Characteristics and Classification of Choroidal Caverns in Patients with Various Retinal and Chorioretinal Diseases
Abstract
1. Introduction
2. Methods
3. Results
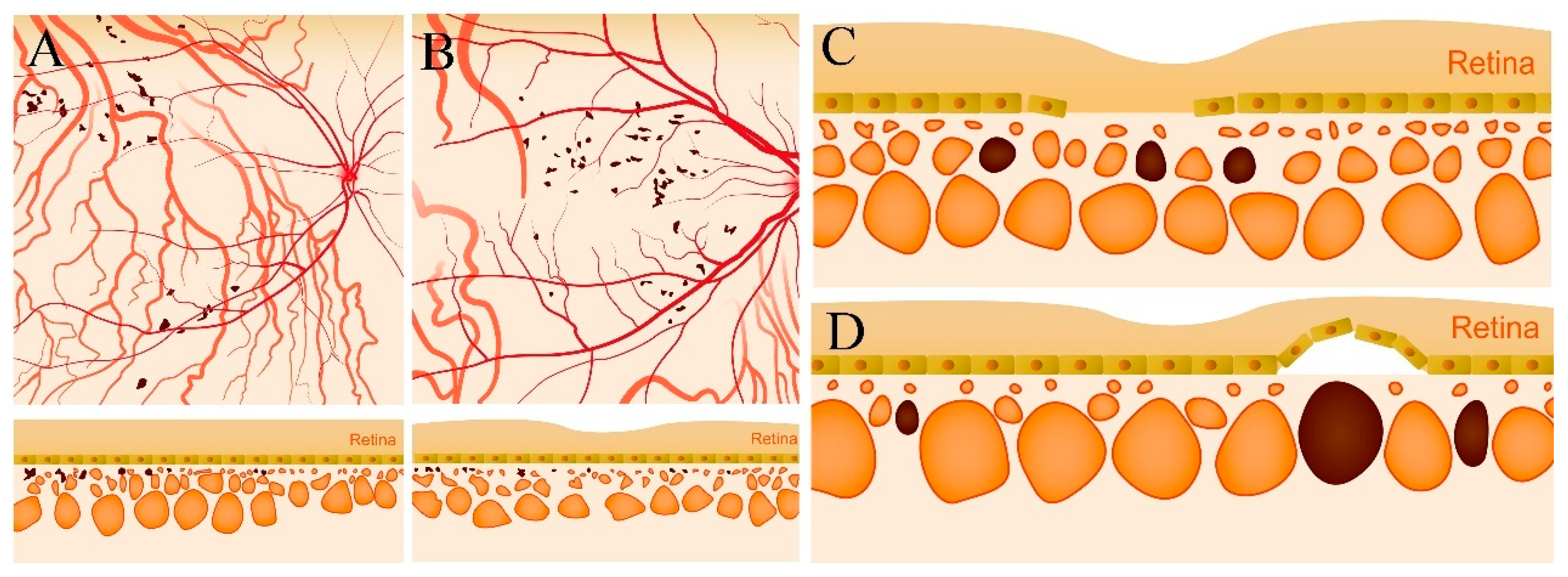
3.1. Characteristics and the Clinical Classification of Choroidal Caverns
- (1)
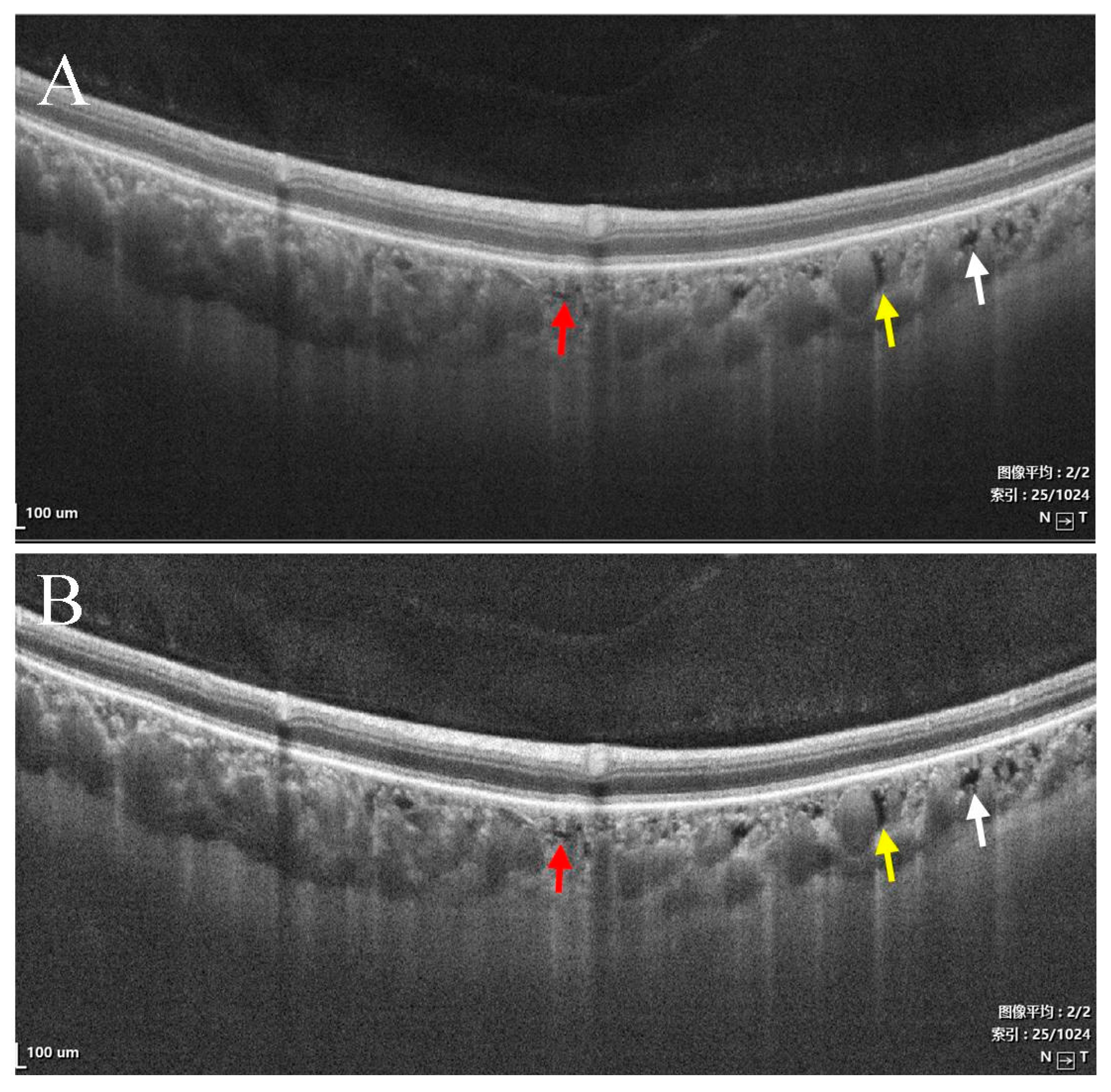
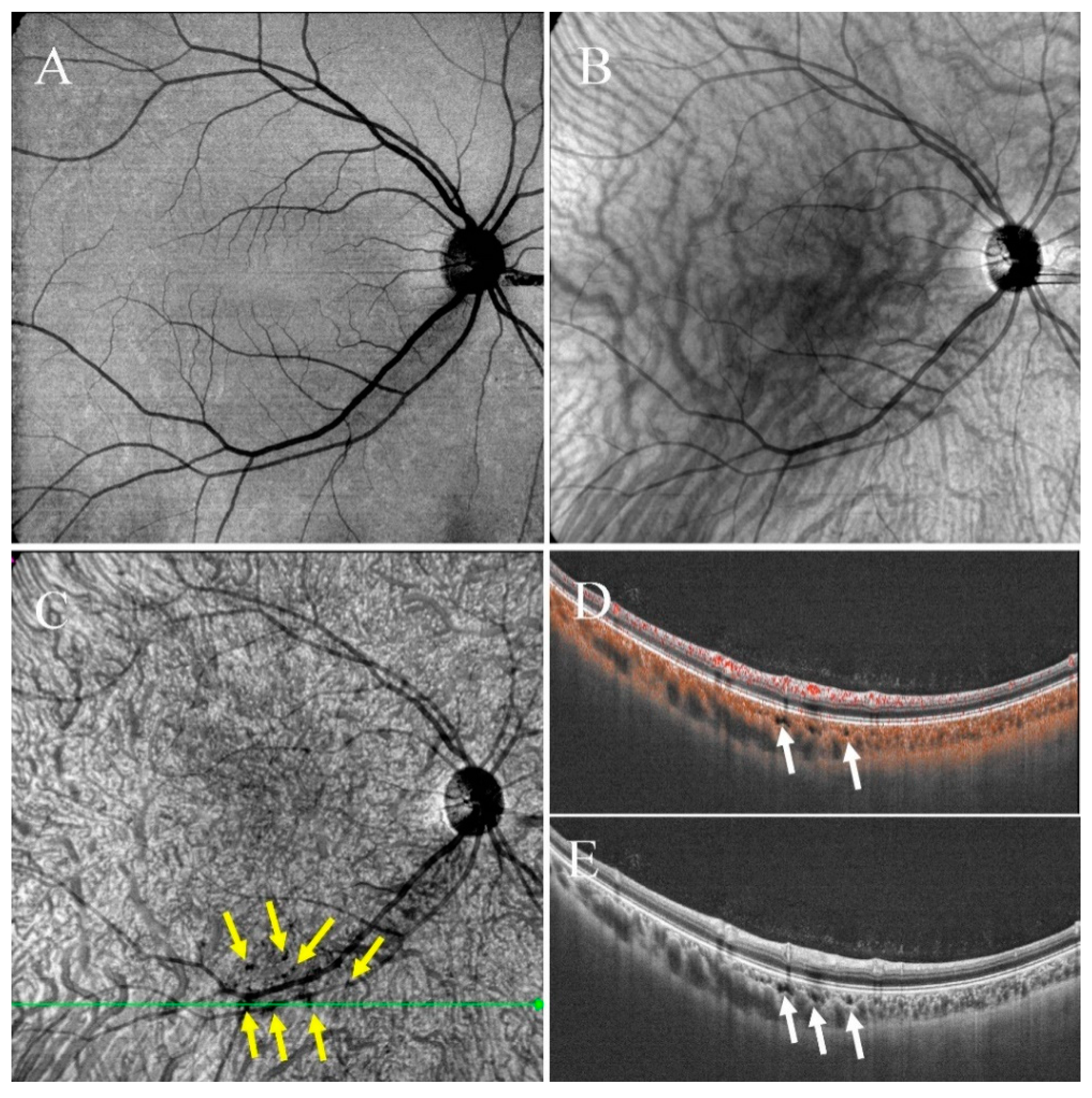
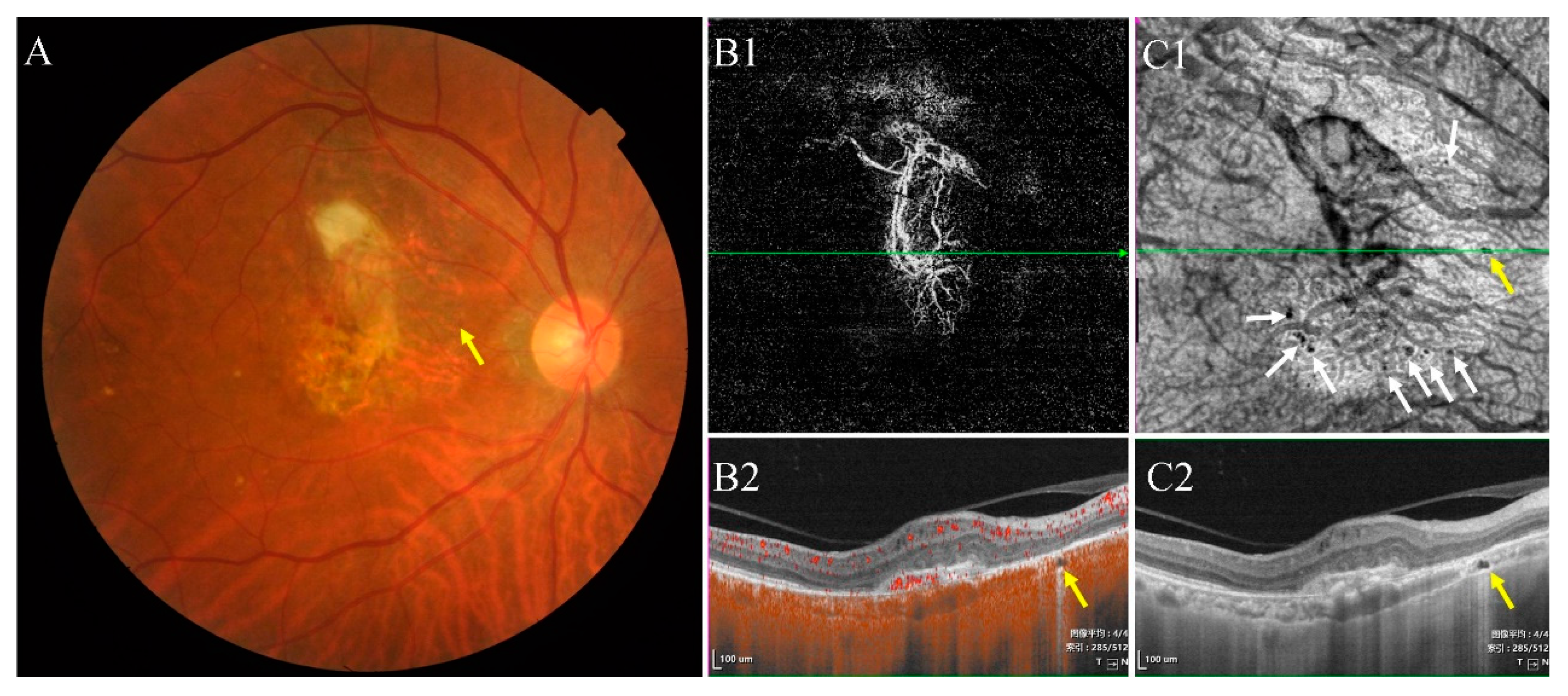
- Type I were small and usually lobulated, occurred in the choroidal capillary and the Sattler’s layers. Depending on the overall choroidal thickness, the upper margin was around 25~50 μm below the Bruch’s membrane (BM) and the lower margin was around 150~200 μm below BM. Furthermore, choroidal caverns outside the macular area were designated subtype Ia (Figure 2) and those within the macular area were designated subtype Ib (Figure 3).
- (2)
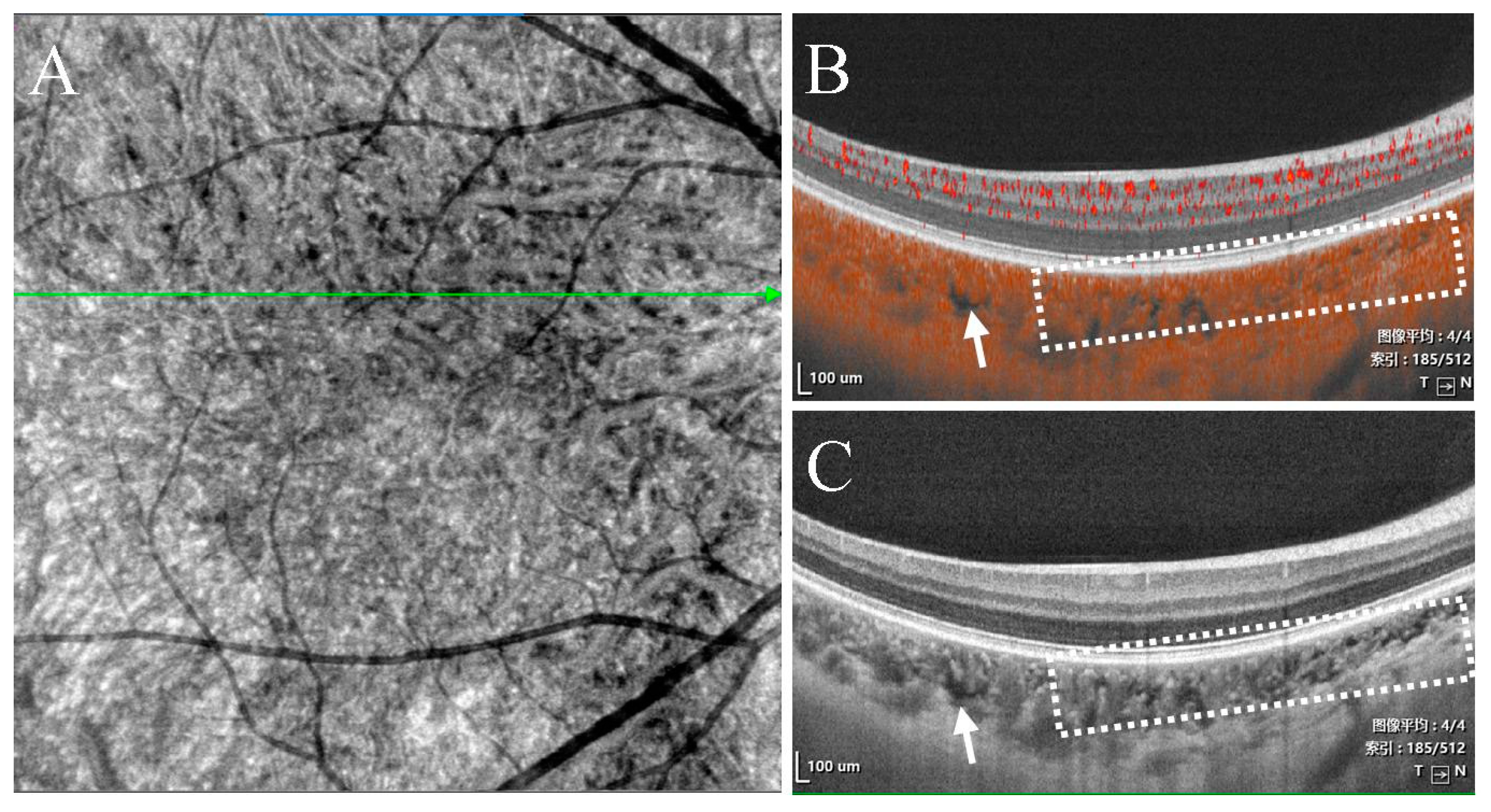
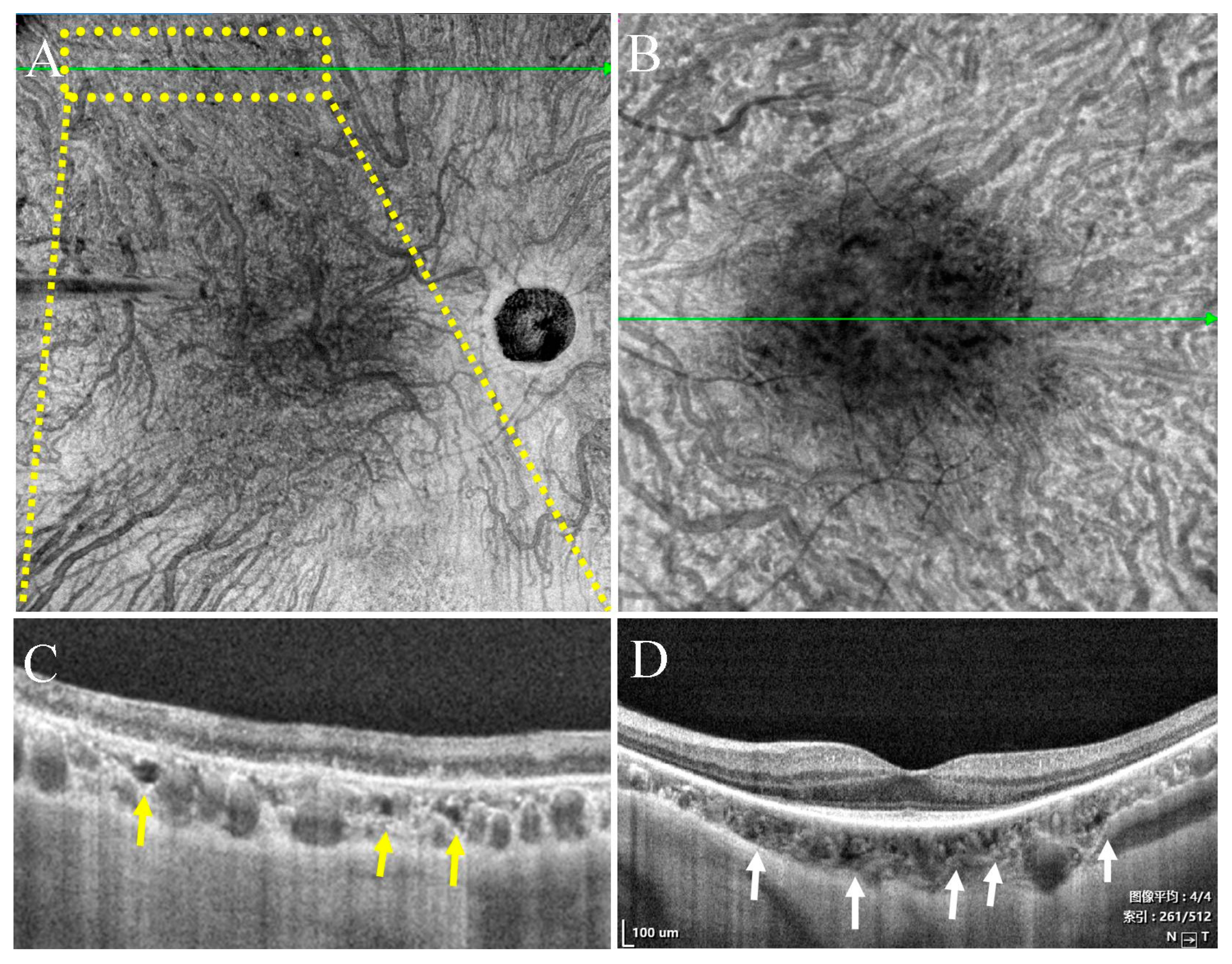
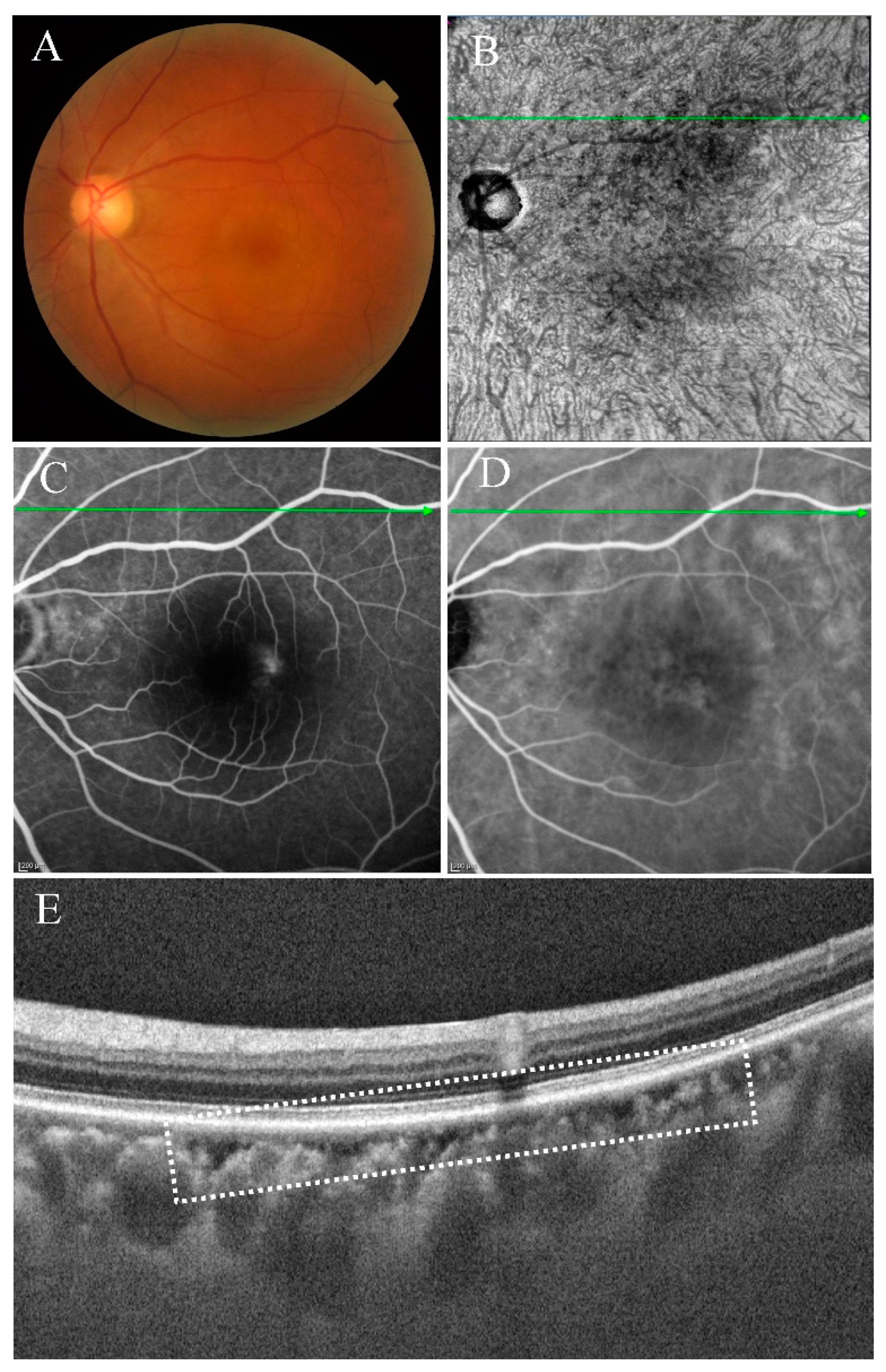
- Type II were large, usually isolated and in the Sattler’s and Haller’s layers. Depending on the overall choroidal thickness of the patients, the upper margin was around 100~200 μm below the BM and the lower margin was around 200~400 μm below the BM (Figure 4).
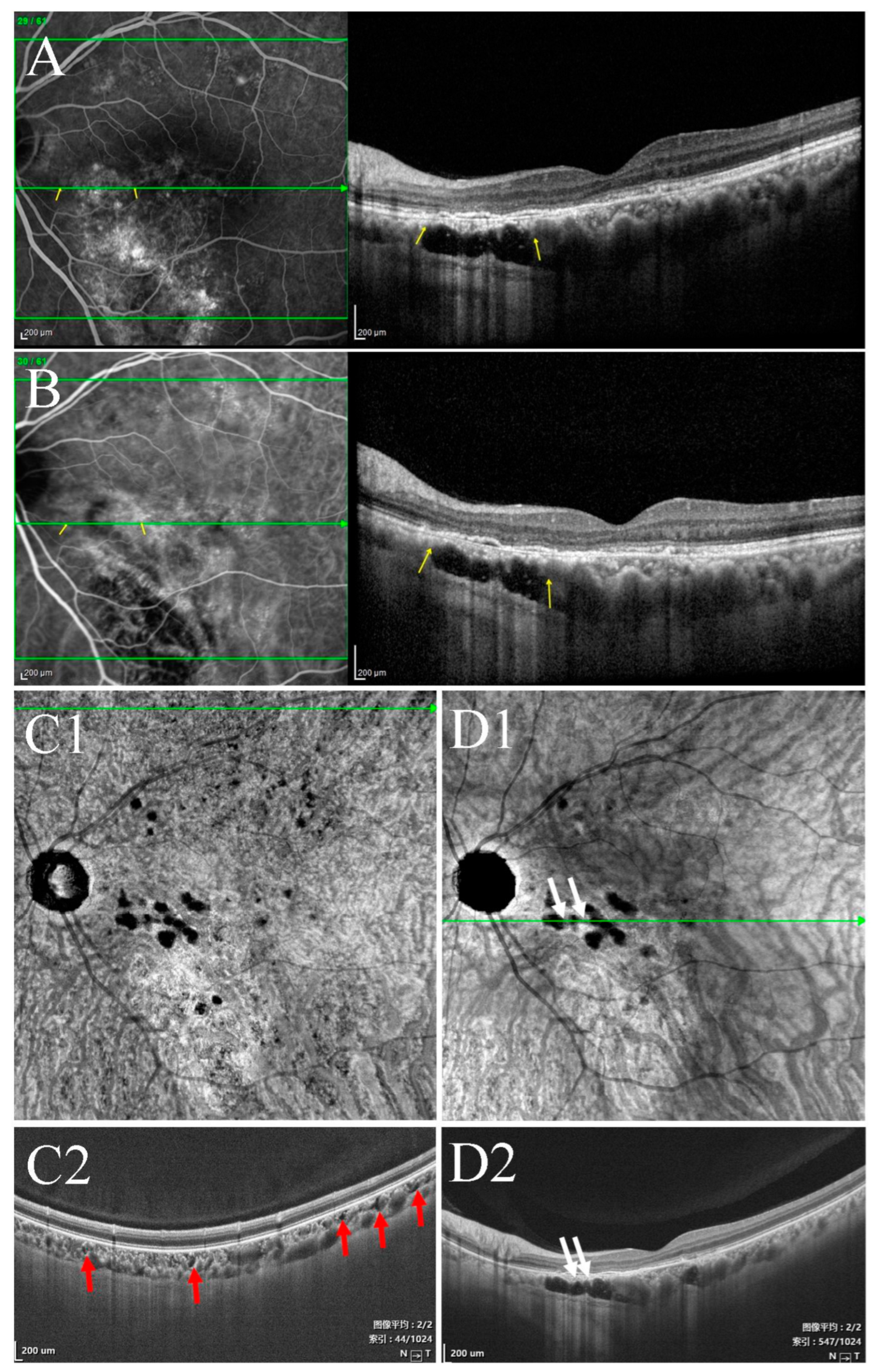
3.2. Characteristics of Type I Choroidal Caverns
3.3. Characteristics of Type II Choroidal Caverns
