The Advantage of Immunohistochemical Staining for Evaluating Lymphovascular Invasion Is Limited for Patients with Esophageal Squamous Cell Carcinoma Invading the Muscularis Mucosa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Group
2.3. ER
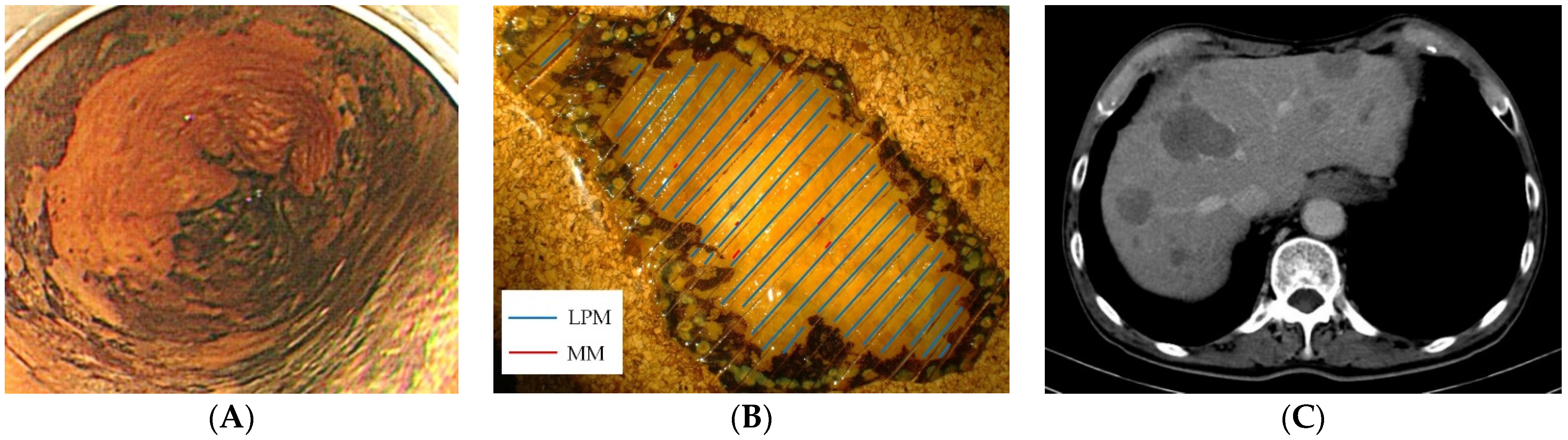
2.4. Histological Evaluation
2.5. Additional Prophylactic Therapy and Follow-Up
2.6. Outcomes
2.7. Statistical Analysis
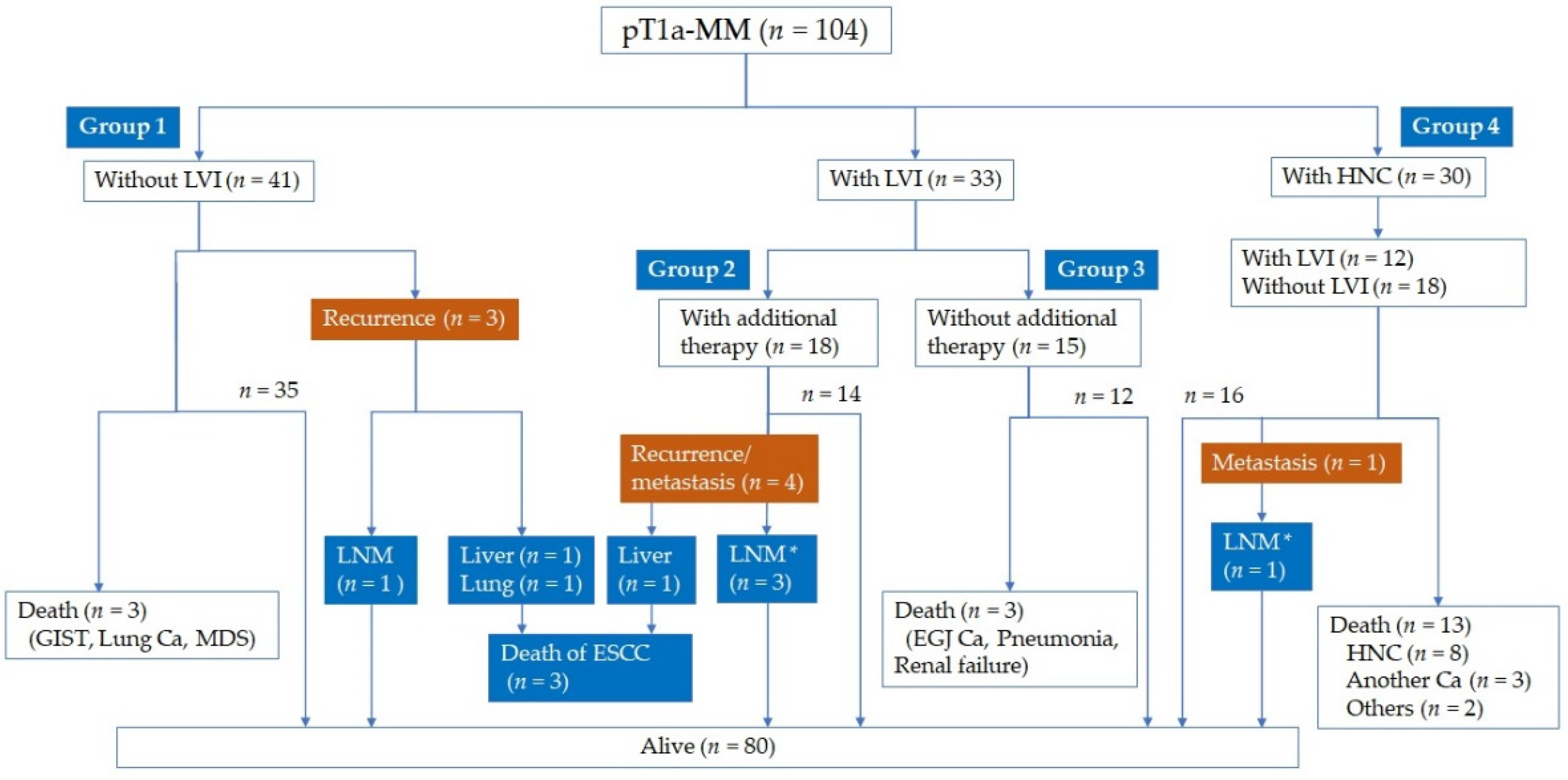
3. Results
3.1. Patient Demographics and Short-Term Outcomes of ER
3.2. Lymph Node or Distant Metastasis Based on LVI Evaluated by IHC
3.3. Clinical Course after ER
3.4. Factors for Metastasis and Survival in pT1a-MM ESCC
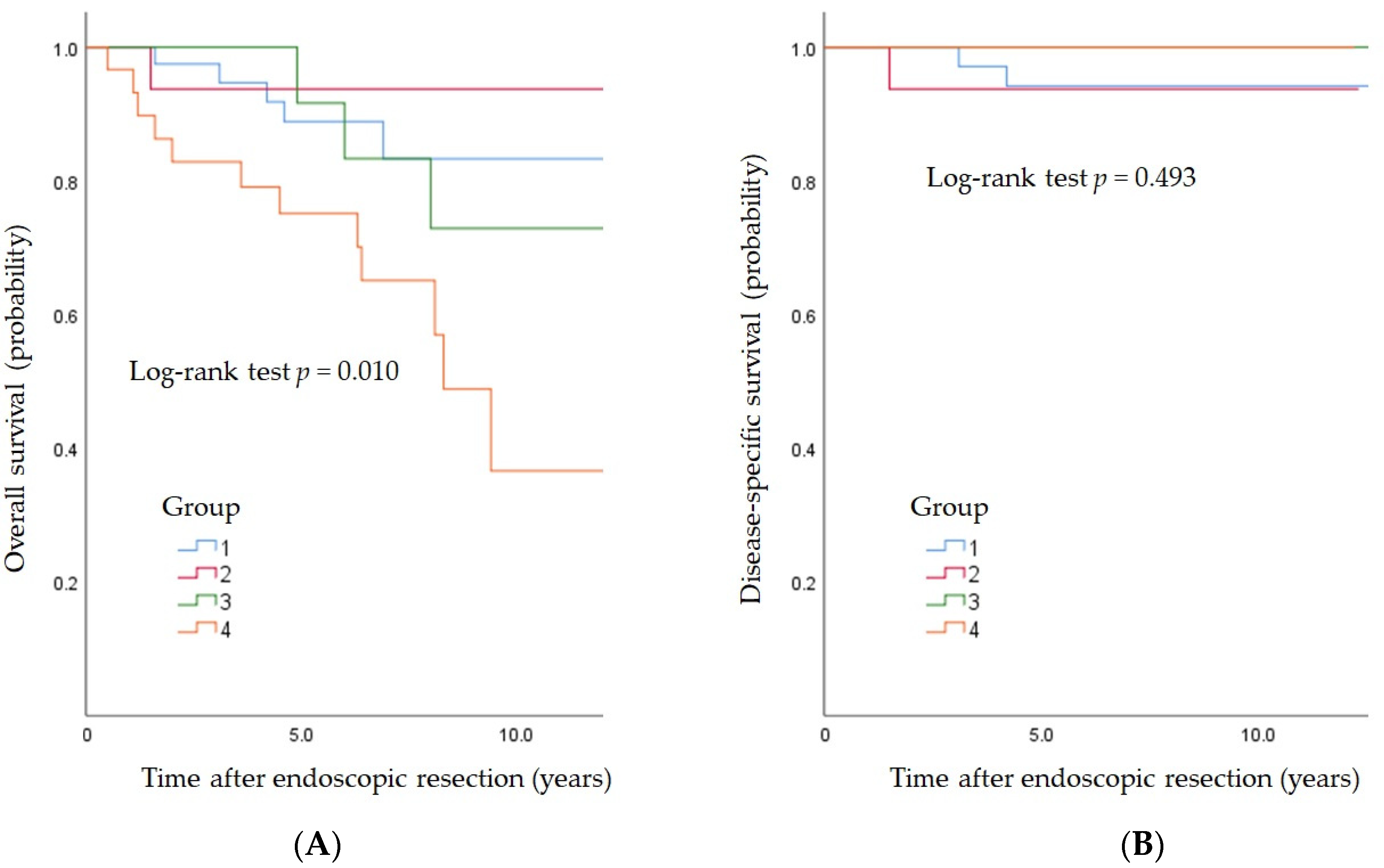
3.5. Long-Term Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asaki, S. Endoscopical polypectomy using high frequency current: Double-snare method of polypectomy for prevention of incidental bleeding and perforation. Tohoku J. Exp. Med. 1982, 136, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Yao, K.; Fujishiro, M.; Oda, I.; Nimura, S.; Yahagi, N.; Iishi, H.; Oka, M.; Ajioka, Y.; Ichinose, M.; et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig. Endosc. 2016, 28, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kashida, H.; Saito, Y.; Yahagi, N.; Yamano, H.; Saito, S.; Hisabe, T.; Yao, T.; Watanabe, M.; Yoshida, M.; et al. Japan Gastroenterological Endoscopy Society guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig. Endosc. 2020, 32, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Yamashina, T.; Ishihara, R.; Nagai, K.; Matsuura, N.; Matsui, F.; Ito, T.; Fujii, M.; Yamamoto, S.; Hanaoka, N.; Takeuchi, Y.; et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. Am. J. Gastroenterol. 2013, 108, 544–551. [Google Scholar] [CrossRef]
