Cerebral Small Vessel Diseases and Outcomes for Acute Ischemic Stroke Patients after Endovascular Therapy
Abstract
1. Introduction
2. Materials and Methods
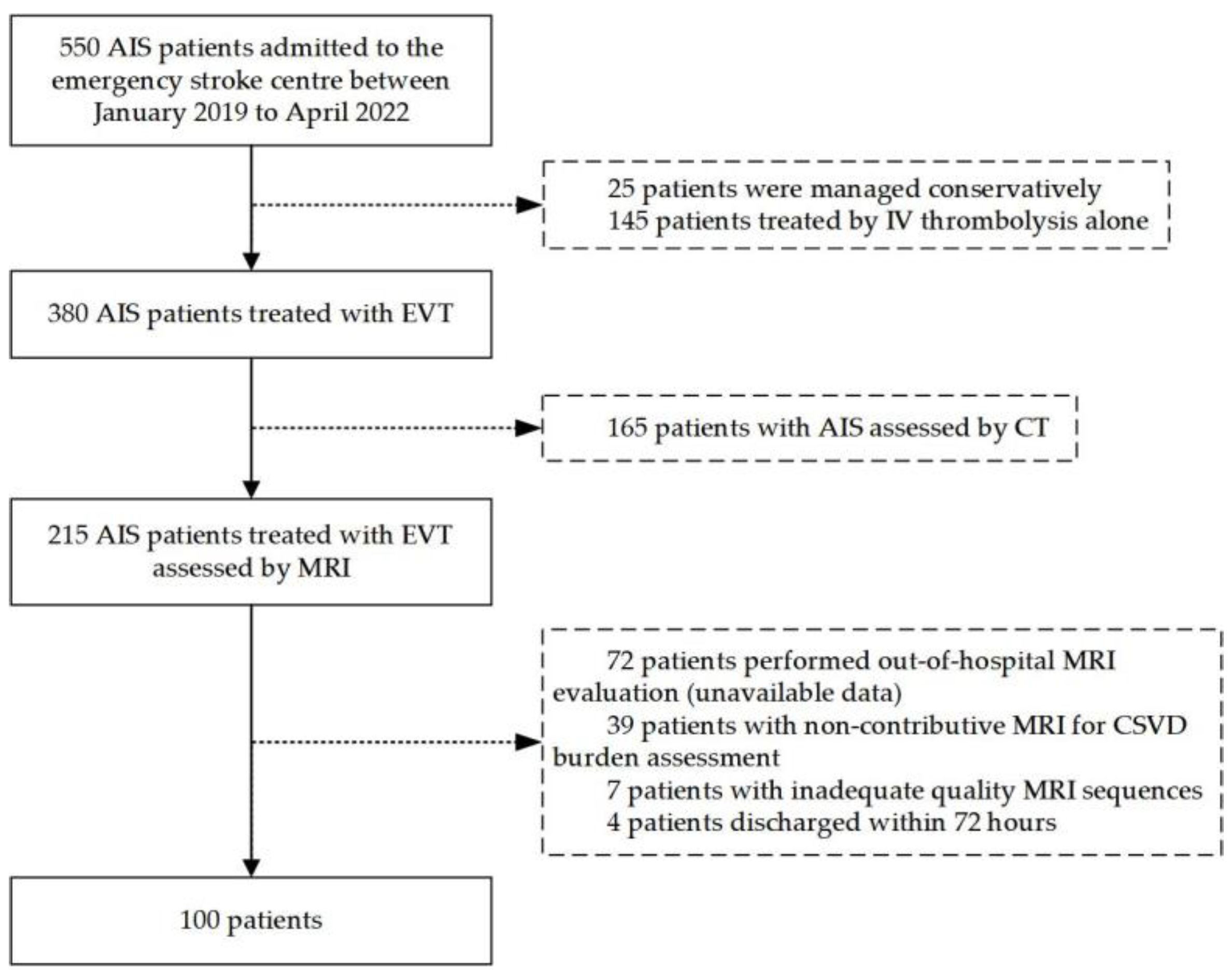
2.1. Study Population
2.2. Clinical Variables
2.3. Imaging Assessment
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Imaging Markers and CSVD Burden
3.2. Association between CSVD Burden and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.-J.; Li, Z.-X.; Gu, H.-Q.; Zhai, Y.; Jiang, Y.; Zhao, X.-Q.; Wang, Y.-L.; Yang, X.; Wang, C.-J.; Meng, X.; et al. China Stroke Statistics 2019: A Report From the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc. Neurol. 2020, 5, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.S.; Lev, M.H.; English, J.D.; Camargo, E.C.; Chou, M.; Johnston, S.C.; Gonzalez, G.; Schaefer, P.W.; Dillon, W.P.; Koroshetz, W.J.; et al. Significance of large vessel intracranial occlusion causing acute ischemic stroke and TIA. Stroke 2009, 40, 3834–3840. [Google Scholar] [CrossRef]
- Berkhemer, O.A.; Fransen, P.S.S.; Beumer, D.; van den Berg, L.A.; Lingsma, H.F.; Yoo, A.J.; Schonewille, W.J.; Vos, J.A.; Nederkoorn, P.J.; Wermer, M.J.H.; et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015, 372, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Chamorro, A.; Cobo, E.; de Miquel, M.A.; Molina, C.A.; Rovira, A.; San Román, L.; Serena, J.; Abilleira, S.; Ribó, M.; et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015, 372, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Goyal, M.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Albers, G.W.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015, 372, 2285–2295. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Balami, J.S.; White, P.M.; McMeekin, P.J.; Ford, G.A.; Buchan, A.M. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int. J. Stroke 2018, 13, 348–361. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Staals, J.; Makin, S.D.J.; Doubal, F.N.; Dennis, M.S.; Wardlaw, J.M. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 2014, 83, 1228–1234. [Google Scholar] [CrossRef]
- Georgakis, M.K.; Duering, M.; Wardlaw, J.M.; Dichgans, M. WMH and long-term outcomes in ischemic stroke: A systematic review and meta-analysis. Neurology 2019, 92, e1298–e1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, T.; Diao, S.; Cai, X.; Kong, Y.; Zhang, L.; Wang, Z.; Li, R.; Zhou, Y.; Fang, Q. The global burden of cerebral small vessel disease related to neurological deficit severity and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Neurol. Sci. 2019, 40, 1157–1166. [Google Scholar] [CrossRef]