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced Depth Imaging Spectral-Domain Optical Coherence Tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Rothenbuehler, S.P.; Maloca, P.; Scholl, H.P.N.; Gyger, C.; Schoetzau, A.; Kuske, L.; Mosimann, N.; Zweifel, S.A.; Barthelmes, D.; Tufail, A.; et al. Three-dimensional analysis of submacular perforating scleral vessels by enhanced depth imaging optical coherence tomography. Retina 2018, 38, 1231–1237. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Li, J.; You, Y.; Liu, C.; Chen, K.; Li, S.; Lei, B. Phenotype Heterogeneity and the Association Between Visual Acuity and Outer Retinal Structure in a Cohort of Chinese X-Linked Juvenile Retinoschisis Patients. Front. Genet. 2022, 13, 322. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, W.; Kang, S.; Li, Y.; Li, W.; Gong, X.; Xiong, K.; Meng, J.; Zhong, P.; Guo, X.; et al. Peripapillary Microvasculature Predicts the Incidence and Development of Diabetic Retinopathy: An SS-OCTA Study. Am. J. Ophthalmol. 2022, 243, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Niederleithner, M.; De Sisternes, L.; Stino, H.; Sedova, A.; Schlegl, T.; Bagherinia, H.; Britten, A.; Matten, P.; Schmidt-Erfurth, U.; Pollreisz, A.; et al. Ultra-widefield OCT Angiography. IEEE Trans. Med. Imaging 2022. [Google Scholar] [CrossRef]