- Takahashi, K.; Hashimoto, S.; Mizuno, K.I.; Kobayashi, T.; Tominaga, K.; Sato, H.; Kohisa, J.; Ikarashi, S.; Hayashi, K.; Takeuchi, M.; et al. Management decision based on lymphovascular involvement leads to favorable outcomes after endoscopic treatment of esophageal squamous cell carcinoma. Endoscopy 2018, 50, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Katada, C.; Muto, M.; Momma, K.; Arima, M.; Tajiri, H.; Kanamaru, C.; Ooyanagi, H.; Endo, H.; Michida, T.; Hasuike, N.; et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy 2007, 39, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Nakanishi, Y.; Shimoda, T.; Iwasaki, M.; Igaki, H.; Tachimori, Y.; Kato, H.; Yamaguchi, H.; Saito, D.; Umemura, S. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: Analysis of 464 surgically resected cases. Mod. Pathol. 2006, 19, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Arima, M.; Iizuka, T.; Oyama, T.; Katada, C.; Kato, M.; Goda, K.; Goto, O.; Tanaka, K.; Yano, T.; et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. 2020, 32, 452–493. [Google Scholar] [CrossRef] [PubMed]
- Min, B.H.; Yang, J.W.; Min, Y.W.; Baek, S.Y.; Kim, S.; Kim, H.K.; Choi, Y.S.; Shim, Y.M.; Choi, Y.L.; Zo, J.I. Nomogram for prediction of lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. J. Gastroenterol. Hepatol. 2020, 35, 1009–1015. [Google Scholar] [CrossRef]
- Ma, D.W.; Jung, D.H.; Kim, J.H.; Park, J.J.; Youn, Y.H.; Park, H. Predicting lymph node metastasis for endoscopic resection of superficial esophageal squamous cell carcinoma. J. Thorac. Cardiovasc. Surg. 2019, 157, 397–402.e1. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.Y.; Huang, H.; Chen, W.Y.; Yan, H.J.; Wei, Z.T.; Wang, X.W.; Li, H.X.; Zheng, X.Y.; Tian, D. Risk factors for lymph node metastasis in T1 esophageal squamous cell carcinoma: A systematic review and meta-analysis. World J. Gastroenterol. 2021, 27, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Yoshio, T.; Ishiyama, A.; Tsuchida, T.; Tokura, J.; Namikawa, K.; Tokai, Y.; Yoshimizu, S.; Horiuchi, Y.; Hirasawa, T.; et al. Long-term outcomes of esophageal squamous cell carcinoma with invasion depth of pathological T1a-muscularis mucosae and T1b-submucosa by endoscopic resection followed by appropriate additional treatment. Dig. Endosc. 2022, 34, 793–804. [Google Scholar] [CrossRef]
