Conjunctival Swabs Reveal Higher Detection Rate Compared to Schirmer Strips for SARS-CoV-2 RNA Detection in Tears of Hospitalized COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
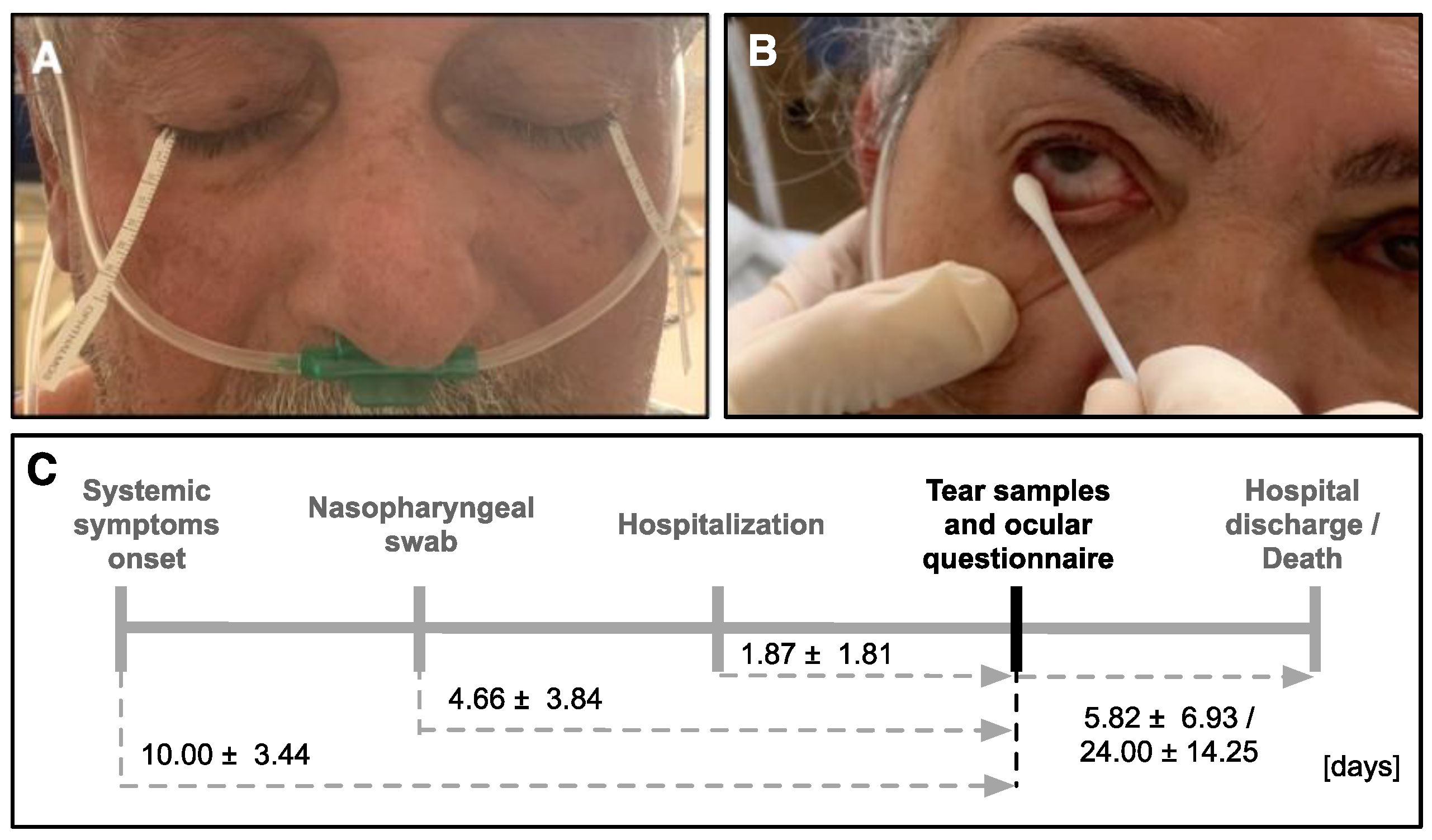
2.2. Tear Sample Collection
2.3. SARS-CoV-2 Viral Genome Detection by Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.4. Statistical Analysis
2.5. Ethical Aspects
3. Results
3.1. Patients’ Demographics
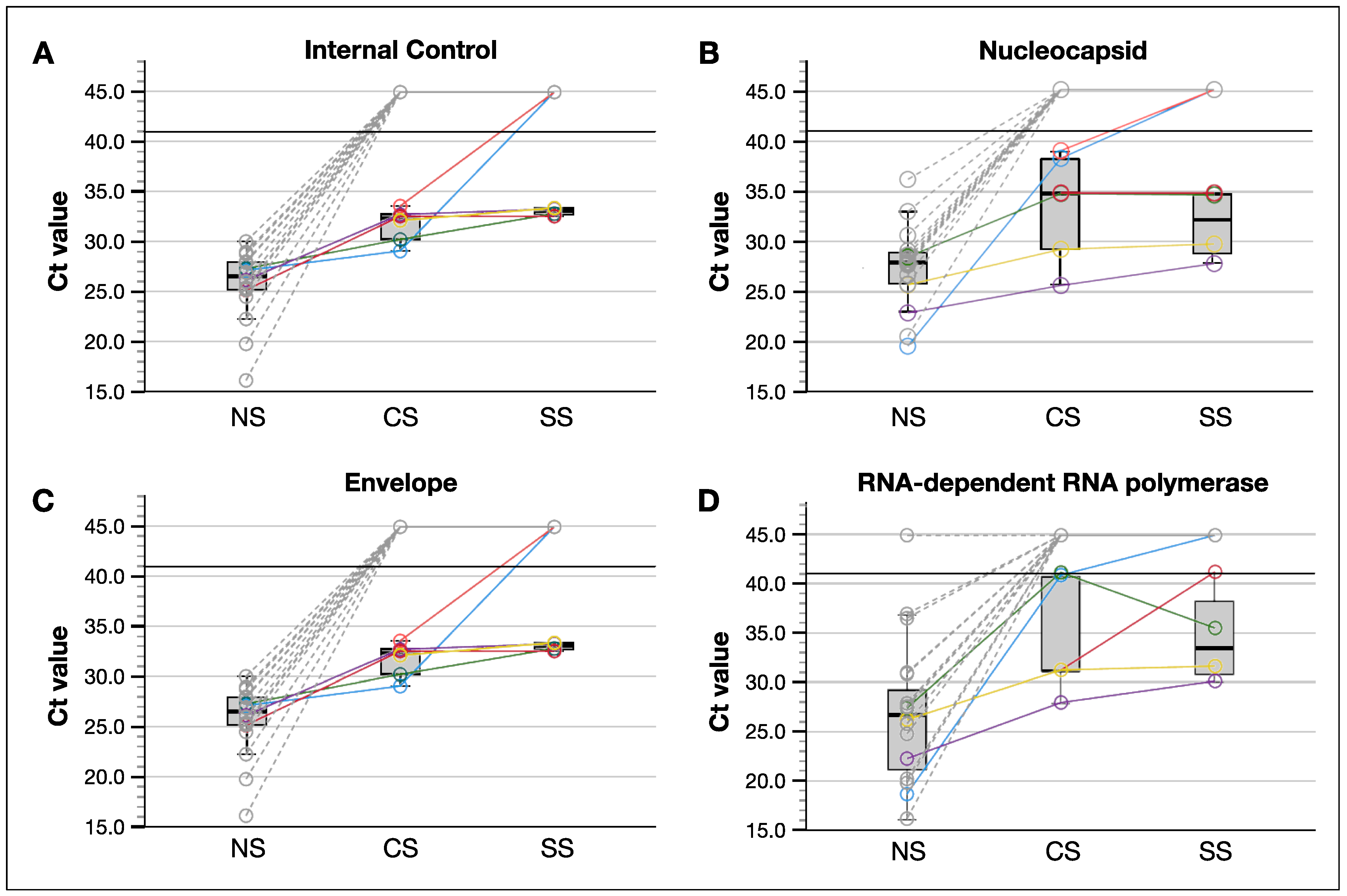
3.2. SARS-CoV-2 Was Better Detected in Tear Samples Collected by Conjunctival Swab Compared to These by Schirmer Strips
3.3. Patients Who Had SARS-CoV-2 Positive Tear Samples Reported Significantly More Subjective Ocular Symptoms, However Objective Measurements Did Not Differ
3.4. Patients with SARS-CoV-2 Positive Tears Had Worse Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Moujahed, A.; Kumar, A.; Chemudupati, T.; Tsang, S.H.; Mahajan, V.B. Telegenetics for inherited retinal diseases in the COVID-19 environment. Int. J. Retin. Vitr. 2021, 7, 25. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Qu, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Lotfi, M.; Hamblin, M.R.