Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis
Abstract
1. Introduction
2. Materials and Methods
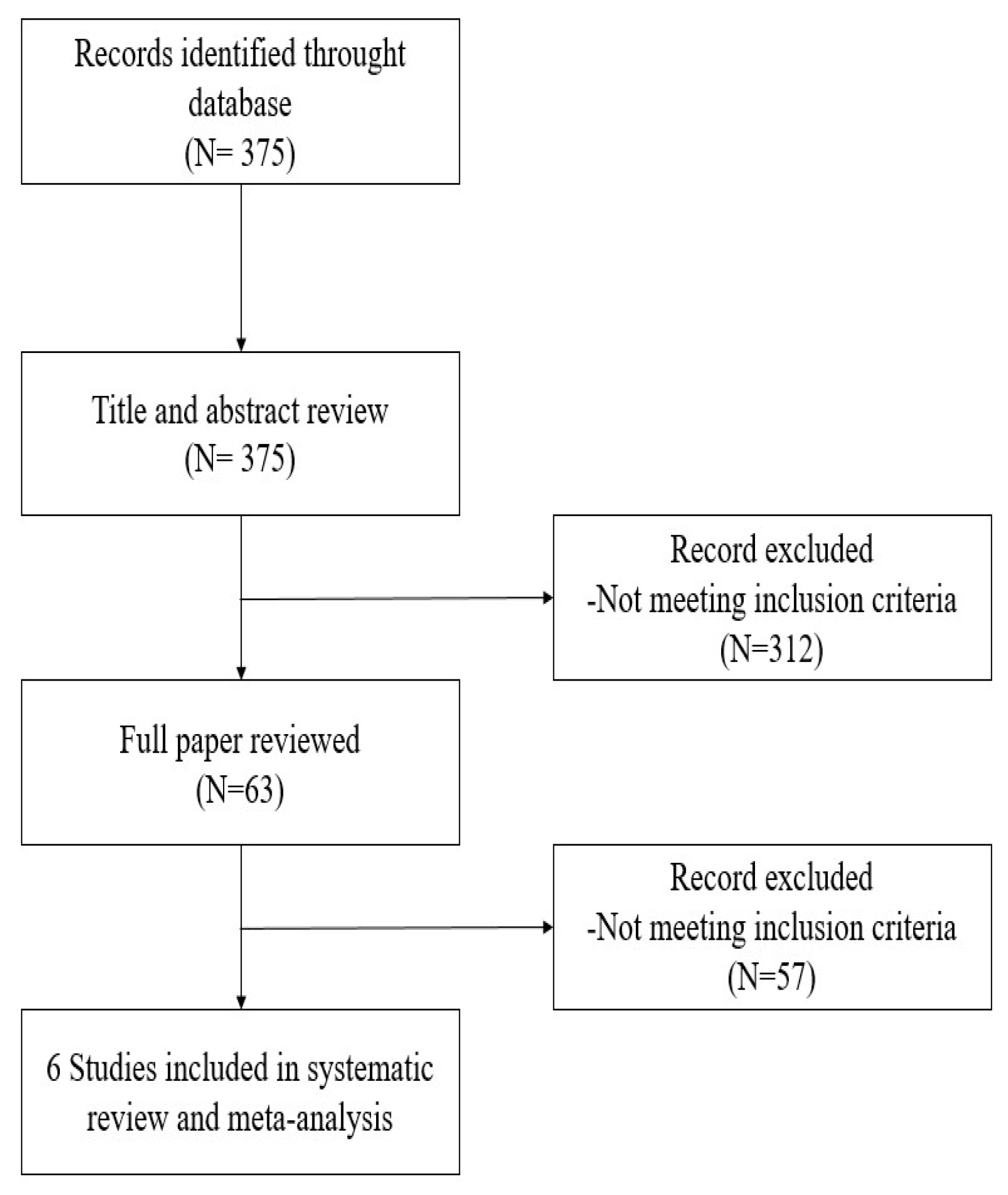
2.1. Search Strategy and Selection Criteria
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Study Characteristics
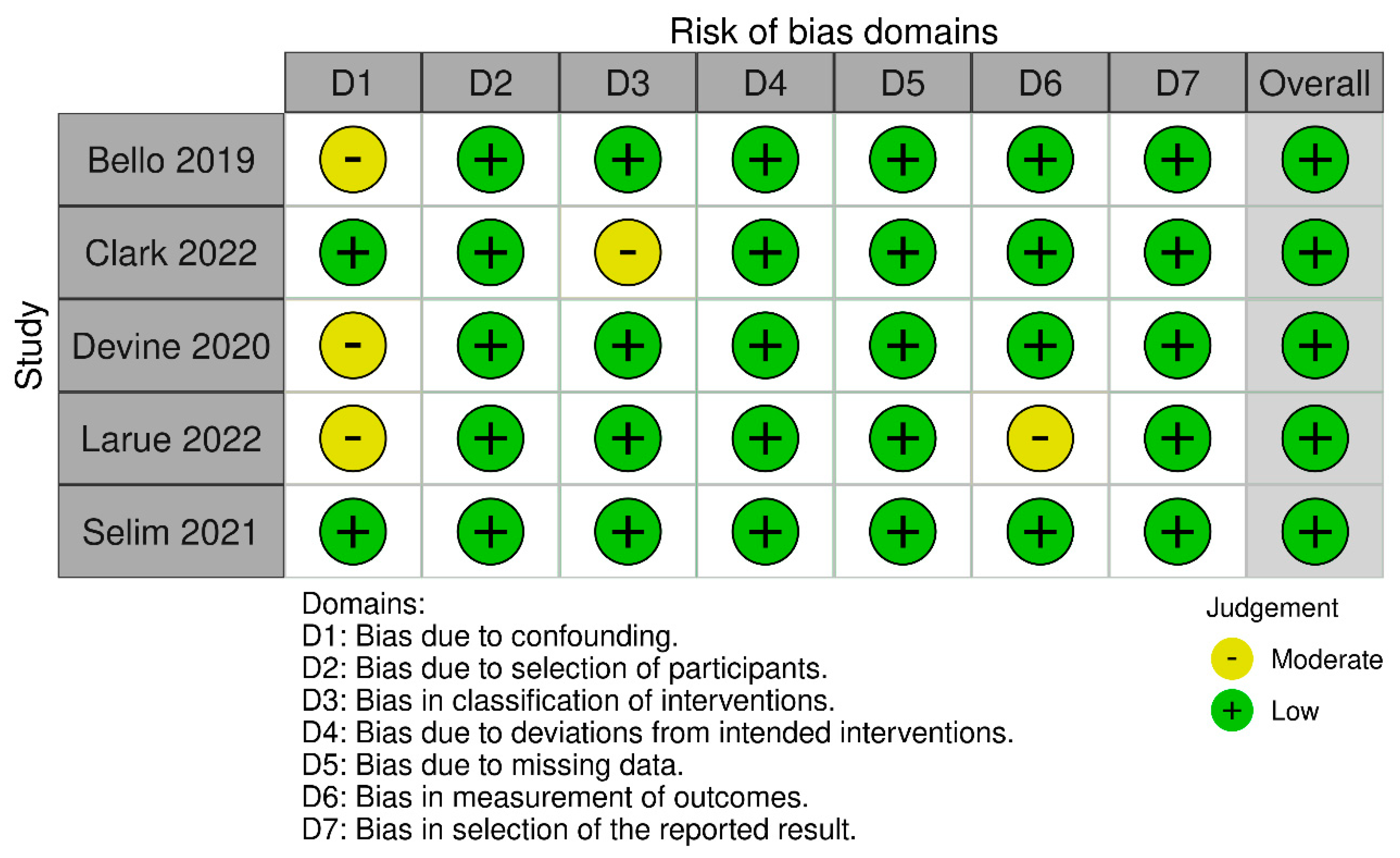
3.2. Risk of Bias
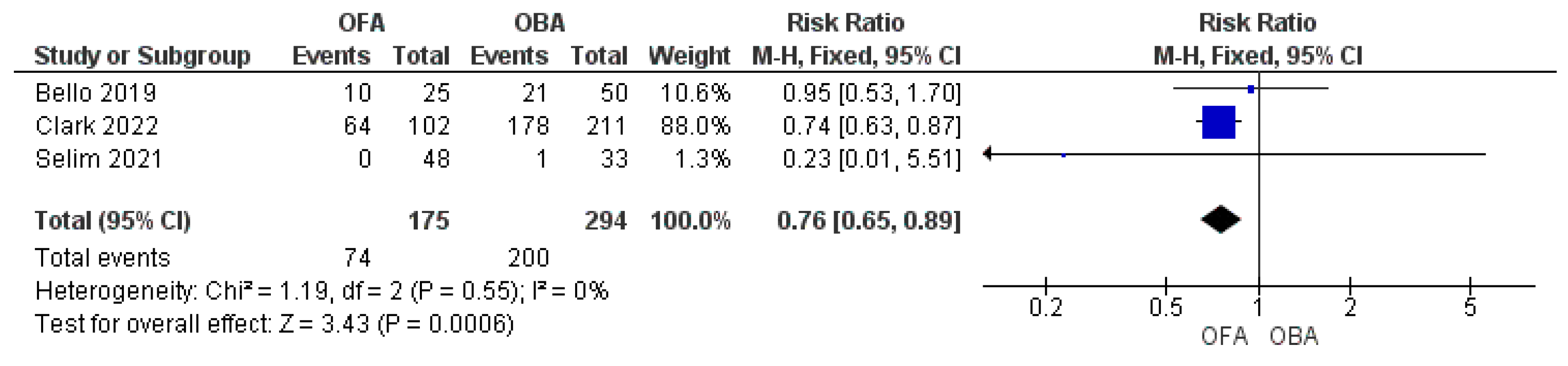
3.3. Complications
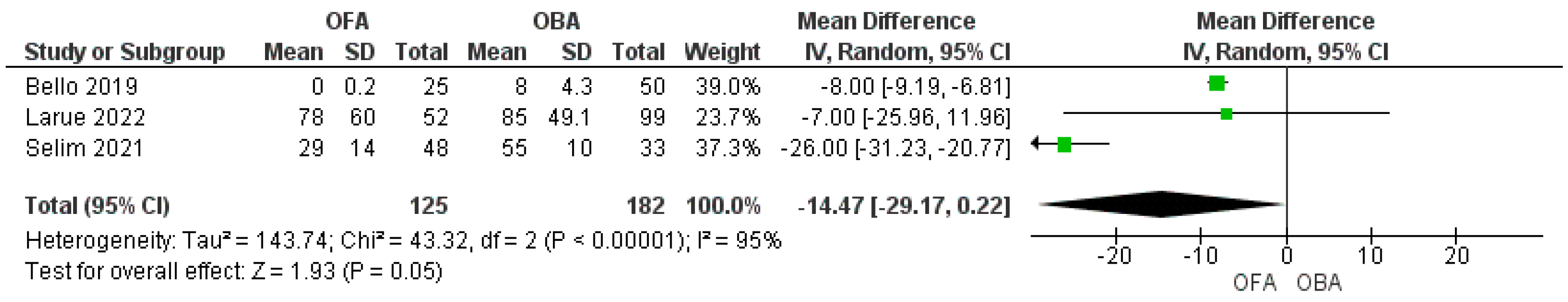
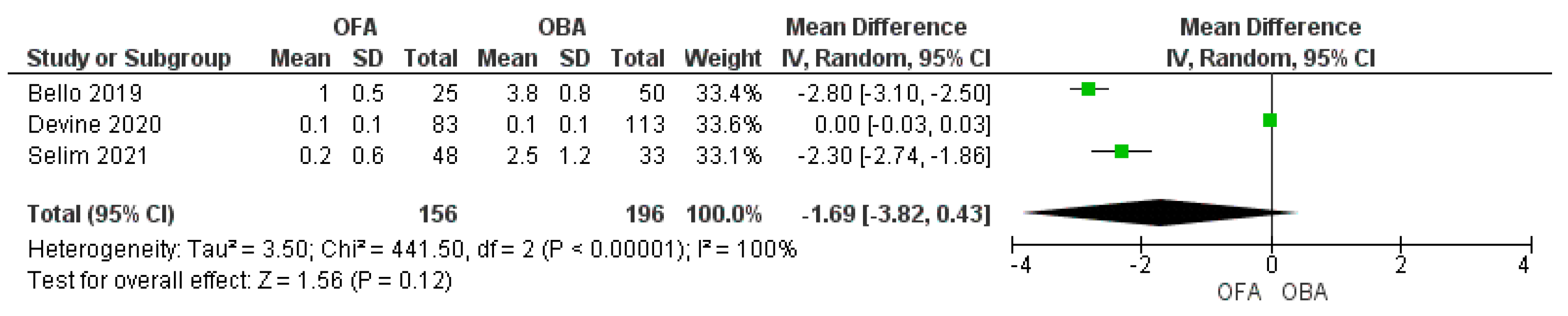
3.4. Morphine Equivalent Consumption
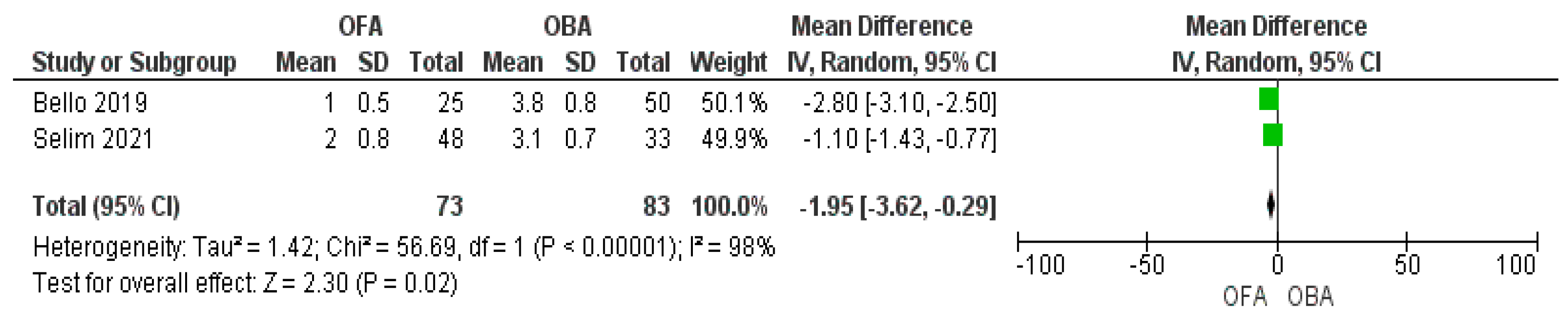
3.5. Pain Score
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Tonner, P.H. Balanced Anaesthesia Today. Best Pract. Res. Clin. Anaesthesiol. 2005, 19, 475–484. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid Complications and Side Effects. Pain Physician 2008, 11 (Suppl. S2), S105–S120. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Kodumudi, V.; Sramcik, J.; Kaye, A.D. The Opioid Crisis: A Comprehensive Overview. Curr. Pain Headache Rep. 2018, 22, 16. [Google Scholar] [CrossRef]
- Hah, J.M.; Bateman, B.T.; Ratliff, J.; Curtin, C.; Sun, E. Chronic Opioid Use after Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesth. Analg. 2017, 125, 1733–1740. [Google Scholar] [CrossRef]
- Patvardhan, C.; Ferrante, M. Opiate Free Anaesthesia and Future of Thoracic Surgery Anaesthesia. J. Vis. Surg. 2018, 4, 253. [Google Scholar] [CrossRef]