- Hatta, W.; Koike, T.; Takahashi, S.; Shimada, T.; Hikichi, T.; Toya, Y.; Tanaka, I.; Onozato, Y.; Hamada, K.; Fukushi, D.; et al. Risk of metastatic recurrence after endoscopic resection for esophageal squamous cell carcinoma invading into the muscularis mucosa or submucosa: A multicenter retrospective study. J. Gastroenterol. 2021, 56, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Mitobe, J.; Ikegami, M.; Urashima, M.; Takahashi, H.; Goda, K.; Tajiri, H. Clinicopathological investigation of lymph node metastasis predictors in superficial esophageal squamous cell carcinoma with a focus on evaluation of lympho-vascular invasion. Scand. J. Gastroenterol. 2013, 48, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Minashi, K.; Yano, T.; Saito, Y.; Oda, I.; Nonaka, S.; Omori, T.; Sugiura, H.; Goda, K.; Kaise, M.; et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: A multicenter randomized controlled trial. J. Clin. Oncol. 2010, 28, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Okada, H.; Kawahara, Y.; Takenaka, R.; Shimizu, S.; Ohno, Y.; Onoda, T.; Sirakawa, Y.; Naomoto, Y.; Yamamoto, K. Lugol-voiding lesions are an important risk factor for a second primary squamous cell carcinoma in patients with esosphageal cancer or head and neck cancer. Am. J. Gastroenterol. 2011, 106, 858–866. [Google Scholar] [CrossRef]
- Oyama, T.; Inoue, H.; Arima, M.; Momma, K.; Omori, T.; Ishihara, R.; Hirasawa, D.; Takeuchi, M.; Tomori, A.; Goda, K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017, 14, 105–112. [Google Scholar] [CrossRef]
- Murata, Y.; Napoleon, B.; Odegaard, S. High-frequency endoscopic ultrasonography in the evaluation of superficial esophageal cancer. Endoscopy 2003, 35, 429–435; discussion 436. [Google Scholar] [CrossRef]
- Inoue, H.; Endo, M.; Takeshita, K.; Yoshino, K.; Muraoka, Y.; Yoneshima, H. A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg. Endosc. 1992, 6, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T.; Tomori, A.; Hotta, K.; Morita, S.; Kominato, K.; Tanaka, M.; Miyata, Y. Endoscopic submucosal dissection of early esophageal cancer. Clin. Gastroenterol. Hepatol. 