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedź, A.; Kawa, M.; Pius-Sadowska, E.; Kuligowska, A.; Ziontkowska, A.; Wrzałek, D.; Parczewski, M.; Safranow, K.; Kozłowski, K.; Machaliński, B.; et al. Evaluating Ocular Symptoms and Tear Film Cytokine Profiles in Symptomatic COVID-19 Patients. J. Clin. Med. 2022, 11, 2647. [Google Scholar] [CrossRef]
- Belser, J.A.; Rota, P.A.; Tumpey, T.M. Ocular Tropism of Respiratory Viruses. Microbiol. Mol. Biol. Rev. 2013, 77, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Summers, W. Virus Infection. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 546–552. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.-Y.; Xie, H.-T.; Zhang, M.-C. Evidence of SARS-CoV-2 Transmission Through the Ocular Route. Clin. Ophthalmol. 2021, 15, 687–696. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544. [Google Scholar] [CrossRef]
- Sabage, L.E.; Mazzo, A.; Sabage, J.; Olivo, T.E.T.; Santos, C.F.; Lourençone, L.F.M. Use of Schirmer strips and conjunctival swabs for virus detection on the ocular surface of adults: A scoping review. Arq. Bras. Oftalmol. 2022, in press. [Google Scholar] [CrossRef]
