Comparison between School-Age Children with and without Obesity in Nutritional and Inflammation Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Laboratory Methods
2.3. Anthropometric Data
2.4. The Dependent Variable
2.5. The Independent Variables
2.6. Statistical Methods
3. Results
Associations of Nutritional and Inflammation Biomarkers with Overweight/Obesity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abarca-Gomez, L.; Abdeen, Z.A.; Abdul Hamid, Z.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bendor, C.D.; Bardugo, A.; Pinhas-Hamiel, O.; Afek, A.; Twig, G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc. Diabetol. 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Reichman, B.; Afek, A.; Derazne, E.; Hamiel, U.; Furer, A.; Gershovitz, L.; Bader, T.; Cukierman-Yaffe, T.; Kark, J.D.; et al. Severe obesity and cardio-metabolic comorbidities: A nationwide study of 2.8 million adolescents. Int. J. Obes. 2019, 43, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Chung, J.; Koo, K.O.; Kim, M.S.; Han, S.N. Hepatic iron storage is related to body adiposity and hepatic inflammation. Nutr. Metab. 2017, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Newfield, R.S.; Koren, I.; Agmon, A.; Lilos, P.; Phillip, M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 416–418. [Google Scholar] [CrossRef]
- Haimi, M.; Lerner, A. Nutritional deficiencies in the pediatric age group in a multicultural developed country, Israel. World J. Clin. Cases 2014, 16, 120–125. [Google Scholar] [CrossRef]
- Densupsoontorn, N.; Jirapinyo, P.; Kangwanpornsiri, C. Micronutrient deficiencies in obese Thai children. Asia Pac. J. Clin. Nutr. 2013, 22, 497–503. [Google Scholar] [CrossRef]
- Nielsen, T.R.H.; Lausten-Thomsen, U.; Fonvig, C.E.; Bojsoe, C.; Pedersen, L.; Bratholm, P.S.; Hansen, T.; Pedersen, O.; Holm, J.C. Dyslipidemia and reference values for fasting plasma lipid concentrations in Danish/North European White children and adolescents. BMC Pediatr. 2017, 17, 116. [Google Scholar] [CrossRef]
- Interator, H.; Lebenthal, Y.; Hoshen, M.; Safra, I.; Balicer, R.; Leshno, M.; Shamir, R. Distinct lipoprotein curves in normal weight, overweight, and obese children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 673–680. [Google Scholar] [CrossRef]
- Interator, H.; Brener, A.; Hoshen, M.; Safra, I.; Balicer, R.; Leshno, M.; Shamir, R.; Lebenthal, Y. Sex, Ethnicity, and socioeconomic status affect on Israeli pediatric lipid testing despite equality in national healthcare services. Isr. Med. Assoc. J. 2019, 21, 369–375. [Google Scholar]
- Schwarzenberg, S.J.; Sinaiko, A.R. Obesity and inflammation in children. Paediatr. Respir. Rev. 2006, 7, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef]
- Vugic, L.; Colson, N.; Nikbakht, E.; Gaiz, A.; Holland, O.J.; Kundur, A.R.; Singh, I. Anthocyanin supplementation inhibits secretion of pro-inflammatory cytokines in overweight and obese individuals. J. Funct. Foods 2020, 64, 103596. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Marginean, C.O.; Melit, L.E.; Ghiga, D.V.; Marginean, M.O. Early inflammatory status related to pediatric obesity. Front. Pediatr. 2019, 7, 241. [Google Scholar] [CrossRef]
- Cobos-Palacios, L.; Munoz-Ubeda, M.; Gallardo-Escribano, C.; Ruiz-Moreno, M.I.; Vilches-Perez, A.; Vargas-Candela, A.; Leiva-Gea, I.; Tinahones, F.J.; Gomez-Huelgas, R.; Bernal-Lopez, M.R. Adipokines profile and inflammation biomarkers in prepubertal population with obesity and healthy metabolic state. Children 2022, 9, 42. [Google Scholar] [CrossRef]
- Zhu, Y.; He, B.; Xiao, Y.; Chen, Y. Iron metabolism and its association with dyslipidemia risk in children and adolescents: A cross-sectional study. Lipids Health Dis. 2019, 18, 50. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.; Kim, H.S.; Kim, D.H.; Park, S.H. Obesity and cardiovascular risk factors in Korean children and adolescents aged 10–18 years from the Korean National Health and Nutrition Examination Survey, 1998 and 2001. Am. J. Epidemiol. 2006, 164, 787–793. [Google Scholar] [CrossRef]
