Clinical Outcomes and Quantitative Margin Analysis of a Universal Adhesive Using a Randomized Clinical Trial over Three Years
Abstract
:1. Introduction
- Both in clinical evaluation (primary outcome) and quantitative marginal analysis (secondary outcome), SBU has an increased performance when compared to that of OFL (adhesive evaluation, application mode, conformity of methods);
- The marginal gap generally increases with the passing of time (gap progression);
- Quantitative margin analysis identifies group differences before they become visible within clinical evaluation parameters (power of methods).
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Restorative Procedure
2.4. Manufacture of the Impression
2.5. Replica Production and Mounting for SEM Imaging
2.6. Study Outcomes
2.6.1. Clinical Assessment
2.6.2. Quantitative Margin Analysis
2.7. Statistical Analysis
2.7.1. Clinical Assessment
2.7.2. Quantitative Margin Analysis
3. Results
3.1. Clinical Assessment
3.2. Quantitative Margin Analysis
3.3. Clinic and QMA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donmez, N.; Belli, S.; Pashley, D.H.; Tay, F.R. Ultrastructural correlates of in vivo/in vitro bond degradation in self-etch adhesives. J. Dent. Res. 2005, 84, 355–359. [Google Scholar] [CrossRef]
- Heintze, S.D. Systematic reviews: I. The correlation between laboratory tests on marginal quality and bond strength. II. The correlation between marginal quality and clinical outcome. J. Adhes. Dent. 2007, 9, 77–106. [Google Scholar]
- Roulet, J.F.; Reich, T.; Blunck, U.; Noack, M. Quantitative margin analysis in the scanning electron microscope. Scanning Microsc. 1989, 3, 147–159. [Google Scholar]
- van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; de Munck, J.; van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Luque, I.; Hass, V.; Reis, A.; Loguercio, A.D.; Bombarda, N.H.C. Immediate bonding properties of universal adhesives to dentine. J. Dent. 2013, 41, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Hanabusa, M.; Mine, A.; Kuboki, T.; Momoi, Y.; van Ende, A.; van Meerbeek, B.; de Munck, J. Bonding effectiveness of a new ‘multi-mode’ adhesive to enamel and dentine. J. Dent. 2012, 40, 475–484. [Google Scholar] [CrossRef]
- Marchesi, G.; Frassetto, A.; Mazzoni, A.; Apolonio, F.; Diolosà, M.; Cadenaro, M.; Di Lenarda, R.; Pashley, D.H.; Tay, F.R.; Breschi, L. Adhesive performance of a multi-mode adhesive system: 1-year in vitro study. J. Dent. 2014, 42, 603–612. [Google Scholar] [CrossRef]
- Peumans, M.; de Munck, J.; Mine, A.; van Meerbeek, B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent. Mater. 2014, 30, 1089–1103. [Google Scholar] [CrossRef]
- Yaseen, S.M.; Reddy, V.V.S. Comparative evaluation of shear bond strength of two self-etching adhesives (sixth and seventh generation) on dentin of primary and permanent teeth: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2009, 27, 33–38. [Google Scholar] [CrossRef]
- Perdigão, J.; Loguercio, A.D. Universal or Multi-mode Adhesives: Why and How? J. Adhes. Dent. 2014, 16, 193–194. [Google Scholar] [CrossRef]
- Oz, F.D.; Kutuk, Z.B.; Ozturk, C.; Soleimani, R.; Gurgan, S. An 18-month clinical evaluation of three different universal adhesives used with a universal flowable composite resin in the restoration of non-carious cervical lesions. Clin. Oral Investig. 2019, 23, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Suárez, C.E.; de Oliveira da Rosa, W.L.; Lund, R.G.; da Silva, A.F.; Piva, E. Bonding performance of universal adhesives: An updated systematic review and meta-analysis. J. Adhes. Dent. 2019, 21, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Niu, L.-N.; Xie, H.; Zhang, Z.-Y.; Zhou, L.-Q.; Jiao, K.; Chen, J.-H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine--Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenberger, R.; Lohbauer, U.; Roggendorf, M.J.; Naumann, M.; Taschner, M. Selective enamel etching reconsidered: Better than etch-and-rinse and self-etch? J. Dent. Res. 2008, 10, 339–344. [Google Scholar]
- Suzuki, T.; Takamizawa, T.; Barkmeier, W.W.; Tsujimoto, A.; Endo, H.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. Influence of etching mode on enamel bond durability of universal adhesive systems. Oper. Dent. 2016, 41, 520–530. [Google Scholar] [CrossRef] [Green Version]