- Telles, R.; Li, W.; Dursch, T.; Lin, M.; Radke, C. Human tear-production rate from closed-eye Schirmer-strip capillary dynamics. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 61–68. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Yen, Y.-F.; Huang, L.-Y.; Chou, P. Manifestations and Virus Detection in the Ocular Surface of Adult COVID-19 Patients: A Meta-Analysis. J. Ophthalmol. 2021, 2021, 9997631. [Google Scholar] [CrossRef]
- Giovanetti, M.; Benedetti, F.; Campisi, G.; Ciccozzi, A.; Fabris, S.; Ceccarelli, G.; Tambone, V.; Caruso, A.; Angeletti, S.; Zella, D.; et al. Evolution patterns of SARS-CoV-2: Snapshot on its genome variants. Biochem. Biophys. Res. Commun. 2021, 538, 88–91. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, H.C.; Cho, A.; Kim, J.; Yun, K.-S.; Kim, J.; Lee, Y.-K. Age-adjusted Charlson comorbidity index score is the best predictor for severe clinical outcome in the hospitalized patients with COVID-19 infection. Medicine 2021, 100, e25900. [Google Scholar] [CrossRef]
- Brasil, M.d.S. SARS-CoV-2 Genomic Surveillance in Brazil. Available online: http://www.genomahcov.fiocruz.br/frequencia-das-principais-linhagens-do-sars-cov-2-por-mes-de-amostragem/ (accessed on 13 July 2022).