- Panichsillaphakit, E.; Suteerojntrakool, O.; Pancharoen, C.; Nuchprayoon, I.; Chomtho, S. The Association between hepcidin and iron status in children and adolescents with obesity. J. Nutr. Metab. 2021, 2021, 9944035. [Google Scholar] [CrossRef]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight children and adolescents: A risk group for iron deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ming-Chen, Z.; Ming, L.; Jiang-Feng, M.; Li-Duo-Si, Y. Relationship between serum uric acid level and metabolic syndrome in Uygur children and adolescents with overweight or obesity. Chin. J. Contemp. Pediatr. 2014, 16, 878–882. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Giannopoulou, E.; Theodosiou, A.; Karaglani, E.; Manios, Y.; Moschonis, G. Adipokines and C-reactive protein as indicators of MetS presence in obese Greek children: The Healthy Growth Study. Toxicol. Rep. 2021, 8, 1645–1650. [Google Scholar] [CrossRef] [PubMed]
- Central Bureau of Statistics (CBS). Statistical Abstract of Israel 2021, No. 72, Jerusalem. 2021. Available online: https://www.cbs.gov.il/en/publications/Pages/2021/Statistical-Abstract-of-Israel-2021-No-72.aspx (accessed on 4 July 2021).
- Haj-Yahya, N.H.; Khalaily, M.; Rudnitzky, A. The Israel democracy institute: Statistical Report on Arab Society in Israel: 2021. 2022. Available online: https://en.idi.org.il/articles/38540 (accessed on 17 March 2022).
- Abu-Much, A.; Nof, E.; Bragazzi, N.L.; Younis, A.; Hochstein, D.; Younis, A.; Shlomo, N.; Fardman, A.; Goldenberg, I.; Klempfner, R.; et al. Ethnic Disparity in Mortality among Ischemic Heart Disease Patients. A-20 Years Outcome Study from Israel. Front. Cardiovasc. Med. 2021, 8, 661390. [Google Scholar] [CrossRef]
- Karkabi, B.; Zafrir, B.; Jaffe, R.; Shiran, A.; Jubran, A.; Adawi, S.; Ben-Dov, N.; Iakobishvili, Z.; Beigel, R.; Cohen, M.; et al. Ethnic Differences among Acute Coronary Syndrome Patients in Israel. Cardiovasc. Revasc. Med. 2020, 21, 1431–1435. [Google Scholar] [CrossRef]
- Israel Center for Disease Control. Mabat Youth Second National Health and Nutrition Survey of 7–12th Grade Students 2015–2016; Ministry of Health: Ramat Gan, Israel, 2017; Volume 373, pp. 16E–19E. [Google Scholar]
- Rubin, L.; Belmaker, I.; Somekh, E.; Urkin, J.; Rudolf, M.; Honovich, M.; Bilenko, N.; Grossman, Z. Building lives: Maternal and child health in Israel. Lancet 2017, 389, 2514–2530. [Google Scholar] [CrossRef]
- Muhsen, K.; Na’amnih, W.; Goldsmith, R.; Maya, M.; Zeidan, N.; Kassem, E.; Ornoy, A. Associations of feeding practices in early life and dietary intake at school age with obesity in 10- to 12-year-old Arab children. Nutrients 2021, 13, 2106. [Google Scholar] [CrossRef]
- Abu-Saad, K.; Murad, H.; Lubin, F.; Freedman, L.S.; Ziv, A.; Alpert, G.; Atamna, A.; Kalter-Leibovici, O. Jews and Arabs in the Same Region in Israel Exhibit Major Differences in Dietary Patterns. J. Nutr. 2012, 142, 2175–2181. [Google Scholar] [CrossRef]
- Muhsen, K.; Jurban, M.; Goren, S.; Cohen, D. Incidence, age of acquisition and risk factors of Helicobacter pylori infection among Israeli Arab infants. J. Trop. Pediatr. 2011, 58, 208–213. [Google Scholar] [CrossRef]
- Muhsen, K.; Masarwa, S.; Guttman, E.; Cohen, D. What are the determinants of early breastfeeding weaning and prolonged breastfeeding in Arab infants? Harefuah 2011, 150, 333–337. (In Hebrew) [Google Scholar]
- Lapidot, Y.; Reshef, L.; Goldsmith, R.; Na’amnih, W.; Kassem, E.; Ornoy, A.; Gophna, U.; Muhsen, K. The Associations between diet and socioeconomic disparities and the intestinal microbiome in preadolescence. Nutrients 2021, 13, 2645. [Google Scholar] [CrossRef] [PubMed]
- NICHD. NICHD Study of early child care and youth development. In Overview of Health and Physical Development Assessment (HPDA) Visit, Operations Manual—Phase IV, Chapter 85.1; RTI International: Research Triangle, NC, USA, 2006. [Google Scholar]
- Butte, N.F.; Garza, C.; de Onis, M. Evaluation of the feasibility of international growth standards for school-aged children and adolescents. J. Nutr. 2007, 137, 153–157. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Growth Reference 5–19 Years, BMI-for-Age (5–19 Years); World Health Organization: Geneva, Switzerland, 2018. Available online: http://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 29 July 2022).