- Querques, G.; Costanzo, E.; Miere, A.; Capuano, V.; Souied, E.H. Choroidal Caverns: A Novel Optical Coherence Tomography Finding in Geographic Atrophy. Investig. Opthalmol. Vis. Sci. 2016, 57, 2578–2582. [Google Scholar] [CrossRef] [PubMed]
- Dolz-Marco, R.; Glover, J.P.; Gal-Or, O.; Litts, K.; Messinger, J.D.; Zhang, Y.; Cozzi, M.; Pellegrini, M.; Freund, K.B.; Staurenghi, G.; et al. Choroidal and Sub-Retinal Pigment Epithelium Caverns. Ophthalmology 2018, 125, 1287–1301. [Google Scholar] [CrossRef]
- Corbelli, E.; Sacconi, R.; De Vitis, L.A.; Carnevali, A.; Rabiolo, A.; Querques, L.; Bandello, F.; Querques, G. Choroidal Round Hyporeflectivities in Geographic Atrophy. PLoS ONE 2016, 11, e0166968. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.R.; Lupidi, M.; Mishra, S.B.; Paez-Escamilla, M.; Querques, G.; Chhablani, J. Unique optical coherence tomographic features in age-related macular degeneration. Surv. Ophthalmol. 2020, 65, 451–457. [Google Scholar] [CrossRef]
- Romano, F.; Airaldi, M.; Cozzi, M.; Oldani, M.; Riva, E.; Bertoni, A.I.; Dautaj, A.; Bertelli, M.; Staurenghi, G.; Salvetti, A.P. Progression of Atrophy and Visual Outcomes in Extensive Macular Atrophy with Pseudodrusen-like Appearance. Ophthalmol. Sci. 2021, 1, 100016. [Google Scholar] [CrossRef]
