcome | ||||
| Hospital stay, days | 13.0 [6.0, 19.0] | 14.0 [9.5, 15.8] | 13.0 [6.0, 19.0] | 0.975 |
| ICU admission | 51/138 (37.0) | 14/36 (38.9) | 37/102 (36.3) | 0.842 |
| Mortality | 35/151 (23.2) | 9/43 (20.9) | 26/108 (24.1) | 0.831 |
| Factor | Survivors | Non-Survivors | p-Value |
|---|---|---|---|
| n = 116 | n = 35 | ||
| Age, years | 52.5 [38.0, 65.0] | 67.0 [55.0, 75.0] | <0.001 |
| Sex | 0.597 | ||
| Female | 19/116 (16.4) | 4/35 (11.4) | |
| Male | 97/116 (83.6) | 31/35 (88.6) | |
| Comorbidity | |||
| Comorbidity present | 52/112 (46.4) | 28/35 (80.0) | <0.001 |
| Obesity | 8/108 (7.4) | 9/35 (25.7) | 0.007 |
| Diabetes mellitus | 11/108 (10.2) | 6/35 (17.1) | 0.366 |
| Hypertension | 23/108 (21.3) | 16/35 (45.7) | 0.008 |
| Respiratory disease | 13/108 (12.0) | 3/35 (8.6) | 0.761 |
| COVID-19 targeted treatments | |||
| Steroid | 45/88 (51.1) | 23/28 (82.1) | 0.004 |
| Convalescent plasma | 6/63 (9.5) | 1/24 (4.2) | 0.668 |
| Intravenous immunoglobulin | 4/66 (6.1) | 0/24 (0.0) | 0.570 |
| Lopinavir/ritonavir | 8/66 (12.1) | 2/24 (8.3) | 1 |
| Remdesivir | 10/68 (14.7) | 9/24 (37.5) | 0.037 |
| Tocilizumab | 11/66 (16.7) | 2/24 (8.3) | 0.501 |
| Hydroxychloroquine * | 20/67 (29.9) | 7/24 (29.2) | 1 |
| Clinical manifestations | |||
| Chest pain | 49/95 (51.6) | 6/28 (21.4) | 0.005 |
| Cough | 29/102 (28.4) | 16/32 (50.0) | 0.032 |
| Fatigue | 3/95 (3.2) | 3/28 (10.7) | 0.131 |
| Fever | 21/108 (19.4) | 29/34 (85.3) | <0.001 |
| Dyspnea | 83/104 (79.8) | 31/32 (96.9) | 0.026 |
| Oxygen saturation (room air), % | 85.0 [80.0, 91.0] | 84.0 [75.0, 89.0] | 0.275 |
| PNx/PMEx characteristics | |||
| Type of disease | 0.038 | ||
| PMEx | 34/116 (29.3) | 9/35 (25.7) | |
| PNx | 63/116 (54.3) | 13/35 (37.1) | |
| Both | 19/116 (16.4) | 13/35 (37.1) | |
| PNx location | 0.029 | ||
| Bilateral | 9/80 (11.2) | 8/22 (36.4) | |
| Left | 32/80 (40.0) | 7/22 (31.8) | |
| Right | 39/80 (48.8) | 7/22 (31.8) | |
| Tension PNx | 15/104 (14.4) | 3/29 (10.3) | 0.762 |
| Subcutaneous emphysema | 28/110 (25.5) | 12/33 (36.4) | 0.269 |
| Chest CT findings | |||
| Emphysema | 7/107 (6.5) | 4/30 (13.3) | 0.256 |
| Ground-glass opacity | 82/102 (80.4) | 24/28 (85.7) | 0.596 |
| Pleural effusion | 6/106 (5.7) | 5/30 (16.7) | 0.065 |
| Visible bullae | 15/106 (14.2) | 1/30 (3.3) | 0.195 |
| Treatments | |||
| Conservative care | 56/105 (53.3) | 17/30 (56.7) | 0.837 |
| Chest tube insertion | 65/104 (62.5) | 17/27 (63.0) | 1 |
| Surgery | 8/108 (7.4) | 0/31 (0.0) | 0.199 |
| Non-invasive ventilation | 6/104 (5.8) | 3/30 (10.0) | 0.418 |
| Invasive mechanical ventilation | 11/111 (9.9) | 19/34 (55.9) | <0.001 |