- Szesz, A.; Parreiras, S.R.A.; Loguerico, A. Selective enamel etching in cervical lesions for self-etch adhesives: A systematic review and meta-analysis. J. Dent. 2016, 53, 1–11. [Google Scholar] [CrossRef]
- Tian, F.; Zhou, L.; Zhang, Z.; Niu, L.; Zhang, L.; Chen, C.; Zhou, J.; Yang, H.; Wang, X.; Fu, B.; et al. Paucity of nanolayering in resin-dentin interfaces of MDP-based adhesives. J. Dent. Res. 2016, 95, 380–387. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Nagaoka, N.; Fukegawa, D.; Hayakawa, S.; Mine, A.; Nakamura, M.; Minagi, S.; Osaka, A.; Suzuki, K.; et al. Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta. Biomater. 2010, 6, 3573–3582. [Google Scholar] [CrossRef] [Green Version]
- Perdigão, J.; Sezinando, A.; Monteiro, P.C. Laboratory bonding ability of a multi-purpose dentin adhesive. Am. J. Dent. 2012, 25, 153–158. [Google Scholar]
- Perdigão, J.; Kose, C.; Mena-Serrano, A.P.; de Paula, E.A.; Tay, L.Y.; Reis, A.; Loguercio, A.D. A new universal simplified adhesive: 18-month clinical evaluation. Oper. Dent. 2014, 39, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Perdigao, J. Current perspectives on dental adhesion: (1) Dentin adhesion—Not there yet. Jpn. Dent. Sci. Rev. 2020, 56, 190–207. [Google Scholar] [CrossRef] [PubMed]
- Haak, R.; Schmidt, P.; Park, K.-J.; Häfer, M.; Krause, F.; Ziebolz, D.; Schneider, H. OCT for early quality evaluation of tooth-composite bond in clinical trials. J. Dent. 2018, 76, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Merle, C.L.; Fortenbacher, M.; Schneider, H.; Schmalz, G.; Challakh, N.; Park, K.-J.; Häfer, M.; Ziebolz, D.; Haak, R. Clinical and OCT assessment of application modes of a universal adhesive in a 12-month RCT. J. Dent. 2022, 119, 104068. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.F.M.; Shinohara, M.S.; Carvalho, P.R.M.A.; Ramos, F.S.E.S.; Oliveira, L.C.; Omoto, É.M.; Fagundes, T.C. Three-year evaluation of different adhesion strategies in non-carious cervical lesion restorations: A randomized clinical trial. J. Appl. Oral Sci. 2021, 29, e20210192. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.V.; Perdigao, J.; Baracco, B.; Giraldes, I.; Caballos, L. Effect of an additional bonding resin on the 5-year performance of a universal adhesive: A randomized clinical trial. Clin. Oral. Investig. 2022, 1–12. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suares, C.E. Bond strength of universal adhesives to dentin: A systematic review and meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef]
- Schneider, H.; Steigerwald-Otremba, A.S.; Häfer, M.; Krause, F.; Scholz, M.; Haak, R. Is optical coherence tomography a potential tool to evaluate marginal adaptation of class III/IV composite restorations in vivo? Oper. Dent. 2019, 44, 242–253. [Google Scholar] [CrossRef]
- Smith, B.G.; Knight, J.K. An index for measuring the wear of teeth. Br. Dent. J. 1984, 156, 435–438. [Google Scholar] [CrossRef]
- Haak, R.; Hähnel, M.; Schneider, H.; Rosolowski, M.; Park, K.-J.; Ziebolz, D.; Häfer, M. Clinical and OCT outcomes of a universal adhesive in a randomized clinical trial after 12 months. J. Dent. 2019, 90, 103200. [Google Scholar] [CrossRef]
- Hickel, R.; Roulet, J.-F.; Bayne, S.; Heintze, S.D.; Mjör, I.A.; Peters, M.; Rousson, V.; Randall, R.; Schmalz, G.; Tyas, M.; et al. Recommendations for conducting controlled clinical studies of dental restorative materials. Science Committee Project 2/98--FDI World Dental Federation study design (Part I) and criteria for evaluation (Part II) of direct and indirect restorations including onlays and partial crowns. J. Adhes. Dent. 2007, 9, 121–147. [Google Scholar]
- Hickel, R.; Peschke, A.; Tyas, M.; Mjör, I.; Bayne, S.; Peters, M.; Hiller, K.-A.; Randall, R.; Vanherle, G.; Heintze, S.D. FDI World Dental Federation: Clinical criteria for the evaluation of direct and indirect restorations-update and clinical examples. Clin. Oral Investig. 2010, 14, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.-H. KHKs jQuantiGap. Available online: http://www.kunzelmann.de/4_software-imagej-quantitative_margin_analysis.html (accessed on 1 April 2019).