- Tempe, D.K.; Sawhney, C. Opioid-Free Anesthesia for Thoracic Surgery: A Step Forward. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3041–3043. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Davies, H.T.; Crombie, I.K.; Tavakoli, M. When Can Odds Ratios Mislead? BMJ 1998, 316, 989–991. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Bello, M.; Oger, S.; Bedon-Carte, S.; Vielstadte, C.; Leo, F.; Zaouter, C.; Ouattara, A. Effect of Opioid-Free Anaesthesia on Postoperative Epidural Ropivacaine Requirement after Thoracic Surgery: A Retrospective Unmatched Case-Control Study. Anaesth. Crit. Care Pain Med. 2019, 38, 499–505. [Google Scholar] [CrossRef]
- Selim, J.; Jarlier, X.; Clavier, T.; Boujibar, F.; Dusséaux, M.-M.; Thill, J.; Borderelle, C.; Plé, V.; Baste, J.-M.; Besnier, E.; et al. Impact of Opioid-Free Anesthesia after Video-Assisted Thoracic Surgery: A Propensity Score Study. Ann. Thorac. Surg. 2022, 114, 218–224. [Google Scholar] [CrossRef]
- Larue, A.; Jacquet-Lagrèze, M.; Ruste, M.; Tronc, F.; Fellahi, J.-L. Opioid-Free Anaesthesia for Video-Assisted Thoracoscopic Surgery: A Retrospective Cohort Study with Propensity Score Analysis. Anaesth. Crit. Care Pain Med. 2022, 41, 101089. [Google Scholar] [CrossRef]
- Devine, G.; Cheng, M.; Martinez, G.; Patvardhan, C.; Aresu, G.; Peryt, A.; Coonar, A.S.; Roscoe, A. Opioid-Free Anesthesia for Lung Cancer Resection: A Case-Control Study. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3036–3040. [Google Scholar] [CrossRef]
- Clark, I.C.; Allman, R.D.; Rogers, A.L.; Goda, T.S.; Smith, K.; Chanas, T.; Oliver, A.L.; Iannettoni, M.D.; Anciano, C.J.; Speicher, J.E. Multimodal Pain Management Protocol to Decrease Opioid Use and to Improve Pain Control after Thoracic Surgery. Ann. Thorac. Surg. 2022, 114, 2008–2014. [Google Scholar] [CrossRef]
