Thoracic UltrasONOgraphy Reporting: The TUONO Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Overview
3.2. Purpose of LUS and Findings Reported
3.3. Comparing SRs to FTRs
3.4. Clinical Considerations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Laursen, C.B.; Sloth, E.; Lassen, A.T.; Christensen, R.D.; Lambrechtsen, J.; Madsen, P.H.; Henriksen, D.P.; Davidsen, J.R.; Rasmussen, F. Point-of-care ultrasonography in patients admitted with respiratory symptoms: A single-blind, randomised controlled trial. Lancet Respir. Med. 2014, 2, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Mongodi, S.; Via, G.; Rouquette, I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology 2015, 122, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Calamai, I.; Greco, M.; Bertolini, G.; Spina, R.; Italian Group for the Evaluation of Interventions in Intensive Care Medicine (GiViTI). Current adoption of lung ultrasound in Intensive Care Units: An Italian multi-center survey. Minerva Anestesiol. 2017, 83, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Mojoli, F.; Elaid Bouhemad, B.; Mongodi, S.; Lichtenstein, D. Lung Ultrasound for Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019, 199, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef]
- Boffelli, S.; Rossi, C.; Anghileri, A.; Giardino, M.; Carnevale, L.; Messina, M.; Neri, M.; Langer, M.; Bertolini, G.; Italian Group for the Evaluation of Inter ventions in Intensive Care Medicine. Continuous quality im provement in intensive care medicine. The GiViTI Margherita Project—Report 2005. Minerva Anestesiol. 2006, 72, 419–432. [Google Scholar]
- Zieleskiewicz, L.; Markarian, T.; Lopez, A.; Taguet, C.; Mohammedi, N.; Boucekine, M.; Baumstarck, K.; Besch, G.; Mathon, G.; Duclos, G.; et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020, 46, 1707–1713. [Google Scholar] [CrossRef]
- Tutino, L.; Cianchi, G.; Barbani, F.; Batacchi, S.; Cammelli, R.; Peris, A. Time needed to achieve completeness and accuracy in bedside lung ultrasound reporting in Intensive Care Unit. Scand. J. Trauma Resusc. Emerg. Med. 2010, 18, 44. [Google Scholar] [CrossRef]
- Via, G.; Storti, E.; Gulati, G.; Neri, L.; Mojoli, F.; Braschi, A. Lung ultrasound in the ICU: From diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol. 2012, 78, 1282–1296. [Google Scholar]
- Gargani, L.; Volpicelli, G. How I do it: Lung ultrasound. Cardiovasc. Ultrasound 2014, 12, 25. [Google Scholar] [CrossRef]