- Häfer, M.; Jentsch, H.; Haak’, R.; Schneider, H. A three-year clinical evaluation of a one-step self-etch and a two-step etch-and-rinse adhesive in non-carious cervical lesions. J. Dent. 2015, 43, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Häfer, M.; Schneider, H.; Rupf, S.; Busch, I.; Fuchß, A.; Merte, I.; Jentsch, H.; Haak, R.; Merte, K. Experimental and clinical evaluation of a self-etching and an etch-and-rinse adhesive system. J. Adhes. Dent. 2013, 15, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Zimmerli, B. Relevance of in vitro tests of adhesive and composite dental materials, a review in 3 parts. Part 1: Approval requirements and standardized testing of composite materials according to ISO specifications. Schweiz. Mon. Zahnmed. 2011, 121, 804–816. [Google Scholar]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef]
- Peumans, M.; de Munck, J.; van Landuyt, K.L.; Lambrechts, P.; van Meerbeek, B. Five-year clinical effectiveness of a two-step self-etching adhesive. J. Adhes. Dent. 2007, 9, 7–10. [Google Scholar]
- Blunck, U. Rasterelektronenmikroskopische Beurteilung von Kompositfüllungsrändern im Dentin in vitro. Dtsch. Zahnarztl. Z. 1988, 43, 939–943. [Google Scholar]
- Frankenberger, R.; Krämer, N.; Lohbauer, U.; Nikolaenko, S.A.; Reich, S.M. Marginal integrity: Is the clinical performance of bonded restorations predictable in vitro? J. Adhes. Dent. 2007, 9 (Suppl. 1), 107–116. [Google Scholar]
- Taylor, M.J.; Lynch, E. Microleakage. J. Dent. 1992, 20, 3–10. [Google Scholar] [CrossRef]
- Roulet, J.F.; Reng, R. Das Problem des Randschlusses bei Kunststofffüllungen. Schweiz. Mon. Zahnheilkd. 1975, 85, 1039–1053. [Google Scholar]
- Federlin, M.; Thonemann, B.; Schmalz, G.; Urlinger, T. Clinical evaluation of different adhesive systems for restoring teeth with erosion lesions. Clin. Oral Investig. 1998, 2, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Haak, R.; Wicht, M.J.; Noack, M.J. Marginal and internal adaptation of extended class I restorations lined with flowable composites. J. Dent. 2003, 31, 231–239. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, J.W.V.; Hörstedt, P. Marginal breakdown of fired ceramic inlays cemented with glass polyalkenoate (ionomer) cement or resin composite. J. Dent. 1994, 22, 265–272. [Google Scholar] [CrossRef] [PubMed]
- van Dijken, J.W.; Hörstedt, P. Marginal adaptation to enamel of a polyacid-modified resin composite (compomer) and a resin-modified glass ionomer cement in vivo. Clin. Oral Investig. 1997, 1, 185–190. [Google Scholar] [CrossRef]
- Mine, A.; de Munck, J.; Cardoso, M.V.; van Landuyt, K.L.; Poitevin, A.; Kuboki, T.; Yoshida, Y.; Suzuki, K.; van Meerbeek, B. Enamel-smear compromises bonding by mild self-etch adhesives. J. Dent. Res. 2010, 89, 1505–1509. [Google Scholar] [CrossRef]