- Sakurada, Y.; Leong, B.C.S.; Parikh, R.; Fragiotta, S.; Freund, K.B. Association between choroidal caverns and choroidal vascular hyperpermeability in eyes with pachychoroid diseases. Retina 2018, 38, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Feng, N.; Hua, R. “Choroidal caverns” spectrum lesions. Eye 2020, 35, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Ayachit, A.; Joshi, S.; Kathyayini, S.; Ayachit, G. Choroidal caverns in pachychoroid neovasculopathy. Indian J. Ophthalmol. 2020, 68, 199–200. [Google Scholar] [CrossRef]
- Pederzolli, M.; Sacconi, R.; Battista, M.; Bandello, F.; Querques, G. Bilateral choroidal caverns in a child with pachychoroid and anxious personality. Am. J. Ophthalmol. Case Rep. 2022, 26, 101505. [Google Scholar] [CrossRef] [PubMed]
- Battista, M.; Borrelli, E.; Veronese, C.; Gelormini, F.; Sacconi, R.; Querques, L.; Prascina, F.; Vella, G.; Ciardella, A.P.; Bandello, F.; et al. Choroidal Rift: A New OCT Finding in Eyes with Central Serous Chorioretinopathy. J. Clin. Med. 2020, 9, 2260. [Google Scholar] [CrossRef] [PubMed]
- Mucciolo, D.P.; Giorgio, D.; Lippera, M.; Dattilo, V.; Passerini, I.; Pelo, E.; Sodi, A.; Virgili, G.; Giansanti, F.; Murro, V. Choroidal Caverns in Stargardt Disease. Investig. Opthalmol. Vis. Sci. 2022, 63, 25. [Google Scholar] [CrossRef]