- Jambrik, Z.; Monti, S.; Coppola, V.; Agricola, E.; Mottola, G.; Miniati, M.; Picano, E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am. J. Cardiol. 2004, 93, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; de Nicolo, A.; Schiavone, M.; Adeniji, A.O.; De Palma, A.; Di Gennaro, F.; Emuveyan, E.E.; Grasso, S.; Henwood, P.C.; Koroma, A.P.; et al. Lung ultrasound for detection of pulmonary complications in critically ill obstetric patients in a resource-limited setting. Am. J. Trop. Med. Hyg. 2021, 104, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Brusasco, C.; Santori, G.; Tavazzi, G.; Via, G.; Robba, C.; Gargani, L.; Mojoli, F.; Mongodi, S.; Bruzzo, E.; Trò, R.; et al. Second-order grey-scale texture analysis of pleural ultrasound images to differentiate acute respiratory distress syndrome and cardiogenic pulmonary edema. J. Clin. Monit. Comput. 2020, 36, 131–140. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology (ESR). The role of lung ultrasound in COVID-19 disease. Insights Imaging 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Gargani, L. A simple, reproducible and accurate lung ultrasound technique for COVID-19: When less is more. Intensive Care Med. 2021, 47, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Dargent, A.; Chatelain, E.; Si-Mohamed, S.; Simon, M.; Baudry, T.; Kreitmann, L.; Quenot, J.-P.; Cour, M.; Argaud, L. Lung ultrasound score as a tool to monitor disease progression and detect ventilator-associated pneumonia during COVID-19-associated ARDS. Heart Lung 2021, 50, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Havelock, T.; Teoh, R.; Laws, D.; Gleeson, F. Pleural procedures and thoracic ultrasound: British Thoracic Society pleural disease guideline 2010. Thorax 2010, 65, i61–i76. [Google Scholar] [CrossRef]
- Via, G.; Vasques, F.; Hussain, A.; Barrett, N.A.; Camporota, L. Bedside noninvasive monitoring of mechanically ventilated patients. Curr. Opin. Crit. Care 2021, 27, 66–75. [Google Scholar] [CrossRef]
- Manivel, V.; Lesnewski, A.; Shamim, S.; Carbonatto, G.; Govindan, T. CLUE: COVID-19 lung ultrasound in emergency department. EMA—Emerg. Med. Australas. 2020, 32, 694–696. [Google Scholar] [CrossRef]
- European Society of Radiology (ESR). ESR paper on structured reporting in radiology. Insights Imaging 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Ernst, B.P.; Hodeib, M.; Strieth, S.; Künzel, J.; Bischof, F.; Hackenberg, B.; Huppertz, T.; Weber, V.; Bahr, K.; Eckrich, J.; et al. Structured reporting of head and neck ultrasound examinations. BMC Med. Imaging 2019, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; Via, G.; Girard, M.; Rouquette, I.; Misset, B.; Braschi, A.; Mojoli, F.; Bouhemad, B. Lung Ultrasound for Early Diagnosis of Ventilator-Associated Pneumonia. Chest 2016, 149, 969–980. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n (%) |
|---|---|
| Patients | 106 |
| Sex—male | 69 (65%) |
| Age (y, SD) | 68 ± 15 |
| SAPS II score | 34 ± 16 |
| SOFA score | 4 ± 3.6 |
| ICU length of stay (d, SD) | 7.1 ± 10.4 |
| Mortality during ICU length of stay | 9 (8.7%) |
| Admission unit: | |
| 53 (56%) |
| 53 (56%) |
| Purpose of admission: | |
| 42 (39%) |
| 64 (64.5%) |
| Respiratory failure on admission | 59 (55%) |
| Ventilatory support during ICU admission: | |
| 74 (69%) |