- Hu, Y.; Chen, T.; Liu, M.; Zhang, L.; Wang, F.; Zhao, S.; Liu, H.; Xia, H.; Wang, Y.; Li, L. Positive detection of SARS-CoV-2 combined HSV1 and HHV6B virus nucleic acid in tear and conjunctival secretions of a non-conjunctivitis COVID-19 patient with obstruction of common lacrimal duct. Acta Ophthalmol. 2020, 98, 859–863. [Google Scholar] [CrossRef]
- Macedo, M.C.F.; Pinheiro, I.M.; Carvalho, C.J.L.; Fraga, H.C.J.R.; Araujo, I.P.C.; Montes, S.S.; Araujo, O.A.C.; Alves, L.A.; Saba, H.; Araújo, M.L.V.; et al. Correlation between hospitalized patients’ demographics, symptoms, comorbidities, and COVID-19 pandemic in Bahia, Brazil. PLoS ONE 2020, 15, e0243966. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Loon, S.-C.; Teoh, S.C.B.; Oon, L.L.E.; Se-Thoe, S.-Y.; Ling, A.-E.; Leo, Y.-S.; Leong, H.-N. The severe acute respiratory syndrome coronavirus in tears. Br. J. Ophthalmol. 2004, 88, 861–863. [Google Scholar] [CrossRef]
- Leong, H.N.; Chan, K.P.; Khan, A.S.; Oon, L.; Se-Thoe, S.Y.; Bai, X.L.; Yeo, P.S.D.; Leo, Y.S.; Ang, B.; Ksiazek, T.G.; et al. Virus-specific RNA and Antibody from Convalescent-phase SARS Patients Discharged from Hospital. Emerg. Infect. Dis. 2004, 10, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.J.L.; Balne, P.K.; Leo, Y.-S.; Tong, L.; Ng, L.F.P.; Agrawal, R. Persistence of Zika virus in conjunctival fluid of convalescence patients. Sci. Rep. 2017, 7, 11194. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Foster, J.; Dai, X.; Gonzales, A.; Zhu, X.; Eberhart, C.; Hsu, W. Prevalence of SARS-CoV-2 in Conjunctival Swab Samples Among Patients Presenting with Conjunctivitis During the COVID-19 Pandemic. Clin. Ophthalmol. 2022, 16, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Miura-Ochiai, R.; Shimada, Y.; Konno, T.; Yamazaki, S.; Aoki, K.; Ohno, S.; Suzuki, E.; Ishiko, H. Quantitative Detection and Rapid Identification of Human Adenoviruses. J. Clin. Microbiol. 2007, 45, 958–967. [Google Scholar] [CrossRef]
- Moore, C.; Gatica, L.; Jones, T.; Matthews, P.; Watkins, J.; Tyson, L.; Keelan, P.; Corden, S.; Phillips, I.; Jones, R. Collect, boil and amplify—A simple approach for the detection of three common viruses associated with epidemic keratoconjunctivitis, conjunctivitis and dendritic ulcers. J. Virol. Methods 2013, 189, 238–241. [Google Scholar] [CrossRef]
- Su, C.S. Detection of Hepatitis B Virus DNA in Tears by Polymerase Chain Reaction. Arch. Ophthalmol. 1994, 112, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, C.; Donati, S.; Premi, E.; Baj, A.; Siracusa, C.; Genoni, A.; Grossi, P.A.; Azzi, L.; Sessa, F.; Dentali, F.; et al. SARS-CoV-2 on Ocular Surfaces in a Cohort of Patients with COVID-19 From the Lombardy Region, Italy. JAMA Ophthalmol. 2021, 139, 956. [Google Scholar] [CrossRef]
- Seah, I.Y.J.; Anderson, D.E.; Kang, A.E.Z.; Wang, L.; Rao, P.; Young, B.E.; Lye, D.C.; Agrawal, R. Assessing Viral Shedding and Infectivity of Tears in Coronavirus Disease 2019 (COVID-19) Patients. Ophthalmology 2020, 127, 977–979. [Google Scholar] [CrossRef] [PubMed]
- Dutescu, R.M.; Banasik, P.; Schildgen, O.; Schrage, N.; Uthoff, D. Detection of Coronavirus in Tear Samples of Hospitalized Patients With Confirmed SARS-CoV-2 From Oropharyngeal Swabs. Cornea 2021, 40, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Troisi, M.; Zannella, C.; Troisi, S.; De Bernardo, M.; Galdiero, M.; Franci, G.; Rosa, N. Ocular Surface Infection by SARS-CoV-2 in COVID-19 Pneumonia Patients Admitted to Sub-Intensive Unit: Preliminary Results. Microorganisms 2022, 10, 347. [Google Scholar] [CrossRef]