| Outcome | |||
| Chest tube indwelling time, days | 5.5 [3.0, 8.8] | 10.0 [6.0, 10.5] | 0.714 |
| Hospital stay, days | 12.0 [6.0, 25.0] | 14.0 [9.3, 18.0] | 0.938 |
| ICU admission | 23/107 (21.5) | 28/31 (90.3) | <0.001 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| Factor | OR (95% CI) | p-Value | OR (95% CI) | p-Value |
| Male (ref. Female) | 1.52 (0.48–4.80) | 0.477 | ||
| Comorbidity present | 4.35 (1.72–11.1) | 0.002 | 3.87 (1.27–11.9) | 0.018 |
| Diabetes mellitus | 1.82 (0.62–5.36) | 0.274 | ||
| Hypertension | 2.80 (1.19–6.57) | 0.018 | 1.22 (0.32–4.61) | 0.772 |
| Bilateral PNx | 3.67 (1.35–9.95) | 0.009 | 4.86 (1.08–21.9) | 0.039 |
| Tension PNx | 0.69 (0.18–2.55) | 0.572 | ||
| Subcutaneous emphysema | 1.67 (0.73–3.83) | 0.222 | ||
| Type of diseases (ref. PMEx) | ||||
| PNx | 0.78 (0.30–2.01) | 0.612 | ||
| PNx with PMEx | 2.58 (0.93–7.16) | 0.068 | ||
| Symptoms–fever | 24.0 (8.31–69.5) | <0.001 | 24.1 (7.75–74.6) | <0.001 |
| Symptoms–cough | 2.52 (1.11–5.69) | 0.026 | 1.13 (0.35–3.65) | 0.838 |
| Symptoms–dyspnea | 7.84 (1.01–60.8) | 0.049 | 3.25 (0.33–31.9) | 0.312 |
| CT findings–GGO | 1.46 (0.46–4.70) | 0.522 | ||
| CT findings–emphysema | 2.20 (0.60–8.08) | 0.236 | ||
| CT findings–pleural effusion | 3.33 (0.94–11.80) | 0.062 | ||
| Training Cohort ¶ | Validation Cohort ⁂ | p-Value | |
|---|---|---|---|
| Factor | n = 151 | n = 133 | |
| Age, years | 56.0 [40.5, 67.5] | 64.0 [51.0, 72.0] | 0.004 |
| Sex | <0.001 | ||
| Female | 23/151 (15.2) | 49/133 (36.8) | |
| Male | 128/151 (84.8) | 84/133 (63.2) | |
| Comorbidities present | 80/147 (54.4) | 101/133 (75.9) | <0.001 |
| Diabetes mellitus | 17/143 (11.9) | 36/133 (27.1) | 0.002 |
| Obesity | 17/143 (11.9) | 32/133 (24.1) | 0.011 |
| Hypertension | 39/143 (27.3) | 69/133 (51.9) | <0.001 |
| Respiratory diseases | 16/143 (11.2) | 19/133 (14.3) | 0.473 |
| Geographical area of data sources | <0.001 | ||
| Africa | 3/151 (2.0) | 0/133 (0.0) | |
| Asia | 41/151 (27.2) | 100/133 (75.2) | |
| Europe | 59/151 (39.1) | 33/133 (24.8) | |
| North America | 42/151 (27.8) | 0/133 (0.0) | |
| South America | 6/151 (4.0) | 0/133 (0.0) | |
| Clinical scenario | <0.001 | ||
| Initial presentation | 68/151 (45.0) | 56/133 (42.1) | |
| During hospitalization | 65/151 (43.9) | 77/133 (57.9) | |
| Recent recovery from COVID-19 | 18/151 (12.2) | 0/133 (0.0) | |
| Presenting symptom of PNx/PMEx | |||
| Chest pain | 55/123 (44.7) | 48/128 (37.5) | 0.251 |
| Cough | 45/134 (33.6) | 86/128 (67.2) | <0.001 |
| Dyspnea | 114/136 (83.8) | 106/128 (82.8) | 0.870 |
| Fever | 50/142 (35.2) | 76/133 (57.1) | <0.001 |