- Nagler, F. Qualitative und Quantitative Randanalyse von Klasse—II—Kavitäten Experimenteller Füllungswerkstoffe unter Verwendung der Selektiven Schmelzätzung und des Selbstätzenden Verfahrens am Rasterelektronenmikroskop: Eine In-Vitro Studie Promotion; Ludwig-Maximilians-Universität zu München: München, Germany, 2020. [Google Scholar]
- Haak, R.; Brückner, A.; Häfer, M.; Scholz, M.; Schneider, H. Is there an association between clinical and SEM quantitative marginal analysis in a 90-month trial? J. Adhes. Dent. 2021, 23, 37–46. [Google Scholar] [CrossRef]
- Haak, R.; Schäfer, P.; Hanßen, B.; Ziebolz, D.; Park, K.-J.; Häfer, M.; Schmalz, G.; Schneider, H. OCT Evaluation of marginal and internal interface integrity of class v composite restorations after 36 to 48 months. J. Adhes. Dent. 2022, 24, 165–174. [Google Scholar]
- Mena-Serrano, A.; Kose, C.; de Paula, E.A.; Tay, L.Y.; Reis, A.; Loguercio, A.D.; Perdigão, J. A new universal simplified adhesive: 6-month clinical evaluation. J. Esthet. Restor. Dent. 2013, 25, 55–69. [Google Scholar] [CrossRef]
- Loguercio, A.D.; de Paula, E.A.; Hass, V.; Luque-Martinez, I.; Reis, A.; Perdigão, J. A new universal simplified adhesive: 36-month randomized double-blind clinical trial. J. Dent. 2015, 43, 1083–1092. [Google Scholar] [CrossRef]
- Çelik, E.U.; Aka, B.; Yilmaz, F. Six-month Clinical Evaluation of a Self-adhesive Flowable Composite in Noncarious Cervical Lesions. J. Adhes. Dent. 2015, 17, 361–368. [Google Scholar] [CrossRef]
- de Paula, E.A.; Tay, L.Y.; Kose, C.; Mena-Serrano, A.; Reis, A.; Perdigão, J.; Loguercio, A.D. Randomized clinical trial of four adhesion strategies in cervical lesions: 12-month results. Int. J. Esthet. Dent. 2015, 10, 122–145. [Google Scholar] [PubMed]
- Peumans, M.; de Munck, J.; van Landuyt, K.L.; Poitevin, A.; Lambrechts, P.; van Meerbeek, B. A 13-year clinical evaluation of two three-step etch-and-rinse adhesives in non-carious class-V lesions. Clin. Oral Investig. 2012, 16, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Wouters, L.; de Munck, J.; van Meerbeek, B.; van Landuyt, K.L. Nine-year Clinical Performance of a HEMA-free One-step Self-etch Adhesive in Noncarious Cervical Lesions. J. Adhes. Dent. 2018, 20, 195–203. [Google Scholar] [CrossRef]
- Dreweck, F.; Burey, A.; de Oliveira Dreweck, M.; Fernandez, E.; Loguercio, A.D.; Reis, A. Challenging the Concept that OptiBond FL and Clearfil SE Bond in NCCLs Are Gold Standard Adhesives: A Systematic Review and Meta-analysis. Oper. Dent. 2021, 46, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.C.; Robles, A.; Fu, C.-C.; Lin, C.P.; Sawlani, K.; Burgess, J.O. Two-year clinical trial of a universal adhesive in total-etch and self-etch mode in non-carious cervical lesions. J. Dent. 2015, 43, 1229–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Landuyt, K.L.; de Munck, J.; Ermis, R.B.; Peumans, M.; van Meerbeek, B. Five-year clinical performance of a HEMA-free one-step self-etch adhesive in noncarious cervical lesions. Clin. Oral. Investig. 