The Effects of the COVID Pandemic on Patients with IBD: Lessons Learned and Future Directions
Abstract
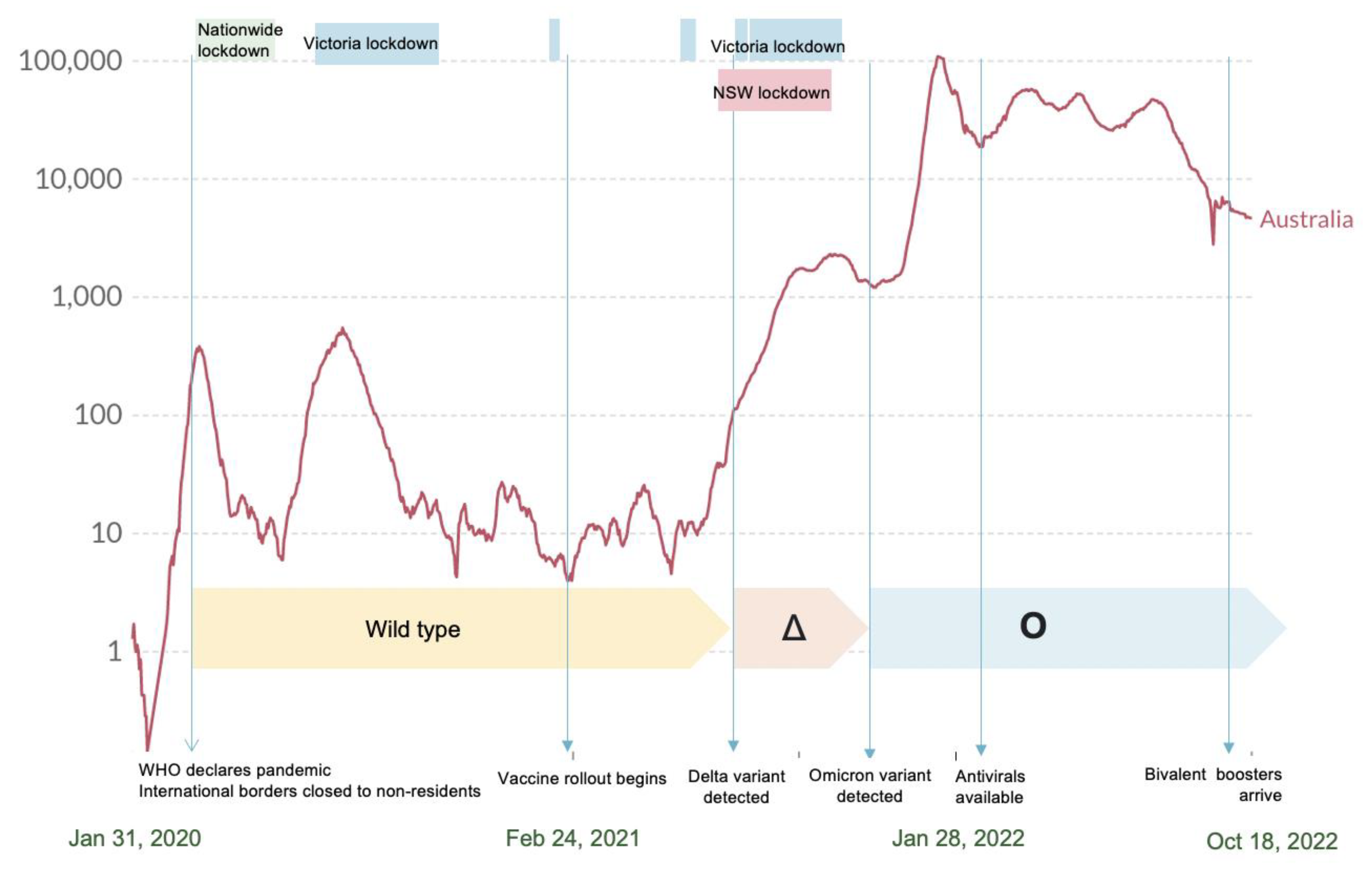
1. Introduction
2. Acquisition
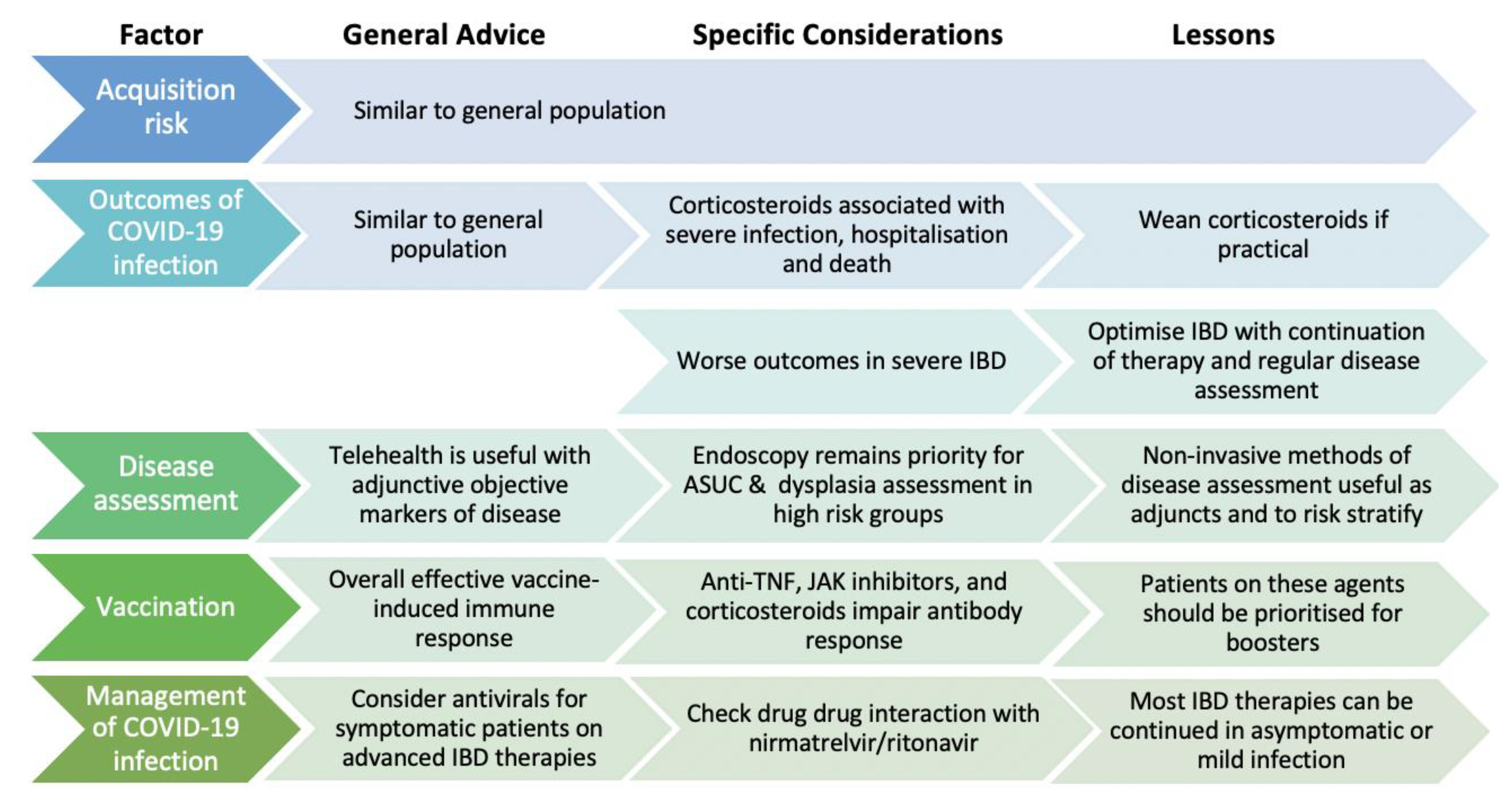
3. The Impact of IBD Medications on Outcomes of COVID-19 Infection
4. Changes in the Delivery of Healthcare
4.1. Endoscopies
4.2. Telemedicine, Non-Invasive Disease Monitoring, and Point of Care Tests
4.3. Switching from Intravenous to Subcutaneous Therapies
5. Immune Response to Vaccination
Omicron and Booster Vaccines
6. Vaccine Uptake Amongst IBD Patients
7. Considerations around COVID-19 Infection in IBD Patient
8. The Interaction of the Gut Microbiome with Infection and Vaccination
9. Conclusions
10. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Ani, A.H.; Prentice, R.E.; Rentsch, C.A.; Johnson, D.; Ardalan, Z.; Heerasing, N.; Garg, M.; Campbell, S.; Sasadeusz, J.; Macrae, F.A.; et al. Review article: Prevention, diagnosis and management of COVID-19 in the IBD patient. Aliment. Pharmacol. Ther. 2020, 52, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Jones, G.-R.; Lamb, C.A.; Appleby, R.; Arnott, I.; Beattie, R.M.; Bloom, S.; Brooks, A.J.; Cooney, R.; Dart, R.J.; et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020, 69, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.W.; Ahmad, T.; Lamb, C.A.; Powell, N.; Din, S.; Cooney, R.; A Kennedy, N.; Ainley, R.; Wakeman, R.; Selinger, C.P. Withdrawal of the British Society of Gastroenterology IBD risk grid for COVID-19 severity. Gut 2022. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jena, A.; Kumar-M, P.; Sharma, V.; Sebastian, S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2020, 9, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 2020, 70, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Grzes, K.M.; Robinson, P.C.; Pearce, E.J. Sulfasalazine: A risk factor for severe COVID-19? Lancet Rheumatol. 2022, 4, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.C.; Brenner, E.J.; Agrawal, M.; Zhang, X.; Kappelman, M.D.; Colombel, J.-F.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; et al. Impact of Medications on COVID-19 Outcomes in Inflammatory Bowel Disease: Analysis of More Than 6000 Patients From an International Registry. Gastroenterology 2022, 162, 316–319.e5. [Google Scholar] [CrossRef]
- Ricciuto, A.; Lamb, C.A.; Benchimol, E.I.; Walker, G.J.; Kennedy, N.A.; Kuenzig, M.E.; Kaplan, G.G.; Kappelman, M.D.; Ungaro, R.C.; Colombel, J.-F.; et al. Inflammatory Bowel Disease Clinical Activity is Associated with COVID-19 Severity Especially in Younger Patients. J. Crohn’s Colitis 2021, 16, 591–600. [Google Scholar] [CrossRef]
- Ungaro, R.C.; Chou, B.; Mo, J.; Ursos, L.; Twardowski, R.; Candela, N.; Colombel, J.-F. Impact of COVID-19 on Healthcare Resource Utilization among Patients with Inflammatory Bowel Disease in the USA. J. Crohn’s Colitis 2022, 16, 1405–1414. [Google Scholar] [CrossRef]
