HER2 Expression in Peritoneal Dissemination of High-Grade Serous Ovarian Carcinoma: A Comparative Study of Immunohistochemical Reactivity Using Four HER2 Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Tissue Samples
2.2. Immunohistochemical Staining and Analysis
2.3. Dual-Color Chromogenic In Situ Hybridization (dc-CISH)
2.4. Statistical Analyses
3. Results
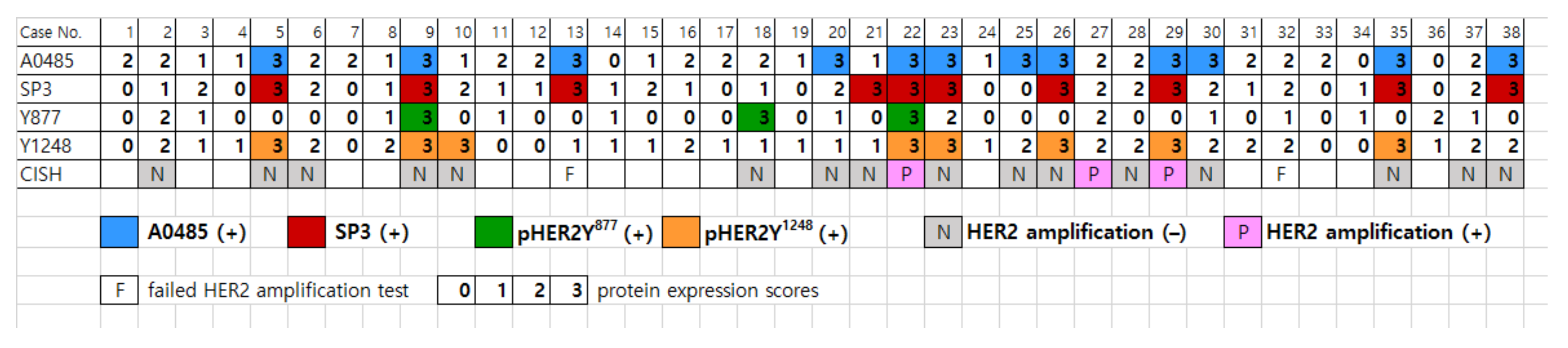
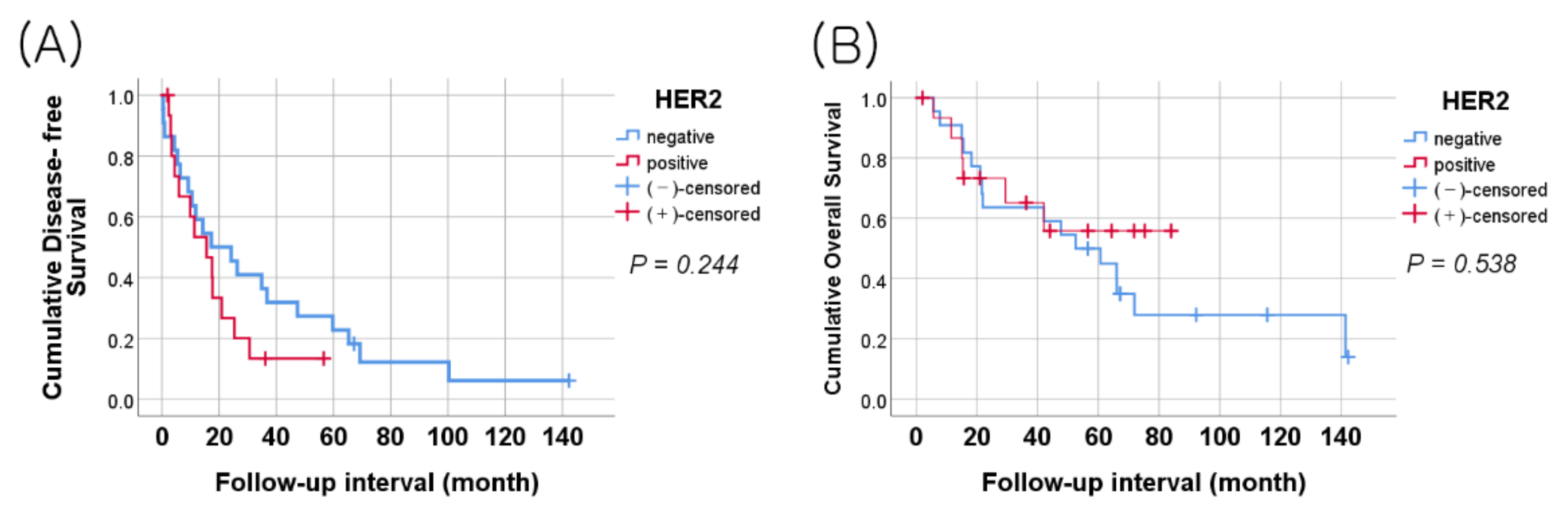
3.1. Relationships between HER2 Expression and Clinicopathological Characteristics
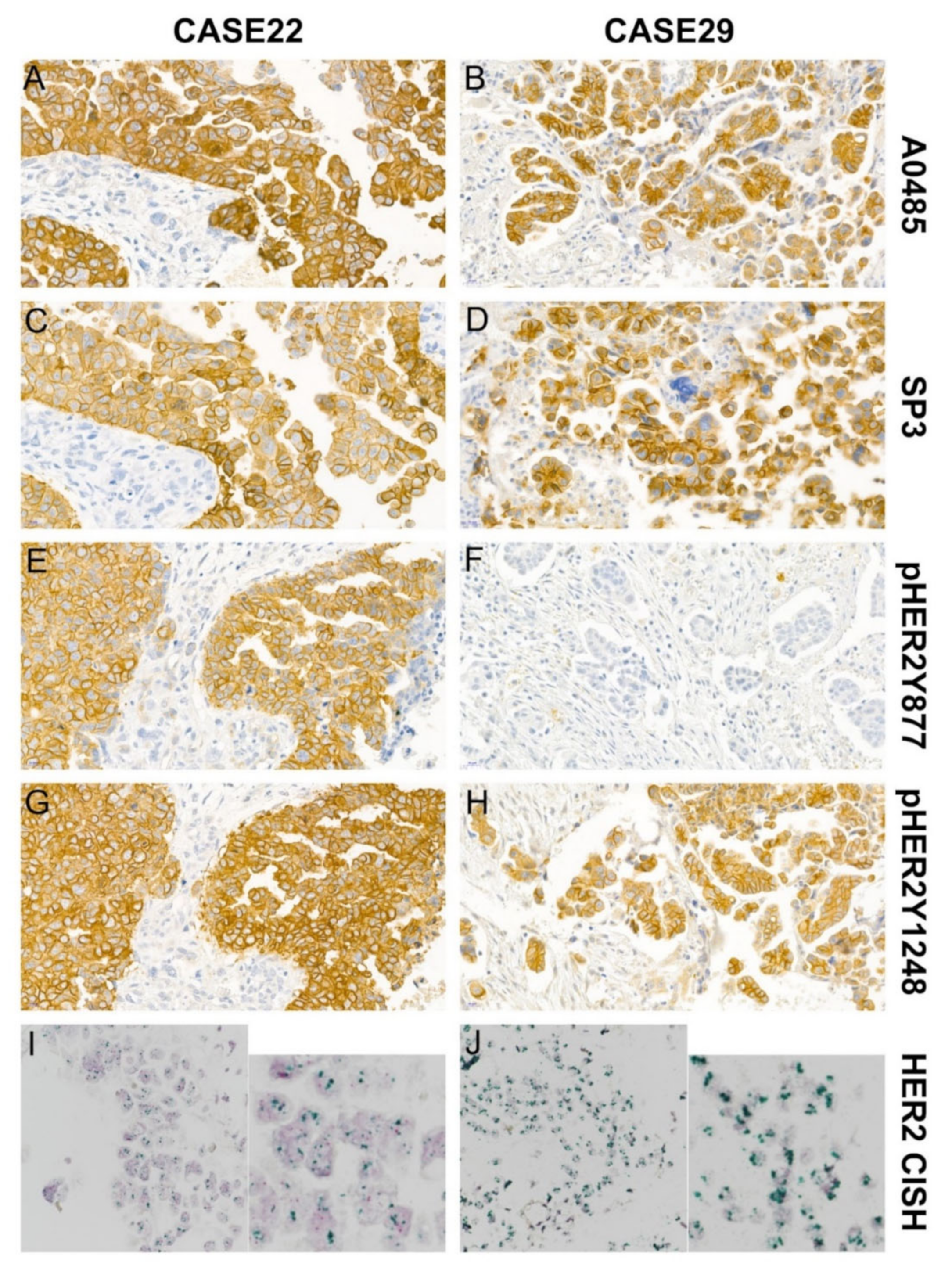
3.2. Comparing HER2 Expression with the Four Anti-HER2 Antibodies in Peritoneal Disseminated HGSOC
3.3. HER2 Gene Amplification by Dual-Color Chromogenic In Situ Hybridization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soslow, R.A.; Brebton, J.D.; Davidson, B.; Folkins, A.K.; Kong, C.S.; Malpica, A.; Soerjomataram, I.; Vang, S.I.R. High-grade serous carcinoma of the ovary. In WHO Classification of Tumours Editorial Board. Female Genital Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, E.Y.; Kim, O.; Schilder, J.M.; Coffey, D.M.; Cho, C.H.; Bast, R.C., Jr. Cell Origins of High-Grade Serous Ovarian Cancer. Cancers 2018, 10, 433. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Bartl, T.; Karacs, J.; Kreuzinger, C.; Pfaffinger, S.; Kendler, J.; Ciocsirescu, C.; Wolf, A.; Reinthaller, A.; Meyer, E.; Brandstetter, M.; et al. Tumor Growth Rate Estimates Are Independently Predictive of Therapy Response and Survival in Recurrent High-Grade Serous Ovarian Cancer Patients. Cancers 2021, 13, 1076. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Carvajal-Hausdorf, D.E.; Schalper, K.A.; Bai, Y.; Black, J.; Santin, A.D.; Rimm, D.L. Objective, domain-specific HER2 measurement in uterine and ovarian serous carcinomas and its clinical significance. Gynecol. Oncol. 2017, 145, 154–158. [Google Scholar] [CrossRef]
- Gordon, M.S.; Matei, D.; Aghajanian, C.; Matulonis, U.A.; Brewer, M.; Fleming, G.F.; Hainsworth, J.D.; Garcia, A.A.; Pegram, M.D.; Schilder, R.J.; et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: Potential predictive relationship with tumor HER2 activation status. J. Clin. Oncol. 2006, 24, 4324–4332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Weng, Z.; Li, Q.; Zhao, L.; Yu, N.; Deng, L.; Xu, W.; Yang, Y.; Zhu, Z.; et al. A new anti-HER2 antibody that enhances the anti-tumor efficacy of trastuzumab and pertuzumab with a distinct mechanism of action. Mol. Immunol. 