2014, 18, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Mahn, E.; Rousson, V.; Heintze, S.D. Meta-analysis of the influence of bonding parameters on the clinical outcome of tooth-colored cervical restorations. J. Adhes. Dent. 2015, 17, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Lührs, A.K.; Jacker-Guhr, S.; Günay, H.; Herrmann, P. Composite restorations placed in non-carious cervical lesions-Which cavity preparation is clinically reliable? Clin. Exp. Dent. Res. 2020, 6, 558–567. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Luque-Martinez, I.V.; Fuentes, S.; Reis, A.; Muñoz, M.A. Effect of dentin roughness on the adhesive performance in non-carious cervical lesions: A double-blind randomized clinical trial. J. Dent. 2018, 69, 60–69. [Google Scholar] [CrossRef]
- Zanatta, R.F.; Lungova, M.; Borges, A.B.; Torres, C.; Sydow, H.-G.; Wiegand, A. Microleakage and shear bond strength of composite restorations under cycling conditions. Oper. Dent. 2017, 42, E71–E80. [Google Scholar] [CrossRef]
- Perdigão, J. Dentin bonding-variables related to the clinical situation and the substrate treatment. Dent. Mater. 2010, 26, e24–e37. [Google Scholar] [CrossRef] [PubMed]
- Peumans, M.; Kanumilli, P.; de Munck, J.; van Landuyt, K.; Lambrechts, P.; van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Luque-Martinez, I.; Malaquias, P.; Hass, V.; Reis, A.; Campanha, N.H.; Loguercio, A.D. In vitro longevity of bonding properties of universal adhesives to dentin. Oper. Dent. 2015, 40, 282–292. [Google Scholar] [CrossRef]
- Yoshihara, K.; Hayakawa, S.; Nagaoka, N.; Okihara, T.; Yoshida, Y.; van Meerbeek, B. Etching Efficacy of Self-Etching Functional Monomers. J. Dent. Res. 2018, 97, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; Yoshida, Y.; van Meerbeek, B. Chemical interaction of glycero-phosphate dimethacrylate (GPDM) with hydroxyapatite and dentin. Dent. Mater. 2018, 34, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, Y.; Li, T.; Pan, Y.; Cui, Y.; Xia, W. Adhesive interfacial characteristics and the related bonding performance of four self-etching adhesives with different functional monomers applied to dentin. J. Dent. 2017, 62, 72–80. [Google Scholar] [CrossRef]
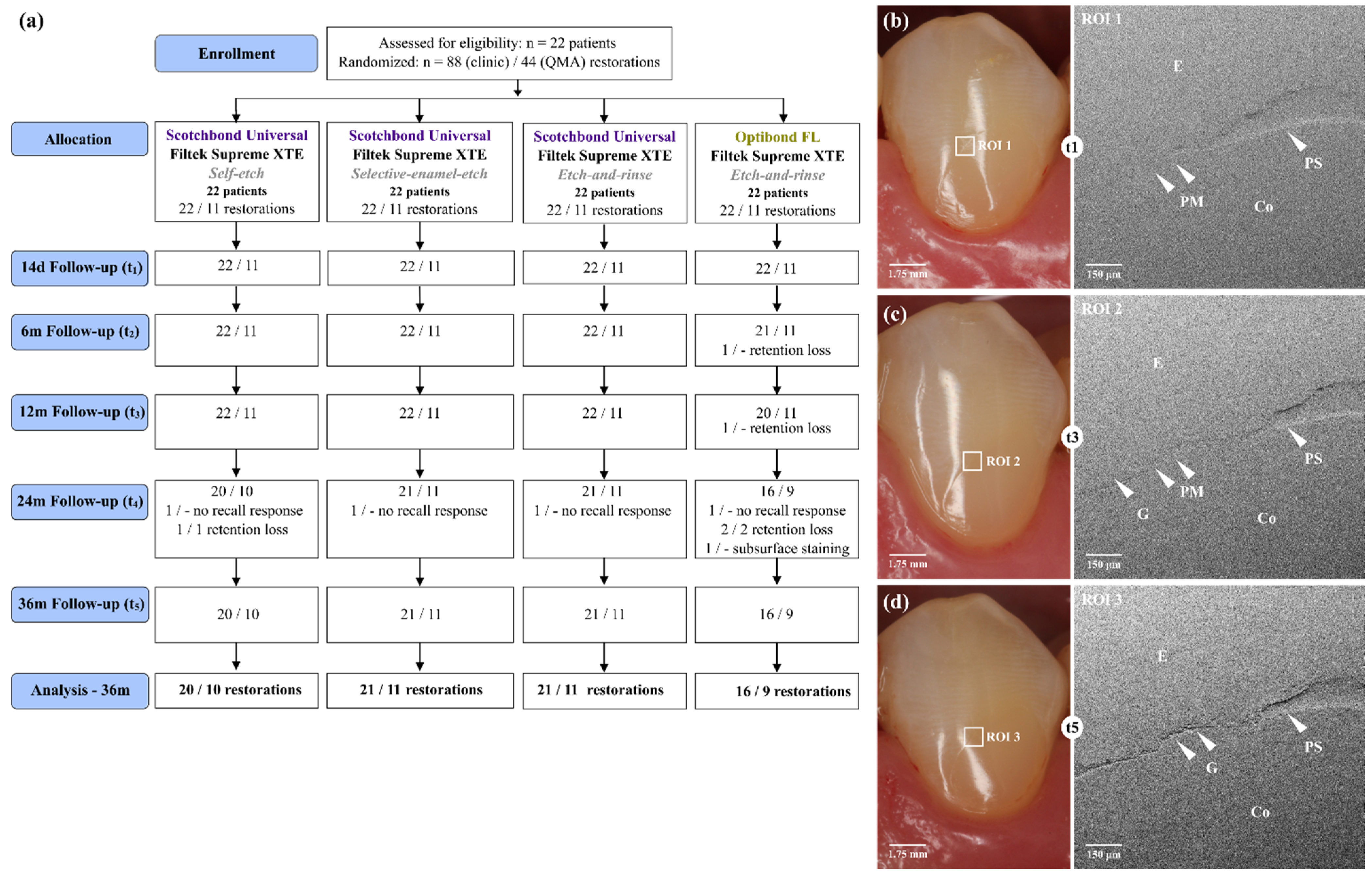
| Group | SBU-SE | SBU-SEE | SBU-ER | OFL-ER |
|---|---|---|---|---|
| NClinic/NQMA | 22/11 | 22/11 | 22/11 | 22/11 |
| Adhesive | Scotchbond Universal (SBU) | OptiBond FL (OFL) | ||
| Application mode | self-etch (SE) | selective-enamel-etch (SEE) | etch-and-rinse (ER) | etch-and-rinse (ER) |
| Composite | nanocomposite Filtek™ Supreme XTE (3M) | |||
| Lost restorations | 1 1 | - | - | 5 2 |
| Location | ||||
| maxilla | 10/4 | 10/5 | 14/7 | 10/6 |
| mandible | 12/7 | 12/6 | 8/4 | 12/5 |
| Lesion borderline | ||||
| enamel | -/- | -/- | -/- | -/- |
| dentin | 2/- | 3/1 | 2/2 | 4/2 |