- Jolliffe, C.J.; Janssen, I. Distribution of lipoproteins by age and gender in adolescents. Circulation 2006, 114, 1056–1062. [Google Scholar] [CrossRef]
- De Jesus, J.M. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128, 213–256. [Google Scholar] [CrossRef]
- Steiner, M.J.; Skinner, A.C.; Perrin, E.M. Fasting might not be necessary before lipid screening: A nationally representative cross-sectional study. Pediatrics 2011, 128, 463–470. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf?ua=1 (accessed on 29 June 2021).
- Sachdev, H.S.; Porwal, A.; Acharya, R.; Ashraf, S.; Ramesh, S.; Khan, N.; Kapil, U.; Kurpad, A.V.; Sarna, A. Haemoglobin thresholds to define anaemia in a national sample of healthy children and adolescents aged 1–19 years in India: A population-based study. Lancet Glob. Health 2021, 9, e822–e831. [Google Scholar] [CrossRef]
- WHO. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Van der Merwe, L.F.; Eussen, S.R. Iron status of young children in Europe. Am. J. Clin. Nutr. 2017, 106, 1663S–1671S. [Google Scholar] [CrossRef]
- Shah, N.; Khadilkar, A.; Gondhalekar, K.; Khadilkar, V. Prevalence of dyslipidemia in Indian children with poorly controlled type 1 diabetes mellitus. Pediatr. Diabetes 2020, 21, 987–994. [Google Scholar] [CrossRef]
- Obita, G.; Alkhatib, A. Disparities in the prevalence of childhood obesity-related comorbidities: A systematic review. Front. Public Health 2022, 10, 923744. [Google Scholar] [CrossRef]
- Mohamed, N.S.; Maher, S.E.; Abozaid, S.M.M.; Moenes, H.M. Anthropometric and metabolic pattern in obese Egyptian children: Its association with C-reactive protein. Egypt. Paediatr. Assoc. 2020, 68, 17. [Google Scholar] [CrossRef]
- Brzezinski, M.; Metelska, P.; Mysliwiec, M.; Szlagatys-Sidorkiewicz, A. Lipid disorders in children living with overweight and obesity-large cohort study from Poland. Lipids Health Dis. 2020, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Bozic, J.; Milic, L.; Unic, I.; Skarabic, V. The prevalence of metabolic syndrome and cardiovascular risk factors in obese children and adolescents in Dalmatia: A hospital based study. Int. J. Endocrinol. 2016, 22, 1888–1895. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, A.; Visiedo-Garcia, F.M.; Dominguez-Riscart, J.; Gonzalez-Dominguez, R.; Mateos, R.M.; Lechuga-Sancho, A.M. Iron metabolism in obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 5529. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Twig, G. The Impact of childhood and adolescent obesity on cardiovascular risk in adulthood: A systematic review. Curr. Diab. Rep. 2018, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Kraynak, T.E.; Marsland, A.L.; Hanson, J.L.; Gianaros, P.J. Retrospectively reported childhood physical abuse, systemic inflammation, and resting corticolimbic connectivity in midlife adults. Brain Behav. Immun. 2019, 82, 203–213. [Google Scholar] [CrossRef]
- Isasi, C.R.; Parrinello, C.M.; Ayala, G.X.; Delamater, A.M.; Perreira, K.M.; Daviglus, M.L.; Elder, J.P.; Marchante, A.N.; Bangdiwala, S.I.; Van-Horn, L.; et al. Sex Differences in Cardiometabolic Risk Factors among Hispanic/Latino Youth. J. Pediatr. 2016, 176, 121–127.e1. [Google Scholar] [CrossRef]
- Rojroongwasinkul, N.; Kijboonchoo, K.; Wimonpeerapattana, W.; Purttiponthanee, S.; Yamborisut, U.; Boonpraderm, A.; Kunapan, P.; Thasanasuwan, W.; Khouw, L. SEANUTS: The nutritional status and dietary intakes of 0.5–12-year-old Thai children. Br. J. Nutr. 2013, 110, S36–S44. [Google Scholar] [CrossRef]
- Halterman, J.S.; Kaczorowski, J.M.; Aligne, C.A.; Auinger, P.; Szilagyi, P.G. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics 2001, 107, 1381–1386. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef]
- Sun, H.L.; Pei, D.; Lue, K.H.; Chen, Y.L. Uric acid levels can predict metabolic syndrome and hypertension in adolescents: A 10-year longitudinal study. PLoS ONE 2015, 10, e0143786. [Google Scholar] [CrossRef]
- Cho, M.H.; Kim, Y.M.; Yoon, J.H.; Kim, D.H.; Lim, J.S. Serum uric acid in Korean children and adolescents: Reference percentiles and association with metabolic syndrome. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Seguin, D.; Kuenzel, E.; Morton, J.B.; Duerden, E.G. School’s out: Parenting stress and screen time use in school-age children during the COVID-19 pandemic. J. Affect. Disord. Rep. 2021, 6, 100217. [Google Scholar] [CrossRef] [PubMed]
- Rifas-Shiman, S.L.; Aris, I.M.; Bailey, C.; Daley, M.F.; Heerman, W.J.; Janicke, D.M.; Lin, P.D.; Petimar, J.; Block, J.P. Changes in obesity and BMI among children and adolescents with selected chronic conditions during the COVID-19 pandemic. Obesity 2022, 30, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Ten-Velde, G.; Lubrecht, J.; Arayess, L.; Van-Loo, C.; Hesselink, M.; Reijnders, D.; Vreugdenhil, A. Physical activity behavior and screen time in Dutch children during the COVID-19 pandemic: Pre-, during- and post-school closures. Pediatr. Obes. 2021, 16, e12779. [Google Scholar] [CrossRef]