- Iacucci, M.; Cannatelli, R.; Labarile, N.; Mao, R.; Panaccione, R.; Danese, S.; Kochhar, G.S.; Ghosh, S.; Shen, B. Endoscopy in inflammatory bowel diseases during the COVID-19 pandemic and post-pandemic period. Lancet Gastroenterol. Hepatol. 2020, 5, 598–606. [Google Scholar] [CrossRef]
- Ho, K.M.A.; Banerjee, A.; Lawler, M.; Rutter, M.D.; Lovat, L.B. Predicting endoscopic activity recovery in England after COVID-19: A national analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Churchhouse, A.M.D.; EL Moffat, V.; Selinger, C.P.; A Lamb, C.; Thornton, M.J.; Penman, I.; Din, S. British Society of Gastroenterology interim framework for addressing the COVID-19-related backlog in inflammatory bowel disease colorectal cancer surveillance. Gut 2022. [Google Scholar] [CrossRef]
- Shah, R.; Wright, E.; Tambakis, G.; Holmes, J.; Thompson, A.; Connell, W.; Lust, M.; Niewiadomski, O.; Kamm, M.; Basnayake, C.; et al. Telehealth model of care for outpatient inflammatory bowel disease care in the setting of the COVID-19 pandemic. Intern. Med. J. 2021, 51, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Shields, S.; Dunlop, A.; Seenan, J.P.; Macdonald, J. Disease monitoring of biologic treatment in IBD: Early impact and future implications of COVID-19 pandemic. Frontline Gastroenterol. 2021, 12, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.M.; Patel, A.; Subramanian, S.; Smith, P.J. From intravenous to subcutaneous infliximab in patients with inflammatory bowel disease: A pandemic-driven initiative. Lancet Gastroenterol. Hepatol. 2021, 6, 88–89. [Google Scholar] [CrossRef]