- An, G.; Zhang, Y.; Chen, N.; Fu, J.; Zhao, B.; Zhao, X. Opioid-Free Anesthesia Compared to Opioid Anesthesia for Lung Cancer Patients Undergoing Video-Assisted Thoracoscopic Surgery: A Randomized Controlled Study. PLoS ONE 2021, 16, e0257279. [Google Scholar] [CrossRef]
- Frauenknecht, J.; Kirkham, K.R.; Jacot-Guillarmod, A.; Albrecht, E. Analgesic Impact of Intra-Operative Opioids vs. Opioid-Free Anaesthesia: A Systematic Review and Meta-Analysis. Anaesthesia 2019, 74, 651–662. [Google Scholar] [CrossRef]
- Salomé, A.; Harkouk, H.; Fletcher, D.; Martinez, V. Opioid-Free Anesthesia Benefit-Risk Balance: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 2069. [Google Scholar] [CrossRef]
- Fletcher, D.; Martinez, V. Opioid-Induced Hyperalgesia in Patients after Surgery: A Systematic Review and a Meta-Analysis. Br. J. Anaesth. 2014, 112, 991–1004. [Google Scholar] [CrossRef]
- Sabanathan, S.; Eng, J.; Mearns, A.J. Alterations in Respiratory Mechanics Following Thoracotomy. J. R. Coll. Surg. Edinb. 1990, 35, 144–150. [Google Scholar]
- Lee, L.A.; Caplan, R.A.; Stephens, L.S.; Posner, K.L.; Terman, G.W.; Voepel-Lewis, T.; Domino, K.B. Postoperative Opioid-induced Respiratory Depression: A Closed Claims Analysis. Anesthesiology 2015, 122, 659–665. [Google Scholar] [CrossRef]
- Haro, G.J.; Sheu, B.; Marcus, S.G.; Sarin, A.; Campbell, L.; Jablons, D.M.; Kratz, J.R. Perioperative Lung Resection Outcomes after Implementation of a Multidisciplinary, Evidence-Based Thoracic ERAS Program. Ann. Surg. 2021, 274, e1008–e1013. [Google Scholar] [CrossRef]