- Di-Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attina, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating habits and lifestyle changes during COVID-19 lockdown: An Italian survey. J. Transl. Med. 2020, 18, 229. [Google Scholar] [CrossRef]
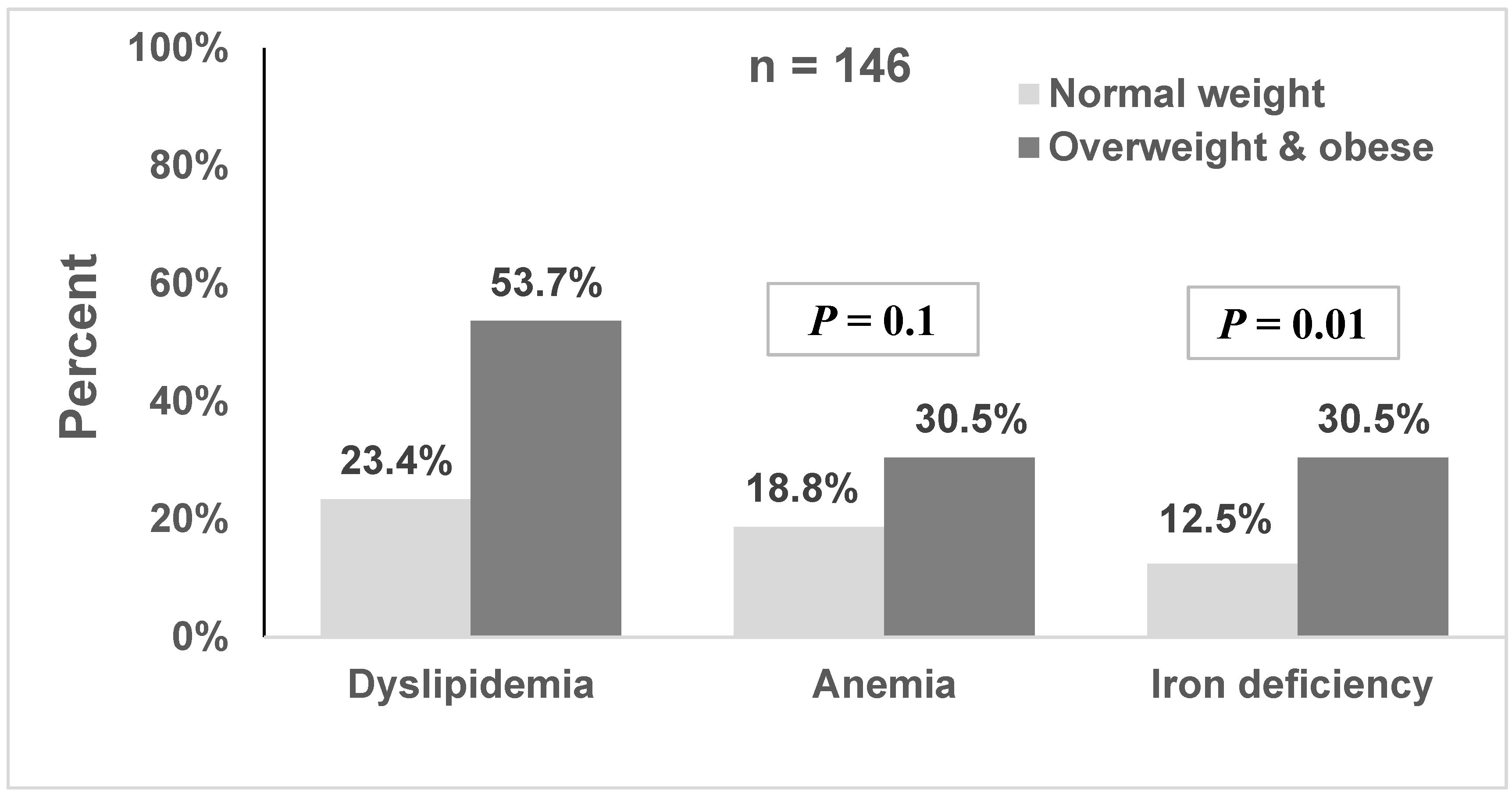
| Overweight/Obesity n = 84 | Normal Weight n = 65 | p-Value a | |
|---|---|---|---|
| Age, mean (SD) | 11.3 (0.5) | 11.2 (0.6) | 0.2 b |
| Sex | 0.9 | ||
| Males | 50 (59.5%) | 38 (58.5%) | |
| Females | 34 (40.5%) | 27 (41.5%) | |
| Village of residence | 0.009 | ||
| Higher SES village | 25 (29.8%) | 33 (50.8%) | |
| Lower SES village | 59 (70.2%) | 32 (49.2%) | |
| Monthly household income | 0.3 | ||
| <5000 NIS | 48 (57.8%) | 43 (67.2%) | |
| >5000 NIS | 35 (42.2%) | 21 (32.8%) | |
| Mean household crowding index (SD) | 1.5 (0.6) | 1.4 (0.8) | 0.5 b |
| Overweight/Obesity n = 82 | Normal Weight n = 64 | p Value a | |
|---|---|---|---|
| White blood cells (103/μL), mean (SD) | 7.9 (2.4) | 7.2 (2.2) | 0.07 |
| Neutrophils (103/μL), mean (SD) | 3.8 (1.6) | 3.5 (1.5) | 0.2 |
| Lymphocytes (103/μL), median (IQR) | 3.0 (1.0) | 2.8 (1.0) | 0.01 b |
| Platelets (103/μL), median (IQR) | 301.0 (101.5) | 282.0 (70.3) | 0.005 b |
| Glucose (mg/dL), mean (SD) | 94.0 (12.6) | 92.13 (16.8) | 0.4 |
| Hemoglobin (g/dL), mean (SD) | 12.3 (0.9) | 12.6 (0.8) | 0.08 |
| Hematocrit (%), mean (SD) | 37.0 (2.5) | 37.0 (2.3) | 0.98 |
| Mean Corpuscular Hemoglobin (pg), median (IQR) | 26.4 (2.5) | 27.3 (2.2) | 0.01 b |
| Mean Corpuscular Volume (fL), median (IQR) | 78.9 (5.6) | 79.6 (7.8) | 0.6 b |
| Iron (mcg/dL), median (IQR) | 56.0 (28.5) | 68.0 (38.8) | 0.002 b |
| Transferrin (mg/dL), mean (SD) | 288.1 (39.2) | 284.1 (34.3) | 0.5 |
| Transferrin-saturated (%), median (IQR) | 15.8 (8.8) | 19.4 (12.1) | 0.002 b |
| Ferritin (ng/mL), median (IQR) | 49.5 (41.8) | 40.0 (25.2) | 0.01 b |
| Vitamin B12 (pg/mL), mean (SD) | 527.6 (196.2) | 527.9 (202.5) | 0.99 |
| Folic Acid (ng/mL), median (IQR) | 6.8 (4.4) | 8.0 (3.2) | 0.2 b |
| Uric Acid (mg/mL), median (IQR) | 4.3 (1.6) | 3.5 (1.1) | <0.001 b |
| Triglycerides (mg/dL), median (IQR) | 104.5 (85.5) | 84.0 (45.8) | 0.001 b |