- Burdge, G.; Hardman, A.; Carbery, I.; Broglio, G.; Greer, D.; Selinger, C.P. Uptake of a Switching Program for Patients Receiving Intravenous Infliximab and Vedolizumab to Subcutaneous Preparations. J. Clin. Med. 2022, 11, 5669. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef]
- Edelman-Klapper, H.; Zittan, E.; Shitrit, A.B.-G.; Rabinowitz, K.M.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; Yanai, H.; et al. Lower Serologic Response to COVID-19 mRNA Vaccine in Patients With Inflammatory Bowel Diseases Treated With Anti-TNFα. Gastroenterology 2022, 162, 454–467. [Google Scholar] [CrossRef]
- Melmed, G.Y.; Botwin, B.G.J.; Sobhani, K.; Li, D.; Prostko, M.J.; Figueiredo, J.; Cheng, S.; Braun, J.; McGovern, D.P. Antibody Responses After SARS-CoV-2 mRNA Vaccination in Adults With Inflammatory Bowel Disease. Ann. Intern. Med. 2021, 174, 1768–1770. [Google Scholar] [CrossRef]
- Alexander, J.L.; Kennedy, N.A.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Seoane, R.C.; Liu, Z.; Nice, R.; Bewshea, C.; D’Mello, A.; et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. ATAGI Recommendations on The Use of a Booster Dose of COVID-19 Vaccine Australian Government Department of Health and Aged Care Website. 2021. Available online: https://www.health.gov.au/resources/publications/atagi-recommendations-on-the-use-of-a-booster-dose-of-covid-19-vaccine (accessed on 23 March 2022).