- Xie, H.-T.; Jiang, S.-Y.; Xu, K.-K.; Liu, X.; Xu, B.; Wang, L.; Zhang, M.-C. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis. 2020, 7, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Tong, J.; Liu, M.; Shen, Y.; Guo, D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J. Med. Virol. 2020, 92, 589–594. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Wang, G.; Chen, L.; Huang, L.; Cao, Y.; Chen, X.; Deng, C.; Chen, X.; Ke, D.; et al. Investigation of SARS-CoV-2 on Ocular Surface of Coronavirus Disease 2019 Patients Using One-Step Reverse-Transcription Droplet Digital PCR. Infect. Drug Resist. 2021, 14, 5395–5401. [Google Scholar] [CrossRef]
- Gijs, M.; Veugen, J.M.J.; Wolffs, P.F.G.; Savelkoul, P.H.M.; Tas, J.; van Bussel, B.C.T.; de Kruif, M.D.; Henry, R.M.A.; Webers, C.A.B.; Dickman, M.M.; et al. In-Depth Investigation of Conjunctival Swabs and Tear Fluid of Symptomatic COVID-19 Patients, an Observational Cohort Study. Transl. Vis. Sci. Technol. 2021, 10, 32. [Google Scholar] [CrossRef]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye 2020, 34, 1220–1223. [Google Scholar] [CrossRef]
- Gross, L.G.; Gasparini, M.S.; dos Santos, L.M.; Hamade, A.M.A.; Favarato, A.P.; Parise, P.L.; Proença-Modena, J.L.; Alves, M. Detection of SARS-CoV-2 virus on the ocular surface of an asymptomatic health-care professional. Arq. Bras. de Oftalmol. 2022, 86, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Li, S.-J.; Wang, W.-L.; Hu, M.; He, S.; Cao, J.; Jiang, L.; Li, Y. Ocular manifestations and SARS-CoV-2 detection in tears and conjunctival scrape from non-severe COVID-19 patients. Int. J. Ophthalmol. 2021, 14, 1133–1137. [Google Scholar] [CrossRef]
- Kaya, H.; Çalışkan, A.; Okul, M.; Sarı, T.; Akbudak, I.H. Detection of SARS-CoV-2 in the tears and conjunctival secretions of Coronavirus disease 2019 patients. J. Infect. Dev. Ctries. 2020, 14, 977–981. [Google Scholar] [CrossRef]
- Daryabari, S.H.; Asadollah, A.; Moghadam, F.A.; Dorostkar, R.; Bahramifar, A.; Aghamollaei, H. Detection of COVID-19 in tears of ICU-admitted patients with SARS-CoV-2 infection. Int. Ophthalmol. 2022, 42, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Athale, A.; Chawhan, A.; Khan, K.; Agarwal, S.; Paul, R.; Iyer, K.; Khadia, A.; Som, V. Detection of SARS-CoV-2 RNA in a conjunctival swab sample in real-time-polymerase chain reaction positive COVID-19 patients and its association with comorbidity and severity at a designated COVID-19 hospital in Central India. Indian J. Ophthalmol. 2021, 69, 3633. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; He, T.; Wei, R.; Shen, Y.; Qi, T.; Han, T.; Song, Z.; Zhu, Z.; Ma, X.; et al. Detection of SARS-CoV-2 in the ocular surface in different phases of COVID-19 patients in Shanghai, China. Ann. Transl. Med. 2021, 9, 100. [Google Scholar] [CrossRef]
- Hanege, F.M.; Kocoglu, E.; Kalcioglu, M.T.; Celik, S.; Cag, Y.; Esen, F.; Bayindir, E.; Pence, S.; Mese, E.A.; Agalar, C. SARS-CoV-2 Presence in the Saliva, Tears, and Cerumen of COVID-19 Patients. Laryngoscope 2021, 131, E1677–E1682. [Google Scholar] [CrossRef]