| Total cholesterol (mg/dL), median (IQR) | 151.5 (32.0) | 147.5 (29.8) | 0.3 b |
| HDL cholesterol (mg/dL), median (IQR) | 44.0 (12.3) | 58.0 (18.0) | <0.001 b |
| LDL cholesterol (mg/dL), mean (SD) | 77.9 (20.3) | 68.9 (17.8) | 0.005 |
| Protein (g/L), mean (SD) | 7.4 (0.3) | 7.2 (0.4) | 0.1 |
| Albumin (g/L), median (IQR) | 4.7 (0.3) | 4.7 (0.2) | 0.1 b |
| Globulin (g/L), median (IQR) | 2.7 (0.4) | 2.5 (0.4) | 0.002 b |
| C-Reactive Protein (mg/L), median (IQR) | 2.6 (4.9) | 0.3 (0.6) | <0.001 b |
| Obesity n = 61 | Overweight n = 21 | Normal Weight n = 64 | p Value | |
|---|---|---|---|---|
| White blood cells (103/μL), mean (SD) | 8.1 (2.1) | 7.60 (3.1) | 7.2 (2.2) | 0.1 |
| Neutrophils (103/μL), mean (SD) | 3.9 (1.4) | 3.66 (2.2) | 3.5 (1.5) | 0.4 |
| Lymphocytes (103/μL), median (IQR) | 3.0 (1.2) | 2.9 (1.0) | 2.8 (1.0) | 0.02 a |
| Platelets (103/μL), median (IQR) | 308.0 (100.0) | 291.0 (107.0) | 282.0 (70.3) | 0.006 b |
| Glucose (mg/dL), mean (SD) | 93.4 (12.8) | 95.7 (12.7) | 92.1 (16.9) | 0.6 |
| Hemoglobin (g/dL), mean (SD) | 12.3 (0.9) | 12.3 (1.0) | 12.64 (0.8) | 0.2 |
| Hematocrit (%), mean (SD) | 37.1 (2.4) | 36.59 (2.8) | 37.01 (2.3) | 0.7 |
| Mean Corpuscular Hemoglobin (pg), median (IQR) | 26.2 (2.6) | 26.6 (2.3) | 27.3 (2.2) | 0.03 c |
| Mean Corpuscular Volume (fL), median (IQR) | 79.4 (5.5) | 78.7 (5.2) | 79.6 (7.8) | 0.7 |
| Iron (mcg/dL), median (IQR) | 52.0 (29.0) | 64.0 (32.0) | 68.0 (38.8) | 0.001 d |
| Transferrin (mg/dL), mean (SD) | 293.3 (39.2) | 273.1 (36.2) | 284.1 (34.3) | 0.08 |
| Transferrin-saturated (%), median (IQR) | 15.0 (8.8) | 18.9 (9.0) | 19.4 (12.1) | <0.001 e |
| Ferritin (ng/mL), median (IQR) | 49.8 (44.5) | 47.2 (36.3) | 40.0 (25.2) | 0.02 b |
| Vitamin B12 (pg/mL), mean (SD) | 485.0 (163.5) | 651.3 (232.7) | 527.9 (202.5) | 0.004 f |
| Folic Acid (ng/mL), median (IQR) | 6.7 (4.2) | 6.78 (4.6) | 7.99 (3.2) | 0.3 |
| Uric Acid (mg/mL), median (IQR) | 4.6 (1.8) | 3.9 (1.5) | 3.5 (1.1) | <0.001 b |
| Triglycerides (mg/dL), median (IQR) | 109.0 (59.5) | 100.0 (55.0) | 84.0 (45.8) | 0.002 b |
| Total cholesterol (mg/dL), median (IQR) | 147.0 (36.5) | 154.0 (27.5) | 147.5 (29.8) | 0.6 |
| HDL cholesterol (mg/dL), median (IQR) | 42.0 (11.0) | 48.0 (15.0) | 58.0 (18.0) | <0.001 g |
| LDL cholesterol (mg/dL), mean (SD) | 78.1 (22.4) | 77.5 (12.9) | 68.9 (17.8) | 0.02 b |
| Protein (g/L), mean (SD) | 7.3 (0.3) | 7.3 (0.3) | 7.2 (0.4) | 0.3 |