- Batchelor, T.J.P.; Rasburn, N.J.; Abdelnour-Berchtold, E.; Brunelli, A.; Cerfolio, R.J.; Gonzalez, M.; Ljungqvist, O.; Petersen, R.H.; Popescu, W.M.; Slinger, P.D.; et al. Guidelines for Enhanced Recovery after Lung Surgery: Recommendations of the Enhanced Recovery after Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur. J. Cardio-Thorac. Surg. 2019, 55, 91–115. [Google Scholar] [CrossRef]
- Buggy, D.J.; Freeman, J.; Johnson, M.Z.; Leslie, K.; Riedel, B.; Sessler, D.I.; Kurz, A.; Gottumukkala, V.; Short, T.; Pace, N.; et al. Systematic Review and Consensus Definitions for Standardised Endpoints in Perioperative Medicine: Postoperative Cancer Outcomes. Br. J. Anaesth. 2018, 121, 38–44. [Google Scholar] [CrossRef]
- Connolly, C.; Buggy, D.J. Opioids and Tumour Metastasis: Does the Choice of the Anesthetic-Analgesic Technique Influence Outcome after Cancer Surgery? Curr. Opin. Anaesthesiol. 2016, 29, 468–474. [Google Scholar] [CrossRef]
- Keijsers, R.; van Delft, R.; van den Bekerom, M.P.J.; de Vries, D.C.A.A.; Brohet, R.M.; Nolte, P.A. Local Infiltration Analgesia Following Total Knee Arthroplasty: Effect on Post-Operative Pain and Opioid Consumption—A Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2015, 23, 1956–1963. [Google Scholar] [CrossRef]
- Olausson, A.; Svensson, C.J.; Andréll, P.; Jildenstål, P.; Thörn, S.-E.; Wolf, A. Total Opioid-Free General Anaesthesia Can Improve Postoperative Outcomes after Surgery, without Evidence of Adverse Effects on Patient Safety and Pain Management: A Systematic Review and Meta-Analysis. Acta Anaesthesiol. Scand. 2022, 66, 170–185. [Google Scholar] [CrossRef]