- Charidimou, A.; Shoamanesh, A. Clinical relevance of microbleeds in acute stroke thrombolysis: Comprehensive meta-analysis. Neurology 2016, 87, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zi, W.; Wan, Y.; Zhang, S.; Sun, B.; Shang, X.; Li, S.; Bai, Y.; Li, Z.; Zheng, D.; et al. Leukoaraiosis severity and outcomes after mechanical thrombectomy with stent-retriever devices in acute ischemic stroke. J. Neurointerv. Surg. 2019, 11, 137–140. [Google Scholar] [CrossRef]
- Boulouis, G.; Bricout, N.; Benhassen, W.; Ferrigno, M.; Turc, G.; Bretzner, M.; Benzakoun, J.; Seners, P.; Personnic, T.; Legrand, L.; et al. White matter hyperintensity burden in patients with ischemic stroke treated with thrombectomy. Neurology 2019, 93, e1498–e1506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Leng, X.; Nie, X.; Yan, H.; Tian, X.; Pan, Y.; Yang, Z.; Wen, M.; Pu, Y.; Gu, W.; et al. Small vessel disease burden may not portend unfavorable outcome after thrombectomy for acute large vessel occlusion. Eur. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Doubal, F.N.; MacLullich, A.M.J.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 2010, 41, 450–454. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Arba, F.; Palumbo, V.; Boulanger, J.-M.; Pracucci, G.; Inzitari, D.; Buchan, A.M.; Hill, M.D. Leukoaraiosis and lacunes are associated with poor clinical outcomes in ischemic stroke patients treated with intravenous thrombolysis. Int. J. Stroke 2016, 11, 62–67. [Google Scholar] [CrossRef]
- Amin Al Olama, A.; Wason, J.M.S.; Tuladhar, A.M.; van Leijsen, E.M.C.; Koini, M.; Hofer, E.; Morris, R.G.; Schmidt, R.; de Leeuw, F.-E.; Markus, H.S. Simple MRI score aids prediction of dementia in cerebral small vessel disease. Neurology 2020, 94, e1294–e1302. [Google Scholar] [CrossRef] [PubMed]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Girot, J.-B.; Richard, S.; Gariel, F.; Sibon, I.; Labreuche, J.; Kyheng, M.; Gory, B.; Dargazanli, C.; Maier, B.; Consoli, A.; et al. Predictors of Unexplained Early Neurological Deterioration After Endovascular Treatment for Acute Ischemic Stroke. Stroke 2020, 51, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Strbian, D.; Meretoja, A.; Putaala, J.; Kaste, M.; Tatlisumak, T. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int. J. Stroke 2013, 8, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Mechtouff, L.; Nighoghossian, N.; Amaz, C.; Buisson, M.; Berthezène, Y.; Derex, L.; Ong, E.; Eker, O.F.; Cho, T.-H. White matter burden does not influence the outcome of mechanical thrombectomy. J. Neurol. 2020, 267, 618–624. [Google Scholar] [CrossRef]
- Shi, Z.-S.; Loh, Y.; Liebeskind, D.S.; Saver, J.L.; Gonzalez, N.R.; Tateshima, S.; Jahan, R.; Feng, L.; Vespa, P.M.; Starkman, S.; et al. Leukoaraiosis predicts parenchymal hematoma after mechanical thrombectomy in acute ischemic stroke. Stroke 2012, 43, 1806–1811. [Google Scholar] [CrossRef]