- Muyldermans, A.; Bjerke, M.; Demuyser, T.; De Geyter, D.; Wybo, I.; Soetens, O.; Weets, I.; Kuijpers, R.; Allard, S.D.; Piérard, D.; et al. SARS-CoV-2 RNA and antibodies in tear fluid. BMJ Open Ophthalmol. 2021, 6, e000733. [Google Scholar] [CrossRef]
- Yan, Y.; Zeng, B.; Zhang, Z.; Hu, C.; Yan, M.; Li, B.; Zhang, X.; Chen, X. Detection of SARS-CoV-2 in Simultaneously Collected Tear and Throat Swab Samples from the Patients with 2019- new SARS-CoV-2 Infection Disease: A Single Center Cross-sectional Study. Ophthalmic Epidemiol. 2021, 28, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Goel, R.; Kumar, S.; Chhabra, M.; Saxena, S.; Manchanda, V.; Pumma, P. Evaluation of SARS-CoV-2 in Tears of Patients with Moderate to Severe COVID-19. Ophthalmology 2021, 128, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Goel, R.; Saxena, S.; Manchanda, V.; Ahmad, M.; Gupta, G.; Chhabra, M.; Kumar, S.; Nguyen, T.M.N.; Payden; et al. Comparative Evaluation of Tears and Nasopharyngeal Swab for SARS-CoV-2 in COVID-19 Dedicated Intensive Care Unit Patients. Ocul. Immunol. Inflamm. 2021, 29, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ares, T.; Lamas-Francis, D.; Treviño, M.; Navarro, D.; Cea, M.; López-Valladares, M.J.; Martínez, L.; Gude, F.; Touriño, R. SARS-CoV-2 in Conjunctiva and Tears and Ocular Symptoms of Patients with COVID-19. Vision 2021, 5, 51. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.; Tang, X.; He, D. The Disease Severity and Clinical Outcomes of the SARS-CoV-2 Variants of Concern. Front. Public Health. 2021, 9, 775224. [Google Scholar] [CrossRef]
- Drain, P.K. Rapid Diagnostic Testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef]
- Dadras, O.; Afsahi, A.M.; Pashaei, Z.; Mojdeganlou, H.; Karimi, A.; Habibi, P.; Barzegary, A.; Fakhfouri, A.; Mirzapour, P.; Janfaza, N.; et al. The relationship between COVID-19 viral load and disease severity: A systematic review. Immunity Inflamm. Dis. 2022, 10, e580. [Google Scholar] [CrossRef]
- da Silva, S.J.R.; de Lima, S.C.; da Silva, R.C.; Kohl, A.; Pena, L. Viral Load in COVID-19 Patients: Implications for Prognosis and Vaccine Efficacy in the Context of Emerging SARS-CoV-2 Variants. Front. Med. 2022, 8, 836826. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Zhang, Y.; Zhang, X.; Jiang, Y.; Wang, X.; Zheng, P.; Chen, Y. Symptoms of Dry Eye Disease in Hospitalized Patients with Coronavirus Disease 2019 (COVID-19). J. Ophthalmol. 2021, 2021, 2678706. [Google Scholar] [CrossRef]
- Asharlous, A.; Hashemi, H.; Yekta, A.A.; Ostadimoghaddam, H.; Gharaee, H.; Khabazkhoob, M. Tear film secretion and stability in welders. Contact Lens Anterior Eye 2018, 41, 426–429. [Google Scholar] [CrossRef]
- Sawant, O.B.; Singh, S.; Wright, R.E.; Jones, K.M.; Titus, M.S.; Dennis, E.; Hicks, E.; Majmudar, P.A.; Kumar, A.; Mian, S.I. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul. Surf. 2020, 19, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.Z.; Møller, R.; Makovoz, B.; Uhl, S.A.; Tenoever, B.R.; Blenkinsop, T.A. SARS-CoV-2 infects human adult donor eyes and hESC-derived ocular epithelium. Cell Stem Cell 2021, 28, 1205–1220.e7. [Google Scholar] [CrossRef] [PubMed]
- Safadi, K.; Kruger, J.M.; Chowers, I.; Solomon, A.; Amer, R.; Aweidah, H.; Frenkel, S.; Mechoulam, H.; Anteby, I.; Ben Eli, H.; et al. Ophthalmology practice during the COVID-19 pandemic. BMJ Open Ophthalmol. 2020, 5, e000487. [Google Scholar] [CrossRef] [PubMed]

| COVID-19 | Non-COVID-19 | |||
|---|---|---|---|---|