2020, 119, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Cicenas, J.; Urban, P.; Kung, W.; Vuaroqueaux, V.; Labuhn, M.; Wight, E.; Eppenberger, U.; Eppenberger-Castori, S. Phosphorylation of tyrosine 1248-ERBB2 measured by chemiluminescence-linked immunoassay is an independent predictor of poor prognosis in primary breast cancer patients. Eur. J. Cancer 2006, 42, 636–645. [Google Scholar] [CrossRef]
- Dokmanovic, M.; Wu, Y.; Shen, Y.; Chen, J.; Hirsch, D.S.; Wu, W.J. Trastuzumab-induced recruitment of Csk-homologous kinase (CHK) to ErbB2 receptor is associated with ErbB2-Y1248 phosphorylation and ErbB2 degradation to mediate cell growth inhibition. Cancer Biol. Ther. 2014, 15, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Adelaide, J.; Goncalves, A.; Repellini, L.; Sircoulomb, F.; Letessier, A.; Finetti, P.; Geneix, J.; Charafe-Jauffret, E.; Bertucci, F.; et al. ERBB2 phosphorylation and trastuzumab sensitivity of breast cancer cell lines. Oncogene 2007, 26, 7163–7169. [Google Scholar] [CrossRef]
- Burguin, A.; Furrer, D.; Ouellette, G.; Jacob, S.; Diorio, C.; Durocher, F. Trastuzumab effects depend on HER2 phosphorylation in HER2-negative breast cancer cell lines. PLoS ONE 2020, 15, e0234991. [Google Scholar] [CrossRef]
- Amin, M.; Edge, S.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017. [Google Scholar]
- Lee, Y.M.; Yeo, M.K.; Seong, I.O.; Kim, K.H. Nuclear Expression of CD133 Is Associated with Good Prognosis in Patients with Colorectal Adenocarcinoma. Anticancer Res. 2018, 38, 4819–4826. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Christ, J.; Fitzgibbons, P.L.; Hamilton, S.R.; Kakar, S.; Shah, M.A.; Tang, L.H.; Troxell, M.L.; Members of the Cancer Biomarker Reporting Committee, College of American Pathologists. Template for Reporting Results of HER2 (ERBB2) Biomarker Testing of Specimens from Patients with Adenocarcinoma of the Stomach or Esophagogastric Junction. Arch. Pathol. Lab. Med. 2015, 139, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.C.; Pintye, M.; Chang, L.C.; Chen, H.Y.; Yeh, K.Y.; Chein, H.P.; Lee, N.; Chen, J.R. Dual-colour chromogenic in-situ hybridization is a potential alternative to fluorescence in-situ hybridization in HER2 testing. Histopathology 2011, 59, 984–992. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, R.; Au, A.; Marquez, A.; Yu, Y.; Shi, Z. Determination of HER2 gene amplification by chromogenic in situ hybridization (CISH) in archival breast carcinoma. Mod. Pathol. 2002, 15, 657–665. [Google Scholar] [CrossRef]