- Zhai, F.-F.; Yang, M.; Wei, Y.; Wang, M.; Gui, Y.; Han, F.; Zhou, L.-X.; Ni, J.; Yao, M.; Zhang, S.-Y.; et al. Carotid atherosclerosis, dilation, and stiffness relate to cerebral small vessel disease. Neurology 2020, 94, e1811–e1819. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Yuan, J.; Chen, Y.; Luo, H. Small Vessel Disease Burden and Outcomes of Mechanical Thrombectomy in Ischemic Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 602037. [Google Scholar] [CrossRef]
- Uniken Venema, S.M.; Postma, A.A.; van den Wijngaard, I.R.; Vos, J.A.; Lingsma, H.F.; Bokkers, R.P.H.; Hofmeijer, J.; Dippel, D.W.J.; Majoie, C.B.; van der Worp, H.B. White Matter Lesions and Outcomes After Endovascular Treatment for Acute Ischemic Stroke: MR CLEAN Registry Results. Stroke 2021, 52, 2849–2857. [Google Scholar] [CrossRef]
- Lau, K.-K.; Li, L.; Lovelock, C.E.; Zamboni, G.; Chan, T.-T.; Chiang, M.-F.; Lo, K.-T.; Küker, W.; Mak, H.K.-F.; Rothwell, P.M. Clinical Correlates, Ethnic Differences, and Prognostic Implications of Perivascular Spaces in Transient Ischemic Attack and Ischemic Stroke. Stroke 2017, 48, 1470–1477. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Pasi, M.; Auriel, E.; van Etten, E.S.; Haley, K.; Ayres, A.; Schwab, K.M.; Martinez-Ramirez, S.; Goldstein, J.N.; et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology 2017, 88, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Best, J.G.; Barbato, C.; Ambler, G.; Du, H.; Banerjee, G.; Wilson, D.; Shakeshaft, C.; Cohen, H.; Yousry, T.A.; Al-Shahi Salman, R.; et al. Association of enlarged perivascular spaces and anticoagulant-related intracranial hemorrhage. Neurology 2020, 95, e2192–e2199. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.K.; Li, L.; Schulz, U.; Simoni, M.; Chan, K.H.; Ho, S.L.; Cheung, R.T.F.; Küker, W.; Mak, H.K.F.; Rothwell, P.M. Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology 2017, 88, 2260–2267. [Google Scholar] [CrossRef] [PubMed]
- Jovin, T.G.; Li, C.; Wu, L.; Wu, C.; Chen, J.; Jiang, C.; Shi, Z.; Gao, Z.; Song, C.; Chen, W.; et al. Trial of Thrombectomy 6 to 24 Hours after Stroke Due to Basilar-Artery Occlusion. N. Engl. J. Med. 2022, 387, 1373–1384. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Benveniste, H.; Nedergaard, M.; Zlokovic, B.V.; Mestre, H.; Lee, H.; Doubal, F.N.; Brown, R.; Ramirez, J.; MacIntosh, B.J.; et al. Perivascular spaces in the brain: Anatomy, physiology and pathology. Nat. Rev. Neurol. 2020, 16, 137–153. [Google Scholar] [CrossRef]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef]
- Kwee, R.M.; Kwee, T.C. Virchow-Robin spaces at MR imaging. Radiographics 2007, 27, 1071–1086. [Google Scholar] [CrossRef]
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, S.; Li, M.; Sun, B.; Shang, X.; Li, S.; Bai, Y.; Li, Z.; Zi, W.; Liu, X. Leukoaraiosis and earlier neurological outcome after mechanical thrombectomy in acute ischemic stroke. J. Neuroradiol. 2020, 47, 428–432. [Google Scholar] [CrossRef]