- Carnevali, A.; Sacconi, R.; Corbelli, E.; Querques, L.; Bandello, F.; Querques, G. Choroidal Caverns: A Previously Unreported Optical Coherence Tomography Finding in Best Vitelliform Dystrophy. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 284–287. [Google Scholar] [CrossRef]
- Arrigo, A.; Bordato, A.; Romano, F.; Aragona, E.; Grazioli, A.; Bandello, F.; Parodi, M.B. Choroidal Patterns in Retinitis Pigmentosa: Correlation withVisual Acuity and Disease Progression. Transl. Vis. Sci. Technol. 2020, 9, 17. [Google Scholar] [CrossRef]
- Guerra, R.L.L.; Arantes, R.C.; Marback, E.F.; Shields, C.L. Novel OCT findings in choroidal osteoma: Brief report. Int. J. Retin. Vitr. 2021, 7, 1–3. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Y.; Wu, Y.; Liu, H.; Ming, S.; Cui, H.; Fan, K.; Li, S.; Lei, B. Detection of superficial and buried optic disc drusen with swept-source optical coherence tomography. BMC Ophthalmol. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Weill, Y.; Brosh, K.; Vineberg, T.L.; Arieli, Y.; Caspi, A.; Potter, M.J.; Zadok, D.; Hanhart, J. Enhanced depth imaging in swept-source optical coherence tomography: Improving visibility of choroid and sclera, a masked study. Eur. J. Ophthalmol. 2019, 30, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Friedman, E.; Smith, T.R. Clinical and Pathological Study of Choroidal Lipid Globules. Arch. Ophthalmol. 