| Oxygen saturation at room air | 85.0 [80.0, 91.0] | 87.0 [80.0, 90.0] | 0.784 |
| Type of PNx/PMEx | 0.004 | ||
| PMEx alone | 43/151 (28.5) | 19/133 (14.3) | |
| PNx alone | 76/151 (50.3) | 69/133 (51.9) | |
| PNx + PMEx | 32/151 (21.2) | 45/133 (33.8) | |
| PNX/PMEx-related characteristics | |||
| Subcutaneous emphysema | 40/143 (28.0) | 62/133 (46.6) | 0.002 |
| Tension PNx | 18/133 (13.5) | 9/103 (8.7) | 0.305 |
| PNx location | 0.282 | ||
| Bilateral | 17/102 (16.7) | 26/107 (24.3) | |
| Left | 39/102 (38.2) | 43/107 (40.2) | |
| Right | 46/102 (45.1) | 38/107 (35.5) | |
| Radiological findings on chest CT | |||
| Visible Bullae | 16/136 (11.8) | 8/128 (6.2) | 0.137 |
| Ground-glass opacity | 106/130 (81.5) | 102/128 (79.7) | 0.754 |
| Pleural effusion | 11/136 (8.1) | 14/128 (10.9) | 0.529 |
| Treatment | |||
| Conservative care | 73/135 (54.1) | 45/129 (34.9) | 0.002 |
| Chest tube drainage | 82/131 (62.6) | 97/133 (72.9) | 0.087 |
| Surgery | 8/139 (5.8) | 2/128 (1.6) | 0.106 |
| Outcome | |||
| Hospital stay, days | 13.0 [6.0, 19.0] | 17.0 [9.0, 26.0] | 0.016 |
| ICU admission | 51/138 (37.0) | 98/133 (73.7) | <0.001 |
| Mortality | 35/151 (23.2) | 83/133 (62.4) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, W.; Kipkorir, V.; Marza, A.M.; Hamouri, S.; Albawaih, O.; Dhali, A.; Kim, W.; Udwadia, Z.F.; Nashwan, A.J.; Shaikh, N.; et al. Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score. J. Clin. Med. 2022, 11, 7132. https://doi.org/10.3390/jcm11237132
Woo W, Kipkorir V, Marza AM, Hamouri S, Albawaih O, Dhali A, Kim W, Udwadia ZF, Nashwan AJ, Shaikh N, et al. Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score. Journal of Clinical Medicine. 2022; 11(23):7132. https://doi.org/10.3390/jcm11237132
Chicago/Turabian StyleWoo, Wongi, Vincent Kipkorir, Adina Maria Marza, Shadi Hamouri, Omar Albawaih, Arkadeep Dhali, Wooshik Kim, Zarir F. Udwadia, Abdulqadir J. Nashwan, Nissar Shaikh, and et al. 2022. "Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score" Journal of Clinical Medicine 11, no. 23: 7132. https://doi.org/10.3390/jcm11237132
APA StyleWoo, W., Kipkorir, V., Marza, A. M., Hamouri, S., Albawaih, O., Dhali, A., Kim, W., Udwadia, Z. F., Nashwan, A. J., Shaikh, N., Belletti, A., Landoni, G., Palumbo, D., Swed, S., Sawaf, B., Buonsenso, D., Pimenta, I., Gonzalez, F. A., Fiorentino, G., ... International COVID-19 Pneumothorax Working Group (ICP-WG). (2022). Prognosis of Spontaneous Pneumothorax/Pneumomediastinum in Coronavirus Disease 2019: The CoBiF Score. Journal of Clinical Medicine, 11(23), 7132. https://doi.org/10.3390/jcm11237132