2005, 3 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th ed.; part II and III. Esophagus 2017, 14, 37–65. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, A.I.; Schoppmann, S.F.; Birner, P. Lymphovascular invasion of tumor cells in lymph node metastases has a negative impact on survival in esophageal cancer. Surgery 2016, 160, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, J.; Zhang, J.; Luo, R.; Wang, Z.; Xu, H.; Huang, B. Risk factors associated with lymph node metastasis for early gastric cancer patients who underwent non-curative endoscopic resection: A systematic review and meta-analysis. J. Gastrointest. Surg. 2019, 23, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, P.; Li, J.; Qi, Q.; Sun, Z.; Shi, S.; Xie, Y.; Liu, S.; Wang, Y.; Du, L.; et al. Exosomal and intracellular miR-320b promotes lymphatic metastasis in esophageal squamous cell carcinoma. Mol. Ther. Oncolytics. 2021, 23, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Du, L.T.; Wang, Y.S.; Gao, S.Y.; Li, J.; Li, P.L.; Sun, Z.W.; Binang, H.; Wang, C.X. Development of a novel serum exosomal microRNA nomogram for the preoperative prediction of lymph node metastasis in esophageal squamous cell carcinoma. Front Oncol. 2020, 10, 573501. [Google Scholar] [CrossRef]
- van Vliet, E.P.; Heijenbrok-Kal, M.H.; Hunink, M.G.; Kuipers, E.J.; Siersema, P.D. Staging investigations for oesophageal cancer: A meta-analysis. Br. J. Cancer. 2008, 98, 547–557. [Google Scholar] [CrossRef]
| Total | Group 1 | Group 2 | Group 3 | Group 4 | p | |
|---|---|---|---|---|---|---|
| Number | 104 | 41 | 18 | 15 | 30 | |
| Sex, male/female | 94/10 | 35/6 | 17/1 | 13/2 | 29/1 | 0.372 |
| Age, mean ± SD, years | 64.8 ± 8.6 | 65.1 ± 8.9 | 65.5 ± 9.9 | 65.7 ± 7.3 | 63.4 ± 8.1 | 0.785 |
| Tumor size, mean ± SD, mm | 29.7 ± 16.4 | 27.8 ± 16.4 | 34.7 ± 12.4 | 32.3 ± 16.3 | 28.1 ± 18.5 | 0.423 |
| Morphological type, IIc | 42 (40.4%) | 15 (36.6%) | 7 (38.9%) | 9 (60%) | 11 (36.7%) | 0.418 |
| Infiltrative growth pattern, c | 2 (1.9%) | 0 | 0 | 0 | 2 (6.7%) | 0.170 |