- Lin, M.P.; Brott, T.G.; Liebeskind, D.S.; Meschia, J.F.; Sam, K.; Gottesman, R.F. Collateral Recruitment Is Impaired by Cerebral Small Vessel Disease. Stroke 2020, 51, 1404–1410. [Google Scholar] [CrossRef]

| Total (n = 380) | Included (n = 100) | Excluded (n = 280) | p-Value | |
|---|---|---|---|---|
| Age, years | 65.27 ± 12.49 | 12.71 ± 0.76 | 11.79 ± 1.18 | 0.146 |
| Gender, male, n (%) | 245 (64.5) | 181 (64.6) | 64 (64.0) | 0.904 |
| Medical history, n (%) | ||||
| Hypertension | 238 (62.6) | 62 (62.0) | 176 (62.9) | 0.904 |
| Diabetes mellitus | 97 (25.5) | 31 (31.0) | 66 (23.6) | 0.145 |
| Coronary heart disease | 75 (19.7) | 22 (22.0) | 53 (18.9) | 0.559 |
| Dyslipidemia | 46 (12.1) | 13 (13.0) | 33 (11.8) | 0.724 |
| Atrial fibrillation | 115 (30.3) | 25 (25.0) | 90 (32.1) | 0.206 |
| General status | ||||
| BMI, kg/m2 | 27.96 ± 34.36 | 34.56 ± 60.76 | 25.54 ± 15.79 | 0.234 |
| Admission SBP, mmHg | 142.64 ± 24.36 | 144.71 ± 23.09 | 141.92 ± 24.81 | 0.378 |
| Admission DBP, mmHg | 82.03 ± 15.17 | 83.19 ± 13.92 | 81.63 ± 15.60 | 0.429 |
| IVT, n (%) | 82 (21.6) | 24 (24.0) | 58 (20.7) | 0.483 |
| Admission NIHSS | 11.43 ± 6.970 | 11.79 ± 6.147 | 11.29 ± 7.277 | 0.543 |
| Onset-to-treatment, h | 16.10 ± 24.02 | 14.48 ± 21.53 | 16.74 ± 24.95 | 0.427 |
| Duration of treatment, min | 125.89 ± 84.18 | 136.84 ± 125.53 | 121.56 ± 60.30 | 0.127 |
| Laboratory examination | ||||
| WBC, cells/mL | 10.02 ± 3.70 | 10.24 ± 3.68 | 9.94 ± 3.72 | 0.510 |
| Hemoglobin, g/dL | 107.85 ± 117.20 | 105.76 ± 116.32 | 108.58 ± 117.75 | 0.840 |
| TG, mmol/L | 1.16 ± 0.71 | 1.13 ± 0.61 | 1.17 ± 0.75 | 0.688 |
| TC, mmol/L | 3.87 ± 0.99 | 3.99 ± 0.90 | 3.82 ± 1.01 | 0.160 |
| LDL, mmol/L | 2.22 ± 0.80 | 2.28 ± 0.78 | 2.20 ± 0.81 | 0.425 |
| HDL, mmol/L | 1.01 ± 0.25 | 1.04 ± 0.28 | 1.00 ± 0.24 | 0.192 |
| D-Dimer, mg/L | 2.48 ± 4.64 | 2.02 ± 2.65 | 2.64 ± 5.15 | 0.301 |
| Hcy, μmol/L | 23.06 ± 15.91 | 24.05 ± 18.12 | 22.71 ± 15.08 | 0.503 |
| Outcome, n (%) | ||||
| sICH | 107 (28.2) | 33 (33.0) | 74 (26.4) | 0.130 |
| Absent-to-Moderate CSVD (n = 69) | Severe CSVD (n = 31) | p-Value | |
|---|---|---|---|
| Age, years | 62.16 ± 12.0 | 67.16 ± 10.7 | 0.049 |
| Gender, male, n (%) | 45 (65.2) | 19 (61.3) | 0.703 |
| Medical history, n (%) | |||
| Hypertension | 37 (53.6) | 25 (80.6) | 0.010 |
| Diabetes mellitus | 23 (33.3) | 8 (25.8) | 0.452 |
| Coronary heart disease | 12 (17.4) | 10 (32.3) | 0.097 |
| Dyslipidemia | 8 (11.6) | 5 (16.1) | 0.533 |
| Atrial fibrillation | 16 (23.2) | 9 (29.0) | 0.533 |
| General status | |||
| BMI, kg/m2 | 24.94 ± 5.05 | 22.59 ± 3.51 | 0.147 |
| Admission SBP, mmHg | 142.23 ± 21.48 | 153.6 ± 21.7 | 0.033 |