1966, 75, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, R.; Borrelli, E.; Marchese, A.; Gelormini, F.; Pennisi, F.; Cerutti, A.; Bandello, F.; Querques, G. Re: Dolz-Marco et al.: Choroidal and sub-retinal pigment epithelium caverns: Multimodal imaging and correspondence with Friedman lipid globules (Ophthalmology. 2018;125:1287-1301). Ophthalmology 2019, 126, e53–e54. [Google Scholar] [CrossRef] [PubMed]
- Young, S.G.; Zechner, R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 2013, 27, 459–484. [Google Scholar] [CrossRef] [PubMed]
- Casaroli-Marano, R.P.; Peinado-Onsurbe, J.; Reina, M.; Staels, B.; Auwerx, J.; Vilaró, S. Lipoprotein lipase in highly vascularized structures of the eye. J. Lipid Res. 1996, 37, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Sakurada, Y.; Parikh, R.; Freund, K.B. Resolution Of A Subfoveal Choroidal Cavern After Half-Dose Photodynamic Therapy For Central Serous Chorioretinopathy. Retin. Cases Brief Rep. 2021, 15, 673–675. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef]
- Spaide, R.F.; Cheung, C.M.G.; Matsumoto, H.; Kishi, S.; Boon, C.J.; van Dijk, E.H.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef]
- Kishi, S.; Matsumoto, H. A new insight into pachychoroid diseases: Remodeling of choroidal vasculature. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 3405–3417. [Google Scholar] [CrossRef]
| Normal | RP | wAMD | Acute CSC | Chronic CSC | Total | |