- Nunes, C.B.; Rocha, R.M.; Buzelin, M.A.; Balabram, D.; de Souza Foureaux, F.; Porto, S.S.; Gobbi, H. False positivity in HER2 testing of breast cancer: Novel paths for approaching an old dilemma. J. Clin. Pathol. 2013, 66, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Wang, Y.H.; Hsu, C.Y.; Chen, Y.J.; Lai, C.R.; Hang, J.F. Analytical validation of human epidermal growth factor receptor 2 immunohistochemistry. by the use of the A0485 antibody versus the 4B5 antibody and breast versus gastric scoring guidelines in ovarian clear cell carcinoma. Histopathology 2021, 79, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Koopman, T.; van der Vegt, B.; Dijkstra, M.; Bart, J.; Duiker, E.; Wisman, G.B.A.; de Bock, G.H.; Hollema, H. HER2 immunohistochemistry in endometrial and ovarian clear cell carcinoma: Discordance between antibodies and with in-situ hybridisation. Histopathology 2018, 73, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.; Kavuri, S.M.; Searleman, A.C.; Shen, W.; Shen, D.; Koboldt, D.C.; Monsey, J.; Goel, N.; Aronson, A.B.; Li, S.; et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013, 3, 224–237. [Google Scholar] [CrossRef]
- Greulich, H.; Kaplan, B.; Mertins, P.; Chen, T.H.; Tanaka, K.E.; Yun, C.H.; Zhang, X.; Lee, S.H.; Cho, J.; Ambrogio, L.; et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc. Natl. Acad. Sci. USA 2012, 109, 14476–14481. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E.; Narasanna, A.; Perez-Torres, M.; Xiang, B.; Wu, F.Y.; Yang, S.; Carpenter, G.; Gazdar, A.F.; Muthuswamy, S.K.; Arteaga, C.L. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006, 10, 25–38. [Google Scholar] [CrossRef]
- Mazieres, J.; Peters, S.; Lepage, B.; Cortot, A.B.; Barlesi, F.; Beau-Faller, M.; Besse, B.; Blons, H.; Mansuet-Lupo, A.; Urban, T.; et al. Lung cancer that harbors an HER2 mutation: Epidemiologic characteristics and therapeutic perspectives. J. Clin. Oncol. 2013, 31, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Zabransky, D.J.; Yankaskas, C.L.; Cochran, R.L.; Wong, H.Y.; Croessmann, S.; Chu, D.; Kavuri, S.M.; Red Brewer, M.; Rosen, D.M.; Dalton, W.B.; et al. HER2 missense mutations have distinct effects on oncogenic signaling and migration. Proc. Natl. Acad. Sci. USA 2015, 112, E6205–E6214. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.H.; Tang, L.; Dong, H.; Dong, Z.; Zhang, L.; Fu, J.; Su, X.; Zhang, T.; Fu, H.; Han, L.; et al. Oncogenic HER2 fusions in gastric cancer. J. Transl. Med. 2015, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Bose, R.; Gao, F.; Freedman, R.A.; Telli, M.L.; Kimmick, G.; Winer, E.; Naughton, M.; Goetz, M.P.; Russell, C.; et al. Neratinib Efficacy and Circulating Tumor DNA Detection of HER2 Mutations in HER2 Nonamplified Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5687–5695. [Google Scholar] [CrossRef]
- Li, B.T.; Ross, D.S.; Aisner, D.L.; Chaft, J.E.; Hsu, M.; Kako, S.L.; Kris, M.G.; Varella-Garcia, M.; Arcila, M.E. HER2 Amplification and HER2 Mutation Are Distinct Molecular Targets in Lung Cancers. J. Thorac. Oncol. 2016, 11, 414–419. [Google Scholar] [CrossRef]
- Zhang, H.; Katerji, H.; Turner, B.M.; Hicks, D.G. HER2-Low Breast Cancers. Am. J. Clin. Pathol. 2022, 157, 328–336. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, Y.; Wu, D.; Lin, E.; Xia, Q. Intratumoral and intertumoral heterogeneity of HER2 immunohistochemical expression in gastric cancer. Pathol. Res. Pract. 2020, 216, 153229. [Google Scholar] [CrossRef]
- Xu, C.; Sun, M.; Jin, M.; Li, Z.; Qin, R.; Ren, G.; Sun, W.; Chen, L.; Luan, L.; Liu, Y.; et al. Dual block HER2 assessment increased HER2 immunohistochemistry positive rate in resected specimens of gastric cancer: A prospective multicenter clinical trial from China. Diagn. Pathol. 2022, 17, 54. [Google Scholar] [CrossRef]
- Lin, C.H.; Wen, C.H.; Liu, C.H.; Yang, C.H. Transferred-tissue Microarray for Fluorescence In Situ Hybridization Test for Human Epidermal Growth Factor Receptor 2 in Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 187–193. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer, C.E., Jr.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencher, L.; et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J. Clin. Oncol. 2020, 38, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.A.A.; Makboul, R.; Elsers, D.A.H.; Elsaba, T.; Thalab, A.; Shaaban, O.M. Pattern of HER-2 Gene Amplification and Protein Expression in Benign, Borderline, and Malignant Ovarian Serous and Mucinous Neoplasms. Int. J. Gynecol. Pathol. 2017, 36, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Karaferic, A.; Jovanovic, D.; Jelic, S. Expression of HER2/neu, estrogen and progesterone receptors, CA 125 and CA19-9 on cancer cell membrane in patients with serous and mucinous carcinoma of the ovary. J. BUON 2009, 14, 635–639. [Google Scholar] [PubMed]