| mixed (enamel/dentin) | 20/11 | 19/10 | 20/9 | 18/9 |
| Lesion depth | ||||
| shallow (<1 mm deep) | -/- | 1/- | -/- | 2/1 |
| medium (1–2 mm deep) | 22/11 | 21/11 | 21/11 | 19/10 |
| deep (>2 mm) | -/- | -/- | 1/- | 1/- |
| Material | Composition | Self-Etch Mode | Selective-Enamel-Etch Mode | Etch-and-Rinse Mode |
|---|---|---|---|---|
| Scotchbond Universal Etchant a | 35% phosphoric acid | 1. Apply etchant for 30 s on the enamel. 2. Rinse with water for 20 s and dry with water- and oil-free air | ||
| Scotchbond Universal a | 10-MDP, HEMA, silane, dimethacrylate resins, Vitrebond™ copolymer, filler, ethanol, water, initiators (LOT 552577) | 1. Actively apply the adhesive to the cavity for 20 s. 2. Gently air-dry the adhesive for approximately 5 s for the solvent to evaporate. 3. Light cure for 10 s (>1000 mW/cm2) 1. | ||
| OptiBond FL b | FL primer: HEMA, GPDM, MMEP, water, ethanol, photoinitiator (CQ), BHT FL adhesive: Bis-GMA, HEMA, GPDM, GDMA, photoinitiator (CQ), ODMAB, fillers, barium aluminoborosilicate (LOT 4964258) | 1. Apply etchant for 15 s (dentin 15 s, enamel 30 s). 2. Rinse thoroughly for 15 s; air dry for 3 s (do not overdry). 3. Actively apply the primer for 15 s; air dry for 5 s. 4. Apply adhesive with a light brushing motion for 15 s; air thin for 3 s; light cure for 20 s (>1000 mW/cm2) 1. | ||
| Filtek Supreme XTE a | Bis-GMA, UDMA, TEGDMA, Bis-EMA, silanated silica, silanated zirconia, photoinitiators (LOT 552577) | 1. Place restorative in increments. 2. Light cure restorative in increments (body, enamel shades 2.0 mm, 20 s. dentin shades 1.5 mm, 40 s, >1000 mW/cm2) 1. | ||
| SBU-SE | SBU-SEE | SBU-ER | OFL | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | 6 m | 12 m | 24 m | 36 m | BL | 6 m | 12 m | 24 m | 36 m | BL | 6 m | 12 m | 24 m | 36 m | BL | 6 m | 12 m | 24 m | 36 m | |
| Restorations assessed, n | 22 | 22 | 22 | 21 | 20 | 22 | 22 | 22 | 21 | 21 | 22 | 22 | 22 | 21 | 21 | 22 | 22 | 21 | 19 | 16 |
| Reassessment rate, % | 100 | 100 | 100 | 95.5 | 90.9 | 100 | 100 | 100 | 95.5 | 95.5 | 100 | 100 | 100 | 95.5 | 95.5 | 100 | 100 | 95.5 | 86.4 | 72.7 |
| Aesthetic Criteria 1 | ||||||||||||||||||||
| Non-acceptable, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5.9 3 | 5.9 3 |
| Functional Criteria 1 | ||||||||||||||||||||
| Non-acceptable, % | 0.0 | 0.0 | 0.0 | 4.8 4 | 4.8 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.5 4 | 9.1 4 | 19.0 4 | 20.0 4 |
| Biological Criteria 1 | ||||||||||||||||||||
| Non-acceptable, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cumulative Failure Rate (Total Score) 2 | ||||||||||||||||||||
| Non-acceptable, % | 0.0 | 0.0 | 0.0 | 4.8 4 | 4.8 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 4.5 4 | 9.1 4 | 23.8 5 | 23.8 5 |
| Groups | Marginal Staining 1 (Score 2) | Marginal Adaptation 2 (Score 2) | Fractures/Retention 3 (Score 5) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | BL | 6 m | 12 m | 24 m | 36 m | BL | 6 m | 12 m | 24 m | 36 m | BL | 6 m | 12 m | 24 m | 36 m | |