|---|---|---|---|---|---|---|
| Eyes/People | 86/49 | 56/29 | 61/51 | 58/58 | 54/33 | 315/220 |
| Male/Female | 20/29 | 14/15 | 37/14 | 41/17 | 30/3 | 142/78 |
| Age, yrs, mean ± SD | 44.3 ± 13.9 | 44.4 ± 20.0 | 69.1 ± 8.6 | 45.5 ± 8.9 | 50.3 ± 8.3 | 51.3 ± 15.5 |
| Refractive error of eyes, diopter, median (range) | −2.5 (−5.0–0) | −1.0 (−5.25–1.75) | −0.5 (−4.75–2.5) | −1.25 (−3.75–1.0) | −1.0 (−4.0–2.25) | - |
| BCVA, median (range) | 20/20 (20/25–20/20) | 20/100 (20/200–20/66) | 20/100 (20/200–20/50) | 20/50 (20/100–20/25) | 20/100 (20/200–20/50) | - |
| Presence with choroidal cavern type I, n (%) | 15 (17.4%) | 11 (19.6%) | 1 (1.6%) | 19 (32.8%) | 46 (85.2%) | 92 (29.2%) |
| choroidal cavern type Ia | 9 | 5 | 0 | 12 | 9 | 35 |
| choroidal cavern type Ib | 6 | 6 | 1 | 4 | 11 | 28 |
| choroidal cavern ype Ia + Ib | 0 | 0 | 0 | 3 | 26 | 29 |
| Presence with choroidal cavern type II, n (%) | 0 | 0 | 13 (21.3%) | 8 (13.8%) | 29 (53.7%) | 50 (15.9%) |
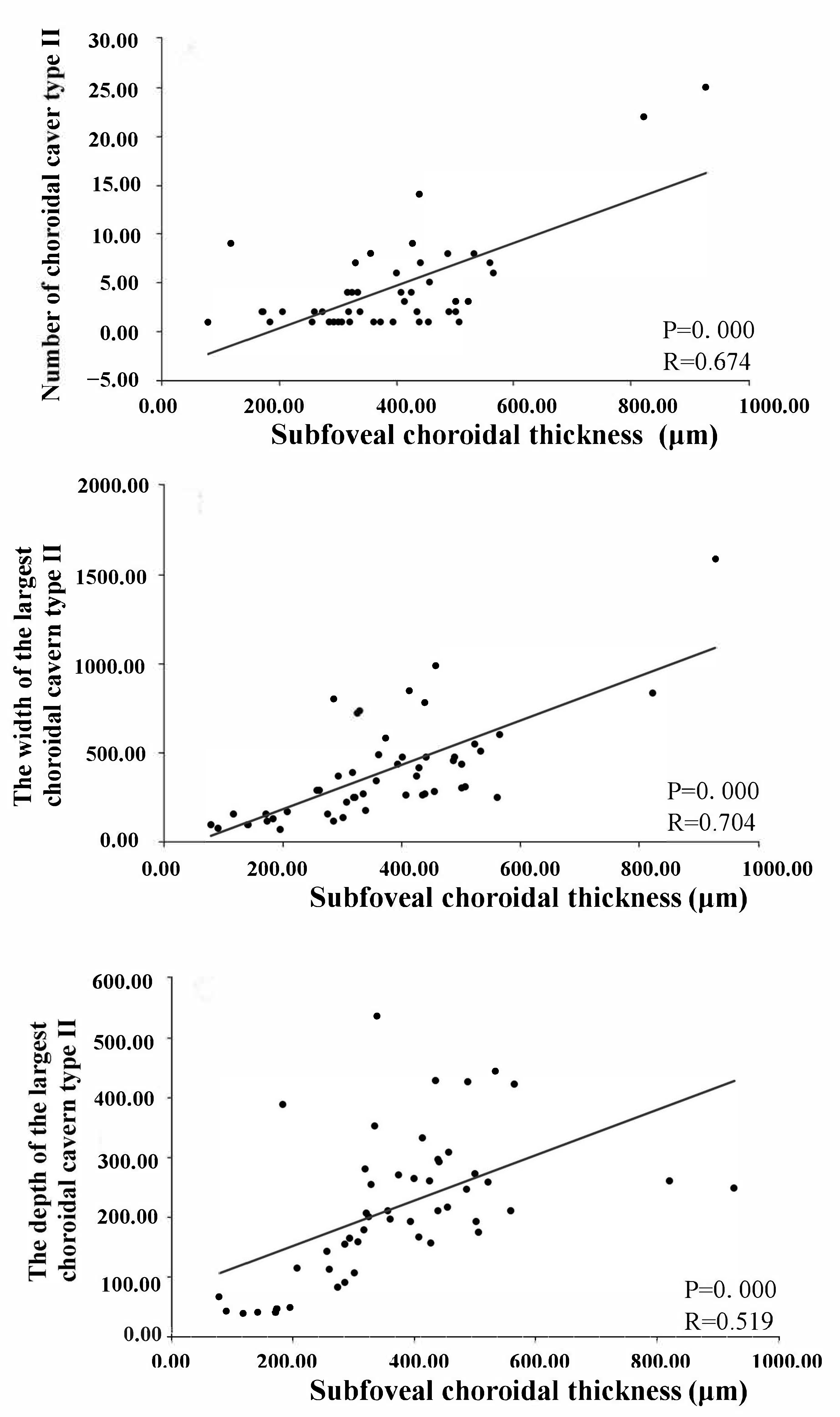
| The width of the largest choroidal cavern type II, (μm, mean ± SD) | 0 | 0 | 197.6 ± 197.7 | 282.0 ± 138.0 | 513.7 ± 294.0 | 394.5 ± 287.1 |
| The depth of the largest choroidal cavern type II, (μm, mean ± SD) | 0 | 0 | 83.7 ± 48.6 | 252.1 ± 134.0 | 265.6 ± 87.3 | 216.1 ± 118.5 |