- Jena, A.; James, D.; Singh, A.K.; Dutta, U.; Sebastian, S.; Sharma, V. Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients With IBD: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 1456–1479.e18. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Kennedy, N.A.; Saifuddin, A.; Sandoval, D.M.; Reynolds, C.; Seoane, R.C.; Kottoor, S.; Pieper, F.; Lin, K.-M.; Butler, D.K.; et al. COVID-19 vaccine-induced antibodies are attenuated and decay rapidly in infliximab treated patients. Nat. Portf. 2021. [Google Scholar] [CrossRef]
- Qui, M.; Le Bert, N.; Chan, W.P.W.; Tan, M.; Hang, S.K.; Hariharaputran, S.; Sim, J.X.Y.; Low, J.G.H.; Ng, W.; Wan, W.Y.; et al. Favorable vaccine-induced SARS-CoV-2–specific T cell response profile in patients undergoing immune-modifying therapies. J. Clin. Investig. 2022, 132, e159500. [Google Scholar] [CrossRef] [PubMed]
- Caldera, F.; Farraye, F.A.; Necela, B.M.; Cogen, D.; Saha, S.; Wald, A.; Daoud, N.D.; Chun, K.; Grimes, I.; Lutz, M.; et al. Higher Cell-Mediated Immune Responses in Patients With Inflammatory Bowel Disease on Anti-TNF Therapy After COVID-19 Vaccination. Inflamm. Bowel Dis. 2022, izac193. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Liu, Z.; Mūnoz Sandoval, D.; Reynolds, C.; Ibraheim, H.; Anandabaskaran, S.; Saifudding, A.; Seoane, R.C.; Anand, N.; Nice, R.; et al. COVID-19 Vaccine-Induced Antibody and T Cell Responses in Immunosuppressed Patients with Inflammatory Bowel Disease after the Third Vaccine Dose: (VIP): A Multicentre, Prospective, Case-Control Study. Lancet Gastroenterol. Hepatol. 2022, 7, 1005–1015. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Aloy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Janjua, M.; Chanchlani, N.; Lin, S.; Bewshea, C.; Nice, R.; McDonald, T.J.; Auckland, C.; Harries, L.W.; Davies, M.; et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic. Gut 2022. [Google Scholar] [CrossRef] [PubMed]
- Karaba, A.H.; Johnston, T.S.; Aytenfisu, T.Y.; Woldemeskel, B.A.; Garliss, C.C.; Cox, A.L.; Blankson, J.N. Low neutralisation of the omicron BA.2 sublineage in boosted individuals who had breakthrough infections. Lancet Microbe 2022, 3, e644. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, A.; Mengesha, E.; Elyanow, R.; Gittelman, R.M.; Chapman, H.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Pozdnyakova, V.; et al. The T-cell clonal response to SARS-CoV-2 vaccination in inflammatory bowel disease patients is augmented by anti-TNF therapy and often deficient in antibody-responders. medRxiv 2021. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against Covid-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Costantino, A.; Noviello, D.; Conforti, F.S.; Aloi, M.; Armuzzi, A.; Bossa, F.; Ficari, F.; Leone, S.; Manguso, F.; Mocci, G.; et al. COVID-19 Vaccination Willingness and Hesitancy in Patients With Inflammatory Bowel Diseases: Analysis of Determinants in a National Survey of the Italian IBD Patients’ Association. Inflamm. Bowel Dis. 2021, 28, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Dalal, R.S.; McClure, E.; Marcus, J.; Winter, R.W.; Hamilton, M.J.; Allegretti, J.R. COVID-19 Vaccination Intent and Perceptions Among Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 1730–1732.e2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Gupta, A.; Al-Ani, A.; Macrae, F.A.; Leong, R.W.; Christensen, B. Misconceptions Drive COVID-19 Vaccine Hesistancy in Individuals with Inflammatory Bowel Disease. Can. J. Gastroenterol. Hepatol. 2022, 2022, 527844. [Google Scholar] [CrossRef] [PubMed]
- Botwin, G.J.; Li, D.; Figueiredo, J.; Cheng, S.; Braun, J.; McGovern, D.P.B.; Melmed, G.Y. Adverse events after SARS-CoV-2 mRNA vaccination among patients with inflammatory bowel disease. Am. J. Gastroenterol. 2021, 116, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus COVID-19 Dashboard. Available online: https://covid19.who.int (accessed on 18 October 2022).
- Selim, R.; Wellens, J.; Brann, S.; Marlow, L.; Adams, A.; Satsangi, J.J. Decrease in uptake of SARS-CoV-2 vaccine in patients with inflammatory bowel disease on intravenous biological therapy. Lancet Gastroenterol. Hepatol. 2022, 7, 984–985. [Google Scholar] [CrossRef]
- Paul, E.; Fancourt, D. Predictors of uncertainty and unwillingness to receive the COVID-19 booster vaccine: An observational study of 22,139 fully vaccinated adults in the UK. Lancet Reg. Health Eur. 2022, 14, 100317. [Google Scholar] [CrossRef]
- The Johns Hopkins University Center for Systems Science and Engineering. COVID-19 Data Repository. 2022. Available online: https://github.com/CSSEGISandData/COVID-19 (accessed on 16 October 2022).