| SBU SE vs. SEE | % pi | 13.6/4.5 0.5 | 13.6/4.5 0.625 | 18.2/4.5 0.375 | 25/9.5 0.375 | 30/19 0.687 | 0/0 n.c. | 22.7/4.5 0.219 | 36.4/18.2 0.344 | 35/23.8 0.687 | 45/23.8 0.344 | 0/0 n.c. | 0/0 n. c. | 0/0 n. c. | 4.8/0 1.0 | 4.8/0 1.0 |
| SBU SE vs.-ER | % pi | 13.6/0 0.25 | 13.6/0 0.25 | 18.2/9.1 0.625 | 25/4.8 0.125 | 30/33.3 1.0 | 0/0 n.c. | 22.7/13 0.687 | 36.4/31.2 1.0 | 35/28.6 1.0 | 45/42.9 1.0 | 0/0 n.c | 0/0 n.c. | 0/0 n.c. | 4.8/0 1.0 | 4.8/0 1.0 |
| SBU-SE vs. OFL | % pi | 13.6/4.5 0.5 | 13.6 /19 1.0 | 18.2/ 25 1.0 | 25/29.4 1.0 | 30/41.2 0.687 | 0/0 n.c. | 22.7/28.6 1.0 | 36.4/35 1.0 | 35/37.5 1.0 | 45/56.3 0.453 | 0/0 n.c. | 0/4.5 1.0 | 0/9.1 0.5 | 4.8/20 0.375 | 4.8/20 0.375 |
| SBU-SEE vs. ER | % pi | 4.5/0 1.0 | 4.5/0 1.0 | 4.5/9.1 1.0 | 9.5/4.8 1.0 | 19./33.3 0.375 | 0/0 n.c. | 4.5/13.6 0.625 | 18.2/31.2 0.549 | 23.8/28.6 1.0 | 23.8/42.9 0.219 | 0/0 n.c. | 0/0 n.c. | 0/0 n.c. | 0/0 n.c. | 0/0 n.c. |
| SBU-SEE vs. OFL | % pi | 4.5/4.5 1.0 | 4.5/19 0.25 | 4.5/25 0.125 | 9.5/29.4 0.25 | 19/41.2 0.063 4 | 0/0 n.c. | 4.5/28.6 0.063 4 | 18.2/35 0.375 | 23.8/37.5 0.453 | 23.8/56.3 0.07 | 0/0 n.c. | 0/4.5 1.0 | 0/9.1 0.5 | 0/20 0.125 | 0/20 0.125 |
| SBU-ER vs. OFL | % pi | 0/4.5 1.0 | 0/19.0 0.125 | 9.1/25 0.375 | 4.8/29.4 0.219 | 33.3/41.2 1.0 | 0/0 n.c. | 13.6/28.6 0.375 | 31.2/35 1.0 | 28.6/37.5 0.727 | 42.9/56.3 0.219 | 0/0 n.c. | 0/4.5 1.0 | 0/9.1 0.5 | 0/20 0.125 | 0/20 0.125 |
| Groups | Time | CFR, % | Confidence Interval (Prevalence) | ||
|---|---|---|---|---|---|
| SBU-SE 3 | 24 m | 4.8 | 0.001–0.238 (0.048) | ||
| 36 m | 4.8 | 0.0013–0.249 (0.050) | |||
| SBU-SEE 1 | 24 m | 0.0 | 0.000–0.161 (0.000) | ||
| 36 m | 0.0 | 0.000–0.161 (0.000) | |||
| SBU-ER 2 | 24 m | 0.0 | 0.000–0.161 (0.000) | ||
| 36 m | 0.0 | 0.000–0.161 (0.000) | |||
| OFL 1,2,3 | 24 m | 23.8 | 0.092–0.512 (0.263) | ||
| 36 m | 23.8 | 0.110–0.587 (0.313) | |||
| Marginal Staining Score 2 1 | ||||||||||
| BL-6 m | 6–12 m | 12–24 m | 24–36 m | BL-12 m | BL-24 m | BL-36 m | 6–24 m | 6–36 m | 12–36 m | |
| SBU-SE | 1.000 | 1.000 | 0.625 | 1.000 | 1.000 | 0.687 | 0.453 | 0.625 | 0.375 | 0.375 |
| SBU-SEE | 1.000 | 1.000 | 1.000 | 0.625 | 1.000 | 1.000 | 0.375 | 1.000 | 0.375 | 0.250 |
| SBU-ER | n.c. | 0.500 | 1.000 | 0.031 | 0.500 | 1.000 | 0.016 | 1.000 | 0.016 | 0.125 |
| OFL | 0.250 | 0.687 | 1.000 | 0.625 | 0.219 | 0.219 | 0.031 | 0.625 | 0.219 | 0.250 |
| Marginal Adaptation Score 2 1 | ||||||||||
| BL-6 m | 6–12 m | 12–24 m | 24–36 m | BL-12 m | BL-24 m | BL-36 m | 6–24 m | 6–36 m | 12–36 m | |
| SBU-SE | 0.063 * | 0.453 | 1.000 | 0.687 | 0.008 | 0.016 | 0.004 | 0.687 | 0.289 | 0.625 |
| SBU-SEE | 1.000 | 0.375 | 1.000 | 1.000 | 0.125 | 0.063 * | 0.063 * | 0.125 | 0.219 | 1.000 |
| SBU-ER | 0.250 | 0.125 | 1.000 | 0.375 | 0.016 | 0.031 | 0.004 | 0.375 | 0.031 | 0.250 |