- Tedesco, D.; Gori, D.; Desai, K.R.; Asch, S.; Carroll, I.R.; Curtin, C.; McDonald, K.M.; Fantini, M.P.; Hernandez-Boussard, T. Drug-Free Interventions to Reduce Pain or Opioid Consumption After Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. JAMA Surg. 2017, 152, e172872. [Google Scholar] [CrossRef]
- Pan, H.; Gu, Z.; Tian, Y.; Jiang, L.; Zhu, H.; Ning, J.; Huang, J.; Luo, Q. Propensity Score-Matched Comparison of Robotic- and Video-Assisted Thoracoscopic Surgery, and Open Lobectomy for Non-Small Cell Lung Cancer Patients Aged 75 Years or Older. Front. Oncol. 2022, 12, 1009298. [Google Scholar] [CrossRef]

| Study | Nation | Surgical Procedure | Intraoperative Analgesic Regimen | Sample Size OFA vs. OBA | Primary Outcome | Secondary Outcome | |
|---|---|---|---|---|---|---|---|
| OBA | OFA | ||||||
| Bello, 2019 [16] | France | Open thoracotomy | TEA Remifentanil Ketamine | TEA Ketamine | 25 vs. 50 | Ropivacaine cumulative administration during the first 48 h post-operatively |
|
| Clark, 2022 [20] | USA | Minimally invasive lobectomy | NR | INB or SAPB Acetominophen Gabapentin | 102 vs. 211 | In-hospital opioid consumption |
|
| Devine, 2020 [19] | Australia | VATS and open thoracotomy | SAPB or PVB Fentanyl Remifentanil and/or morphine paracetamol and parecoxib | PVB Lidocaine Magnesium Paracetamol Parecoxib | 83 vs. 104 | Postoperative pain scores at 0, 1 and 24 h |
|
| An, 2021 [21] | China | VATS | PVB Sufentanil Remifentanil | PVB Dexmedetomidine Ketorolac | 49 vs. 48 | Intraoperative PTI |