| 19 (10%) |
| Mechanical ventilation duration (d, IQR) | 1 [1,2,3,4,5,6,7] |
| Tot % | SRs, n (%) | FTRs, n (%) | p-Value * | |
|---|---|---|---|---|
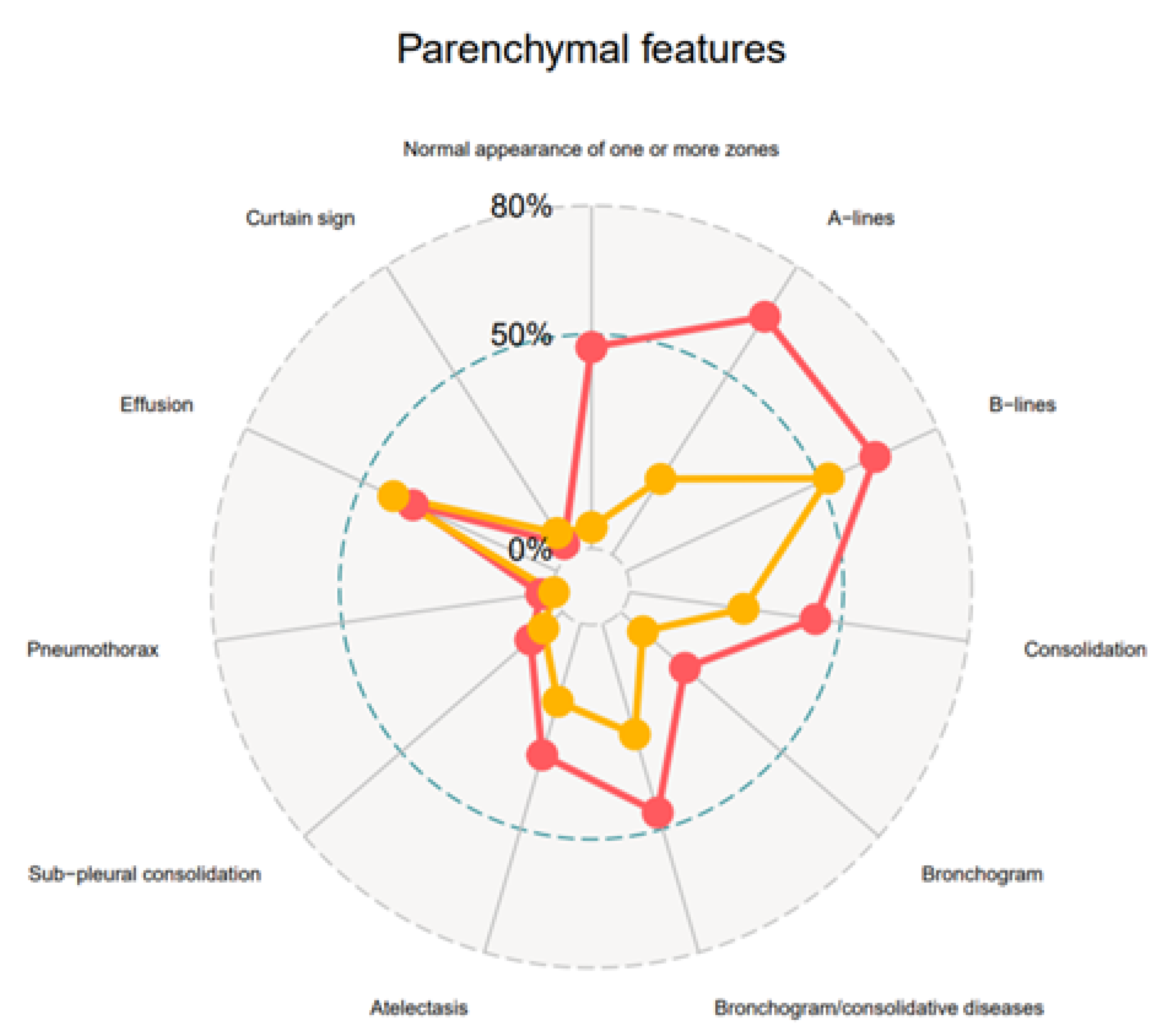
| Normal appearance of one or more zones | 34 (2%) | 28 (47%) | 6 (5%) | <0.0001 |
| A-lines | 63 (37%) | 39 (66%) | 24 (21%) | <0.0001 |
| B-lines | 96 (56%) | 38 (64%) | 58 (52%) | 0.1446 |
| 28/96 (29%) | 17/38 (45%) | 11/58 (19%) | 0.0109 |
| 31/96 (32%) | 13/38 (34%) | 18/58 (31%) | 0.8246 |
| 37/96 (39%) | 10/38 (26%) | 27/58 (47%) | 0.0555 |
| 16/69 (17%) | 5/38 (13%) | 11/58 (19%) | 0.5796 |
| Consolidation tissue-like | 56 (33%) | 26 (44%) | 30 (27%) | 0.0264 |
| 8/56 (14%) | 5/26 (19%) | 3/30 (10%) | 0.4507 |
| 2/56 (4%) | 1/26 (4%) | 1/30 (3%) | 1.0000 |
| Subpleural consolidation | 13 (8%) | 6 (10%) | 7 (6%) | 0.3750 |
| Bronchogram | 20 (12%) | 12 (20%) | 8 (7%) | 0.0218 |
| Bronchogram/consolidations | 20/56 (36%) | 12/26 (46%) | 8/30 (27%) | 0.1666 |
| Atelectasis | 40 (23%) | 19 (32%) | 21 (19%) | 0.0582 |
| 9/40 (23%) | 4/19 (21%) | 5/21 (24%) | 1.0000 |
| Pneumonia | 12 (7%) | 11 (19%) | 1 (1%) | <0.0001 |
| NA | NA | NA | |
| Pneumothorax | 2 (1%) | 2 (3%) | 0 (0) | 0.1177 |
| Effusion | 69 (40%) | 22 (37%) | 47 (42%) | 0.6239 |
| 3/69 (4%) | 3/22 (14%) | 0/47 (0) | 0.0294 |
| 44/69 (64%) | 10/22 (45%) | 34/47 (72%) | 0.0363 |
| 31/69 (42%) | 11/22 (50%) | 20/47 (43%) | 0.6106 |
| 14/69 (20%) | 13/22 (59%) | 1/47 (2%) | <0.0001 |
| 5/69 (7%) | 0/22 | 5/47 (11%) | 0.1694 |
| Curtain sign | 9 (5%) | 2 (3%) | 7 (6%) | 0.7203 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calamai, I.; Greco, M.; Finazzi, S.; Savi, M.; Vitiello, G.; Garbero, E.; Spina, R.; Montisci, A.; Mongodi, S.; Bertolini, G., on behalf of the TUONO study investigators. Thoracic UltrasONOgraphy Reporting: The TUONO Study. J. Clin. Med. 2022, 11, 7126. https://doi.org/10.3390/jcm11237126
Calamai I, Greco M, Finazzi S, Savi M, Vitiello G, Garbero E, Spina R, Montisci A, Mongodi S, Bertolini G on behalf of the TUONO study investigators. Thoracic UltrasONOgraphy Reporting: The TUONO Study. Journal of Clinical Medicine. 2022; 11(23):7126. https://doi.org/10.3390/jcm11237126
Chicago/Turabian StyleCalamai, Italo, Massimiliano Greco, Stefano Finazzi, Marzia Savi, Gaia Vitiello, Elena Garbero, Rosario Spina, Andrea Montisci, Silvia Mongodi, and Guido Bertolini on behalf of the TUONO study investigators. 2022. "Thoracic UltrasONOgraphy Reporting: The TUONO Study" Journal of Clinical Medicine 11, no. 23: 7126. https://doi.org/10.3390/jcm11237126
APA StyleCalamai, I., Greco, M., Finazzi, S., Savi, M., Vitiello, G., Garbero, E., Spina, R., Montisci, A., Mongodi, S., & Bertolini, G., on behalf of the TUONO study investigators. (2022). Thoracic UltrasONOgraphy Reporting: The TUONO Study. Journal of Clinical Medicine, 11(23), 7126. https://doi.org/10.3390/jcm11237126