| Total | SARS-CoV-2 Detection in Tears | No SARS-CoV-2 Detection in Tears | Total | |
| Number of Patients | 33 | 6 | 27 | 28 |
| Sex | ||||
| Male | 57.6% (19) | 33.3% (2) | 62.9% (17) | 42.9% (12) |
| Female | 42.4% (14) | 66.7% (4) | 37.1% (10) | 57.1% (16) |
| Mean age (range) | 58.6 (23–87) | 68.7 (56–82) | 56.3 (23–87) | 59.4 (24–86) |
| Mean CCI (range) | 62.6% (0.0–98.0%) | 34.0% (0.0–77.0%) | 67.6% (0.0–98.0%) | 55.0% (0.0–98.0%) |
| SAH | 48.5% (16) | 83.3% (5) | 40.7% (11) | 39.3% (11) |
| DM2 | 24.2% (8) | 50.0% (3) | 18.5% (5) | 17.8% (5) |
| Obesity | 24.2% (8) | 33.3% (2) | 22.2% (6) | 25.0% (7) |
| Dyslipidemia | 12.1% (4) | 16.7% (1) | 11.1% (3) | 3.6% (1) |
| Vaccine | ||||
| CoronaVac 3rd dose | 6.6% (2) | 33.3% (2) | 0.0% (0) | 0.0% * (0) |
| CoronaVac 2nd dose | 36.4% (12) | 50.0% (3) | 33.3% (9) | 42.9% * (6) |
| CoronaVac 1st dose | 9.1% (3) | 0.0% (0) | 11.1% (3) | 0.0% * (0) |
| Oxford’s AZD1222 2nd dose | 0.0% (0) | 0.0% (0) | 0.0% (0) | 7.1% * (1) |
| Oxford’s AZD1222 1st dose | 9.1% (3) | 0.0% (0) | 11.1% (3) | 14.3% * (2) |
| No vaccine | 38.8% (13) | 16.7% (1) | 44.5% (12) | 35.7% * (5) |
| Unknown | 0 | 0 | 0 | 14 |
| SARS-CoV-2 testing in tears (conjunctival swab and Schirmer strips) | 100.0% (33) | 100.0% (6) | 100.0% (27) | 50.0% (14) |
| Total (33) | SARS-CoV-2 Tear Samples | χ2 | ||
|---|---|---|---|---|
| Positive (6) | Negative (27) | |||
| Reported ocular symptoms | 48.5% (16) | 50.0% (3) | 48.1% (13) | ns |
| Foreign body sensation | 30.3% (10) | 16.6% (1) | 33.3% (9) | ns |
| Tearing | 9.1% (3) | 33.3% (2) | 6.9% (1) | 0.022 |
| Red eye | 12.1% (4) | 16.6% (1) | 11.1% (3) | ns |
| Pain | 12.1% (4) | 0.0% (0) | 14.8% (4) | ns |
| Previous eye surgery | 21.2% (7) | 16.6% (1) | 22.2% (6) | ns |
| Use of eye drops | 0.0% (0) | 0.0% (0) | 0.0% (0) | ns |
| Use of O2 (nasal cannula) | 72.7% (24) | 100.0% (6) | 66.6% (18) | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabage, L.E.; Sun, Y.J.; Wolf, J.; Sabage, J.; Mazzo, A.; Santos, C.F.; Mahajan, V.B.; Manzoni Lourençone, L.F. Conjunctival Swabs Reveal Higher Detection Rate Compared to Schirmer Strips for SARS-CoV-2 RNA Detection in Tears of Hospitalized COVID-19 Patients. J. Clin. Med. 2022, 11, 6929. https://doi.org/10.3390/jcm11236929
Sabage LE, Sun YJ, Wolf J, Sabage J, Mazzo A, Santos CF, Mahajan VB, Manzoni Lourençone LF. Conjunctival Swabs Reveal Higher Detection Rate Compared to Schirmer Strips for SARS-CoV-2 RNA Detection in Tears of Hospitalized COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(23):6929. https://doi.org/10.3390/jcm11236929
Chicago/Turabian StyleSabage, Luís Expedito, Young Joo Sun, Julian Wolf, Josmar Sabage, Alessandra Mazzo, Carlos Ferreira Santos, Vinit B. Mahajan, and Luiz Fernando Manzoni Lourençone. 2022. "Conjunctival Swabs Reveal Higher Detection Rate Compared to Schirmer Strips for SARS-CoV-2 RNA Detection in Tears of Hospitalized COVID-19 Patients" Journal of Clinical Medicine 11, no. 23: 6929. https://doi.org/10.3390/jcm11236929
APA StyleSabage, L. E., Sun, Y. J., Wolf, J., Sabage, J., Mazzo, A., Santos, C. F., Mahajan, V. B., & Manzoni Lourençone, L. F. (2022). Conjunctival Swabs Reveal Higher Detection Rate Compared to Schirmer Strips for SARS-CoV-2 RNA Detection in Tears of Hospitalized COVID-19 Patients. Journal of Clinical Medicine, 11(23), 6929. https://doi.org/10.3390/jcm11236929