| OFL | 0.031 | 0.625 | 1.000 | 0.375 | 0.016 | 0.031 | 0.004 | 0.500 | 0.063 * | 0.125 |
| Fractures/Retention Score 5 2 | ||||||||||
| BL-6 m | 6–12 m | 12–24 m | 24–36 m | BL-12 m | BL-24 m | BL-36 m | 6–24 m | 6–36 m | 12–36 m | |
| SBU-SE | n.c. | n.c. | 1.000 | 1.000 | n.c. | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| SBU-SEE | n.c. | |||||||||
| SBU-ER | n.c. | |||||||||
| OFL | 1.000 | 1.000 | 0.500 | 1.000 | 0.500 | 0.125 | 0.125 | 0.250 | 0.250 | 0.500 |
| Time | SBU-SE vs. SBU-SEE | SBU-SE vs. SBU-ER | SBU-SE vs. OFL | SBU-SEE vs. SBU-ER | SBU-SEE vs. OFL | SBU-ER vs. OFL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gap, % | pi | % | pi | % | pi | % | pi | % | pi | % | pi | |
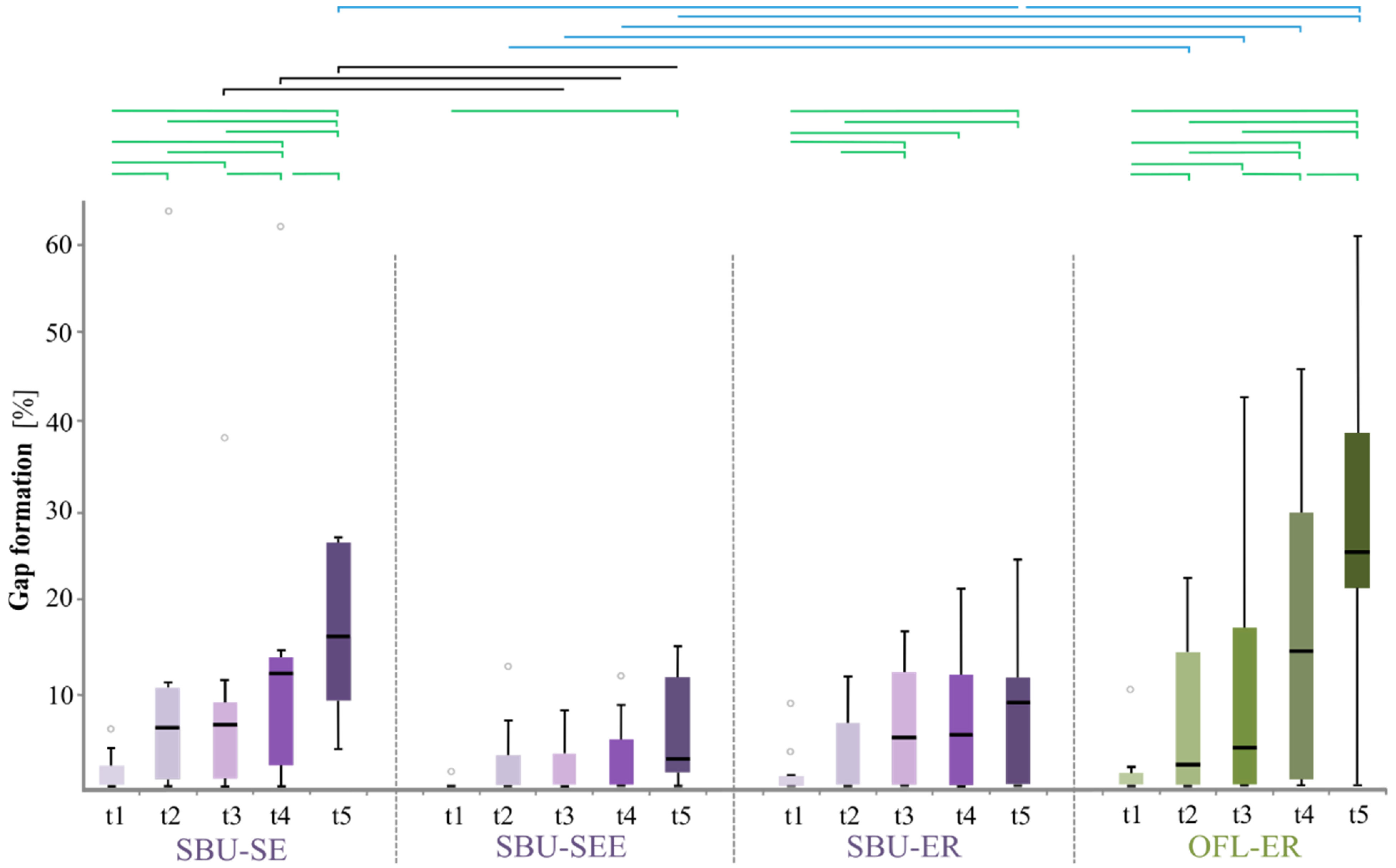
| BL | 1.4/0.1 | 0.125 | 1.4/1.3 | 0.844 | 1.4/2.9 | 0.831 | 0.1/1.3 | 0.375 | 0.1/2.9 | 0.063 1 | 1.3/2.9 | 0.469 |
| 6 m | 9.2/2.4 | 0.156 | 9.2/3.5 | 0.156 | 9.2/9.7 | 0.627 | 2.4/3.5 | 0.625 | 2.4/9.7 | 0.023 | 3.5/9.7 | 0.223 |
| 12 m | 9.6/1.9 | 0.016 | 9.6/6.0 | 0.820 | 9.6/14.6 | 0.557 | 1.9/6.0 | 0.039 | 1.9/14.6 | 0.027 | 6.0/14.6 | 0.275 |
| 24 m | 12.2/3.2 | 0.016 | 12.2/7.4 | 0.055 1 | 12.2/17.1 | 0.320 | 3.2/7.4 | 0.195 | 3.2/17.1 | 0.006 | 7.4/17.1 | 0.066 1 |
| 36 m | 19.5/5.5 | 0.007 | 19.5/7.8 | 0.001 | 19.5/29.2 | 0.215 | 5.5/7.8 | 0.742 | 5.5/29.2 | 0.003 | 7.8/29.2 | 0.005 |
| Parameter | Times | SBU-SE | SBU-SEE | SBU-ER | OFL |
|---|---|---|---|---|---|
| gap | BL vs. 6 m | 0.016 | 0.125 | 0.063 1 | 0.008 |
| BL vs. 12 m | 0.008 | 0.063 1 | 0.016 | 0.008 | |
| BL vs. 24 m | 0.008 | 0.063 1 | 0.016 | 0.004 | |
| BL vs. 36 m | 0.001 | 0.031 | 0.016 | 0.002 | |
| 6 m vs. 12 m | 1.000 | 0.844 | 0.016 | 0.063 1 | |
| 6 m vs. 24 m | 0.023 | 0.438 | 0.016 | 0.004 | |
| 6 m vs. 36 m | 0.001 | 0.297 | 0.078 | 0.002 | |
| 12 m vs. 24 m | 0.008 | 0.313 | 0.297 | 0.004 | |
| 12 m vs. 36 m | 0.001 | 0.156 | 0.547 | 0.002 | |
| 24 m vs. 36 m | 0.001 | 0.094 | 0.578 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haak, R.; Werner, M.S.; Schneider, H.; Häfer, M.; Schulz-Kornas, E. Clinical Outcomes and Quantitative Margin Analysis of a Universal Adhesive Using a Randomized Clinical Trial over Three Years. J. Clin. Med. 2022, 11, 6910. https://doi.org/10.3390/jcm11236910
Haak R, Werner MS, Schneider H, Häfer M, Schulz-Kornas E. Clinical Outcomes and Quantitative Margin Analysis of a Universal Adhesive Using a Randomized Clinical Trial over Three Years. Journal of Clinical Medicine. 2022; 11(23):6910. https://doi.org/10.3390/jcm11236910
Chicago/Turabian StyleHaak, Rainer, Melissa Sophie Werner, Hartmut Schneider, Matthias Häfer, and Ellen Schulz-Kornas. 2022. "Clinical Outcomes and Quantitative Margin Analysis of a Universal Adhesive Using a Randomized Clinical Trial over Three Years" Journal of Clinical Medicine 11, no. 23: 6910. https://doi.org/10.3390/jcm11236910