|
| Larue, 2022 [18] | France | VATS | SAPB/ESPB/PVB Sufentanil Ketamine Magnesium Desametasone | SAPB/ESPB/PVB Dexmetedonimine Ketamine Magnesium Desametasone | 52 vs. 99 | Opioid consumption at 48 h | Postoperative NRS |
| Selim, 2021 [17] | France | VATS or RATS | PVB/SAPB Remifentanil Ketamine Nefopam Ketoprofen | PVB/SAPB Dexmedetomidine Lidocaine Ketamine Nefopam Ketoprofen Paracetamol | 48 vs. 33 | Postoperative consumption of morphine within the first 48 h |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, F.; Barucco, G.; Licheri, M.; Valsecchi, G.; Zaraca, L.; Mucchetti, M.; Zangrillo, A.; Monaco, F. Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis. J. Clin. Med. 2022, 11, 6955. https://doi.org/10.3390/jcm11236955
D’Amico F, Barucco G, Licheri M, Valsecchi G, Zaraca L, Mucchetti M, Zangrillo A, Monaco F. Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis. Journal of Clinical Medicine. 2022; 11(23):6955. https://doi.org/10.3390/jcm11236955
Chicago/Turabian StyleD’Amico, Filippo, Gaia Barucco, Margherita Licheri, Gabriele Valsecchi, Luisa Zaraca, Marta Mucchetti, Alberto Zangrillo, and Fabrizio Monaco. 2022. "Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis" Journal of Clinical Medicine 11, no. 23: 6955. https://doi.org/10.3390/jcm11236955
APA StyleD’Amico, F., Barucco, G., Licheri, M., Valsecchi, G., Zaraca, L., Mucchetti, M., Zangrillo, A., & Monaco, F. (2022). Opioid Free Anesthesia in Thoracic Surgery: A Systematic Review and Meta Analysis. Journal of Clinical Medicine, 11(23), 6955. https://doi.org/10.3390/jcm11236955