- Nofech-Mozes, S.; Khalifa, M.A.; Ismiil, N.; Saad, R.S.; Hanna, W.M.; Covens, A.; Ghorab, Z. Immunophenotyping of serous carcinoma of the female genital tract. Mod. Pathol. 2008, 21, 1147–1155. [Google Scholar] [CrossRef]
- Lanitis, E.; Dangaj, D.; Hagemann, I.S.; Song, D.G.; Best, A.; Sandaltzopoulos, R.; Coukos, G.; Powell, D.J., Jr. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS ONE 2012, 7, e49829. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Koo, J.S. Differential expression of lipid metabolism-related proteins in different breast cancer subtypes. PLoS ONE 2015, 10, e0119473. [Google Scholar] [CrossRef]
- Ravacci, G.R.; Brentani, M.M.; Tortelli, T.C.; Torrinhas, R.S.; Santos, J.R.; Logullo, A.F.; Waitzberg, D.L. Docosahexaenoic Acid Modulates a HER2-Associated Lipogenic Phenotype, Induces Apoptosis, and Increases Trastuzumab Action in HER2-Overexpressing Breast Carcinoma Cells. BioMed Res. Int. 2015, 2015, 838652. [Google Scholar] [CrossRef] [PubMed]
- Bonello, M.; Sims, A.H.; Langdon, S.P. Human epidermal growth factor receptor targeted inhibitors for the treatment of ovarian cancer. Cancer Biol. Med. 2018, 15, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Ohmichi, M.; Shibuya, T.; Takahashi, T.; Tsutsumi, S.; Takahashi, K.; Kurachi, H. Gefitinib (ZD1839) increases the efficacy of cisplatin in ovarian cancer cells. Cancer Biol. Ther. 2012, 13, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Hirte, H.; Oza, A.; Swenerton, K.; Ellard, S.L.; Grimshaw, R.; Fisher, B.; Tsao, M.; Seymour, L. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149). Gynecol. Oncol. 2010, 118, 308–312. [Google Scholar] [CrossRef]
- Tjulandin, S.; Moiseyenko, V.; Semiglazov, V.; Manikhas, G.; Learoyd, M.; Saunders, A.; Stuart, M.; Keilholz, U. Phase I, dose-finding study of AZD8931, an inhibitor of EGFR (erbB1), HER2 (erbB2) and HER3 (erbB3) signaling, in patients with advanced solid tumors. Investig. New Drugs 2014, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the Gynecologic Oncology Group. J. Clin. Oncol. 2003, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Akita, R.W.; Fox, W.D.; Lewis, G.D.; Higgins, B.; Pisacane, P.I.; Lofgren, J.A.; Tindell, C.; Evans, D.P.; Maiese, K.; et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002, 2, 127–137. [Google Scholar] [CrossRef] [PubMed]
| A0485 | SP3 | pHER2Y877 | pHER2Y1248 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (−) | (+) | p * | (−) | (+) | p * | (−) | (+) | p * | (−) | (+) | p * | |