| Albumin (g/L), median (IQR) | 4.6 (0.2) | 4.7 (0.3) | 4.7 (0.2) | 0.05 a |
| Globulin (g/L), median (IQR) | 2.7 (0.4) | 2.6 (0.5) | 2.5 (0.4) | 0.005 b |
| C-Reactive Protein (mg/L), median (IQR) | 3.9 (5.4) | 1.0 (1.6) | 0.3 (0.6) | <0.001 h |
| Model 1 Adjusted OR (95% CI) | p Value a | Model 2 Adjusted OR (95% CI) | p Value b | |
|---|---|---|---|---|
| Village | ||||
| Lower SES village | 8.57 (2.48–29.59) | 0.001 | 6.44 (1.96–21.09) | 0.002 |
| Higher SES village | Reference | Reference | ||
| SEX | ||||
| Males | 1.87 (0.68–5.13) | 0.2 | 2.26 (0.86–5.93) | 0.1 |
| Females | Reference | Reference | ||
| Lymphocytes (103/μL) | 2.00 (1.07–3.75) | 0.03 | 2.10 (1.18–3.96) | 0.02 |
| Platelets (103/μL) | 1.00 (0.99–1.01) | 0.7 | 1.002 (0.99–1.01) | 0.6 |
| Iron (mcg/dL) | 0.97 (0.95–0.99) | 0.004 | Not included | |
| Transferrin-saturated (%) | Not included | 0.95 (0.89–1.01) | 0.1 | |
| Ferritin (ng/mL) | 1.02 (0.99–1.04) | 0.1 | 1.02 (0.99–1.04) | 0.06 |
| Uric Acid (mg/mL) | 1.66 (1.01–2.72) | 0.04 | 2.01 (1.22–3.29) | 0.006 |
| Triglycerides (mg/dL) | 1.01 (0.99–1.02) | 0.08 | 1.01 (1.003–1.02) | 0.01 |
| LDL cholesterol (mg/dL) | 1.04 (1.01–1.07) | 0.004 | 1.03 (1.003–1.06) | 0.03 |
| HDL cholesterol (mg/dL) | 0.92 (0.88–0.97) | 0.001 | Not included | |
| C-Reactive Protein (mg/L) | Not included | 1.39 (1.10–1.76) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, E.; Na’amnih, W.; Shapira, M.; Ornoy, A.; Muhsen, K. Comparison between School-Age Children with and without Obesity in Nutritional and Inflammation Biomarkers. J. Clin. Med. 2022, 11, 6973. https://doi.org/10.3390/jcm11236973
Kassem E, Na’amnih W, Shapira M, Ornoy A, Muhsen K. Comparison between School-Age Children with and without Obesity in Nutritional and Inflammation Biomarkers. Journal of Clinical Medicine. 2022; 11(23):6973. https://doi.org/10.3390/jcm11236973
Chicago/Turabian StyleKassem, Eias, Wasef Na’amnih, Maanit Shapira, Asher Ornoy, and Khitam Muhsen. 2022. "Comparison between School-Age Children with and without Obesity in Nutritional and Inflammation Biomarkers" Journal of Clinical Medicine 11, no. 23: 6973. https://doi.org/10.3390/jcm11236973
APA StyleKassem, E., Na’amnih, W., Shapira, M., Ornoy, A., & Muhsen, K. (2022). Comparison between School-Age Children with and without Obesity in Nutritional and Inflammation Biomarkers. Journal of Clinical Medicine, 11(23), 6973. https://doi.org/10.3390/jcm11236973