| Number of choroidal cavern type II, (n, mean ± SD) | 0 | 0 | 2.0 ± 2.2 | 1.5 ± 1.0 | 5.9 ± 5.8 | 4.2 ± 4.9 |
| Choroidal thickness (μm, mean ± SD) | 259.8 ± 80.2 | 211.9 ± 61.3 | 200.9 ± 80.4 | 365.4 ± 90.7 | 440.9 ± 134.5 | 290.3 ± 126.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhou, Y.; Gu, C.; Wu, Y.; Liu, H.; Chang, Q.; Lei, B.; Wang, M. Characteristics and Classification of Choroidal Caverns in Patients with Various Retinal and Chorioretinal Diseases. J. Clin. Med. 2022, 11, 6994. https://doi.org/10.3390/jcm11236994
Guo X, Zhou Y, Gu C, Wu Y, Liu H, Chang Q, Lei B, Wang M. Characteristics and Classification of Choroidal Caverns in Patients with Various Retinal and Chorioretinal Diseases. Journal of Clinical Medicine. 2022; 11(23):6994. https://doi.org/10.3390/jcm11236994
Chicago/Turabian StyleGuo, Xiaohong, Yao Zhou, Chenyang Gu, Yingjie Wu, Hui Liu, Qing Chang, Bo Lei, and Min Wang. 2022. "Characteristics and Classification of Choroidal Caverns in Patients with Various Retinal and Chorioretinal Diseases" Journal of Clinical Medicine 11, no. 23: 6994. https://doi.org/10.3390/jcm11236994
APA StyleGuo, X., Zhou, Y., Gu, C., Wu, Y., Liu, H., Chang, Q., Lei, B., & Wang, M. (2022). Characteristics and Classification of Choroidal Caverns in Patients with Various Retinal and Chorioretinal Diseases. Journal of Clinical Medicine, 11(23), 6994. https://doi.org/10.3390/jcm11236994