| Lymphovascular invasion | 45 (43.3%) | 0 | 18 (100%) | 15 (100%) | 12 (40%) | <0.001 |
| Additional prophylactic therapy | 24 (23.1%) | 0 | 18 (100%) | 0 | 6 (20%) | <0.001 |
| Distant or lymph node metastasis | 8 (7.7%) | 3 (7.3%) | 4 (22.2%) | 0 | 1 (3.3%) | 0.056 |
| Death from all causes | 22 (21.2%) | 5 (12.2%) | 1 (5.6%) | 3 (20%) | 13 (43.3%) | 0.004 |
| Death from esophageal cancer | 3 (2.9%) | 2 (4.9%) | 1 (5.6%) | 0 | 0 | 0.498 |
| Only HE (n = 33) | HE + IHC (n = 33) | |
|---|---|---|
| Lymphoid invasion | 5 | 10 |
| Vascular invasion | 1 | 8 |
| Lymphoid or vascular invasion | 5 (15.1%) | 13 (39.4%) |
| LVI (−) Additional Therapy (−) | LVI (+) Additional Therapy (+) | LVI (+) Additional Therapy (−) | |
|---|---|---|---|
| Rate of metastasis | 5.1% (3/59) * | 20.8% (5/24) | 0% (0/21) |
| 11.1% (5/45) * | |||
| Case | Age | Sex | Location | Size (mm) | Type | ER | ly | v | Additional Therapy | Duration of Recurrence (Months) | Metastasis | Additional Therapy after Surgery or Recurrence | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73 | M | Ut | 46 | IIb | ESD | 0 | 1 | SR | - | LN (#105) | CT | Alive |
| 2 | 46 | M | Mt | 36 | IIb | ESD | 1 | 0 | SR | - | LN (#1) | CT | Alive |
| 3 | 64 | M | Lt | 45 | IIc | ESD | 1 | 0 | SR | - | LN (#2) | None | Alive |
| 4 | 61 | M | Lt | 94 | IIb | ESD | 1 | 0 | SR | - | LN (#9) | CT | Alive |
| 5 | 60 | M | Mt | 27 | IIb | ESD | 1 | 0 | RT | 10 | Liver LN (#110) | CT | Death of ESCC |
| 6 | 66 | M | Lt | 56 | IIb | ESD | 0 | 0 | - | 31 | Liver | CT | Death of ESCC |
| 7 | 63 | F | Lt | 25 | IIa | ESD | 0 | 0 | - | 28 | Lung | CT | Death of ESCC |
| 8 | 60 | M | Mt | 33 | IIa | ESD | 0 | 0 | - | 15 | LN (#2) | CT + SR | Alive |
| Disease-Free Survival, Hazard Ratio (95% CI) | Overall Survival, Hazard Ratio (95% CI) | |
|---|---|---|
| Age | 0.98 (0.93–1.03) | 1.03 (0.97–1.10) |
| Tumor size, per 10 mm | 1.31 (1.01–1.67) | 1.12 (0.81–1.53) |
| Morphological type, IIc | 0.75 (0.29–1.87) | 1.04 (0.34–3.21) |
| Infiltrative growth pattern, c | 1.47 (0.19–7.81) | 0.92 (0.11–5.67) |