| Case no. | 26 | 12 | 28 | 10 | 35 | 3 | 30 | 8 | ||||
| Age | 0.337 ** | 0.460 | 0.081 | 0.709 | ||||||||
| <60 | 13 | 8 | 14 | 7 | 21 | 0 | 16 | 5 | ||||
| ≥60 | 13 | 4 | 14 | 3 | 14 | 3 | 14 | 3 | ||||
| pT stage | 0.270 | 0.709 | 0.538 | 1.000 | ||||||||
| 2 | 7 | 6 | 9 | 4 | 13 | 0 | 10 | 3 | ||||
| 3 | 19 | 6 | 19 | 6 | 22 | 3 | 20 | 5 | ||||
| pNode | 0.395 | 0.156 | 1.000 | 0.307 | ||||||||
| (−) | 20 | 11 | 21 | 10 | 28 | 3 | 23 | 8 | ||||
| (+) | 6 | 1 | 7 | 0 | 7 | 0 | 7 | 0 | ||||
| pTNM stage | 0.270 *** | 0.709 *** | 0.538 *** | 1.000 *** | ||||||||
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| II | 7 | 6 | 9 | 4 | 13 | 0 | 10 | 3 | ||||
| III | 17 | 5 | 16 | 6 | 19 | 3 | 17 | 5 | ||||
| IV | 2 | 1 | 3 | 0 | 3 | 0 | 3 | 0 | ||||
| A0485 | |||
|---|---|---|---|
| Matched Pairs | Negative (26 cases) | Positive (12 cases) | p * |
| SP3 (no.) | 0.625 | ||
| negative | 25 | 3 | |
| positive | 1 | 9 | |
| pHER2Y877 | 0.012 | ||
| negative | 25 | 10 | |
| positive | 1 | 2 | |
| pHER2Y1248 | 0.219 | ||
| negative | 25 | 5 | |
| positive | 1 | 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, M.-K.; Kim, S.; Yoo, H.J.; Suh, K.-S.; Kim, K.-H. HER2 Expression in Peritoneal Dissemination of High-Grade Serous Ovarian Carcinoma: A Comparative Study of Immunohistochemical Reactivity Using Four HER2 Antibodies. J. Clin. Med. 2022, 11, 6963. https://doi.org/10.3390/jcm11236963
Yeo M-K, Kim S, Yoo HJ, Suh K-S, Kim K-H. HER2 Expression in Peritoneal Dissemination of High-Grade Serous Ovarian Carcinoma: A Comparative Study of Immunohistochemical Reactivity Using Four HER2 Antibodies. Journal of Clinical Medicine. 2022; 11(23):6963. https://doi.org/10.3390/jcm11236963
Chicago/Turabian StyleYeo, Min-Kyung, Sup Kim, Heon Jong Yoo, Kwang-Sun Suh, and Kyung-Hee Kim. 2022. "HER2 Expression in Peritoneal Dissemination of High-Grade Serous Ovarian Carcinoma: A Comparative Study of Immunohistochemical Reactivity Using Four HER2 Antibodies" Journal of Clinical Medicine 11, no. 23: 6963. https://doi.org/10.3390/jcm11236963
APA StyleYeo, M.-K., Kim, S., Yoo, H. J., Suh, K.-S., & Kim, K.-H. (2022). HER2 Expression in Peritoneal Dissemination of High-Grade Serous Ovarian Carcinoma: A Comparative Study of Immunohistochemical Reactivity Using Four HER2 Antibodies. Journal of Clinical Medicine, 11(23), 6963. https://doi.org/10.3390/jcm11236963