- Rubin, D.T.; Feuerstein, J.D.; Wang, A.Y.; Cohen, R.D. AGA Clinical Practice Update on Management of Inflammatory Bowel Disease During the COVID-19 Pandemic: Expert Commentary. Gastroenterology 2020, 159, 350–357. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Burki, T.K. The role of antiviral treatment in the COVID-19 pandemic. Lancet Respir. Med. 2022, 10, e18. [Google Scholar] [CrossRef]
- The University of Liverpool. COVID-19 Drug Interactions. 2022. Available online: https://www.covid19-druginteractions.org/checker (accessed on 18 October 2022).
- Ng, S.C.; Peng, Y.; Zhang, L.; Mok, C.K.P.; Zhao, S.; Li, A.; Ching, Y.L.J.; Liu, Y.; Yan, S.; Chan, D.L.S.; et al. Gut microbiota composition is associated with SARS-CoV-2 vaccine immunogenicity and adverse events. Gut 2022, 71, 1106–1116. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e948. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed]
| Agent | Acquisition | Hospitalisation | Severe COVID | Death |
|---|---|---|---|---|
| Systemic corticosteroids |  1.64 (1–2.7) |  2.4 (1.83–3.31) |  3.41 (2.62–4.64) |  4.77 (3.36–6.77) |
| 5-ASA |  1.89 (1.23–2.93) |  1.02 (0.83–1.26) |  1.03 (0.74–1.83) |  1.09 (0.65–1.82) |
| Methotrexate |  Pooled data immunomodulator 1.55 (0.97–2.48) |  1.26 (1.0–1.57) |  1.04 (0.39–2.81) |  0.79 (0.20–3.08) |
| Thiopurines |  0.96 (0.80–1.15) |  1.34 (0.90–2.0) |  0.93 (0.53–1.65) | |
| Anti-TNF |  1.06 (0.68–1.71) |  0.58 (0.50–0.69) |  0.50 (0.33–0.78) |  0.44 (0.26–0.76) |
| Ustekinumab |  3.16 (0.55–18.7) |  0.44 (0.36–0.54) |  0.43 (0.26–0.71) |  0.55 (0.28–1.11) |
| Vedolizumab |  2.31 (1–5.3) |  0.66 (0.56–0.76) |  0.72 (0.42–1.24) |  0.50 (0.32–0.78) |
| Tofacitinib | N/A |  0.48 (0.30–0.76) |  0.50 (0.14–1.86) |  0.83 (0.10–7.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, E.; Christensen, B.; Macrae, F.A.; Leong, R. The Effects of the COVID Pandemic on Patients with IBD: Lessons Learned and Future Directions. J. Clin. Med. 2022, 11, 7002. https://doi.org/10.3390/jcm11237002
Zhang E, Christensen B, Macrae FA, Leong R. The Effects of the COVID Pandemic on Patients with IBD: Lessons Learned and Future Directions. Journal of Clinical Medicine. 2022; 11(23):7002. https://doi.org/10.3390/jcm11237002
Chicago/Turabian StyleZhang, Eva, Britt Christensen, Finlay Alistair Macrae, and Rupert Leong. 2022. "The Effects of the COVID Pandemic on Patients with IBD: Lessons Learned and Future Directions" Journal of Clinical Medicine 11, no. 23: 7002. https://doi.org/10.3390/jcm11237002
APA StyleZhang, E., Christensen, B., Macrae, F. A., & Leong, R. (2022). The Effects of the COVID Pandemic on Patients with IBD: Lessons Learned and Future Directions. Journal of Clinical Medicine, 11(23), 7002. https://doi.org/10.3390/jcm11237002




