| Lymphovascular invasion | 1.48 (0.50–4.17) | 2.77 (0.83–9.39) |
| Additional prophylactic therapy | 1.14(0.37–3.49) | 0.50 (0.10–1.96) |
| Head and neck cancer | 2.22 (0.85–5.41) | 5.16 (1.64–16.89) |
| Chemotherapy for head and neck cancer | 2.01 (0.46–7.85) | 5.06 (1.01–24.44) |
| Lymph node metastasis or recurrence | - | 45.41 (6.93–304.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobashi, A.; Aizawa, D.; Hara, Y.; Furuhashi, H.; Matsui, H.; Futakuchi, T.; Ono, S.; Toyoizumi, H.; Bazerbachi, F.; Yamauchi, T.; et al. The Advantage of Immunohistochemical Staining for Evaluating Lymphovascular Invasion Is Limited for Patients with Esophageal Squamous Cell Carcinoma Invading the Muscularis Mucosa. J. Clin. Med. 2022, 11, 6969. https://doi.org/10.3390/jcm11236969
Dobashi A, Aizawa D, Hara Y, Furuhashi H, Matsui H, Futakuchi T, Ono S, Toyoizumi H, Bazerbachi F, Yamauchi T, et al. The Advantage of Immunohistochemical Staining for Evaluating Lymphovascular Invasion Is Limited for Patients with Esophageal Squamous Cell Carcinoma Invading the Muscularis Mucosa. Journal of Clinical Medicine. 2022; 11(23):6969. https://doi.org/10.3390/jcm11236969
Chicago/Turabian StyleDobashi, Akira, Daisuke Aizawa, Yuko Hara, Hiroto Furuhashi, Hiroaki Matsui, Toshiki Futakuchi, Shingo Ono, Hirobumi Toyoizumi, Fateh Bazerbachi, Takashi Yamauchi, and et al. 2022. "The Advantage of Immunohistochemical Staining for Evaluating Lymphovascular Invasion Is Limited for Patients with Esophageal Squamous Cell Carcinoma Invading the Muscularis Mucosa" Journal of Clinical Medicine 11, no. 23: 6969. https://doi.org/10.3390/jcm11236969
APA StyleDobashi, A., Aizawa, D., Hara, Y., Furuhashi, H., Matsui, H., Futakuchi, T., Ono, S., Toyoizumi, H., Bazerbachi, F., Yamauchi, T., Suka, M., & Sumiyama, K. (2022). The Advantage of Immunohistochemical Staining for Evaluating Lymphovascular Invasion Is Limited for Patients with Esophageal Squamous Cell Carcinoma Invading the Muscularis Mucosa. Journal of Clinical Medicine, 11(23), 6969. https://doi.org/10.3390/jcm11236969