| Admission NIHSS | 11.41 ± 6.13 | 12.65 ± 6.2 | 0.354 |
| Laboratory examination | |||
| WBC, cells/mL | 9.37 ± 4.42 | 10.42 ± 3.95 | 0.275 |
| Hemoglobin, g/dL | 106.37 ± 117.0 | 129.27 ± 130.17 | 0.390 |
| TG, mmol/L | 1.12 ± 0.34 | 1.13 ± 0.74 | 0.926 |
| TC, mmol/L | 3.52 ± 1.02 | 3.67 ± 0.66 | 0.454 |
| LDL, mmol/L | 2.26 ± 0.83 | 2.21 ± 0.68 | 0.837 |
| HDL, mmol/L | 0.98 ± 0.27 | 1.14 ± 0.34 | 0.033 |
| D-Dimer, mg/L | 3.42 ± 10.13 | 2.13 ± 2.69 | 0.511 |
| Hcy, μmol/L | 21.22 ± 14.27 | 31.13 ± 23.49 | 0.016 |
| Occlusion site | |||
| ICA | 25 (36.2) | 9 (29.0) | 0.482 |
| MCA | 36 (52.2) | 16 (51.6) | 0.959 |
| PCA/BA | 8 (11.6) | 6 (19.4) | 0.301 |
| Treatment details and process times | |||
| Onset-to-treatment, h | 11.41 ± 14.30 | 15.12 ± 13.87 | 0.229 |
| Duration of treatment, min | 142.80 ± 114.19 | 123.13 ± 64.96 | 0.477 |
| IVT, n (%) | 17 (24.6) | 7 (22.6) | 0.824 |
| Thrombectomy | 56 (81.2) | 22 (73.3) | 0.381 |
| Stent implantation | 30 (43.5) | 13 (43.3) | 0.989 |
| Balloon dilatation | 27 (39.1) | 14 (46.7) | 0.484 |
| contrast, ml | 215.8 ± 101.12 | 243.0 ± 103.8 | 0.225 |
| TICI 2b/3, n (%) | 54(78.3) | 24 (77.4) | 0.925 |
| Outcome, n (%) | |||
| sICH | 23 (33.3) | 10 (32.3) | 0.916 |
| END | 11 (15.9) | 7 (22.6) | 0.424 |
| MCE | 8 (11.6) | 6 (20.0) | 0.270 |
| In-hospital death | 5 (5.8) | 0 | 0.178 |
| mRS > 2 | 24 (40.7) | 17 (56.7) | 0.153 |
| Univariate Analysis OR (95%CI) | Multivariate Analysis | |||
|---|---|---|---|---|
| Model 1 OR (95%CI) | Model 2 OR (95%CI) | Model 3 OR (95%CI) | ||
| mRS > 2 | 1.907 (0.784, 4.642) | 2.453 (0.869, 6.711) | 2.656 (0.824, 8.557) | 2.636 (0.780, 8.905) |
| sICH | 0.952 (0.386, 2.352) | 0.958 (0.365, 2.519) | 1.036 (0.328, 3.265) | 0.997 (0.313, 3.180) |
| END | 1.538 (0.533, 4.440)) | 1.618 (0.520, 5.035) | 1.911 (0.504, 7.242) | 1.773 (0.460, 6.839) |
| MCE | 1.906 (0.598, 6.075) | 2.604 (0.713, 9.517) | 2.036 (0.476, 8.716) | 1.808 (0.408, 8.001) |
| mRS > 2 | sICH | END | MCE | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Adjusted * | OR (95% CI) | Adjusted * | OR (95% CI) | Adjusted * | OR (95% CI) | Adjusted * | |
| PWMHs | 1.250 (0.50, 3.12) | 2.981 (0.79, 11.32) | 0.947 (0.37, 2.41) | 0.853 (0.19, 3.98) | 0.625 (0.08, 4.96) | 0.402 (0.03, 5.30) | 1.078 (0.31, 3.78) | 1.2 (0.23, 6.23) |
| DWMHs | 2.316 (0.98, 5.46) | 5.524 (1.49, 20.52) | 0.905 (0.39, 2.12) | 0.468 (0.10, 2.25) | 2.06 (0.73, 5.77) | 2.273 (0.65, 7.93) | 1.13 (0.36, 3.53) | 1.11 (0.22, 5.49) |
| EPVS-BG | 0.827 (0.36, 1.92) | 1.073 (0.34, 3.36) | 1.097 (0.48, 2.53) | 2.61 (0.59, 11.52) | 1.13 (0.41, 3.16) | 0.473 (0.11, 2.0) | 0.64 (0.2, 1.99) | 0.52 (0.12, 2.28) |
| EPVS-CS | 2.063 (0.78, 5.49) | 1.024 (0.31, 3.43) | 2.054 (0.734, 5.72) | 3.893 (0.73, 20.73) | 7.89 (1.0, 62.53) | 9.391 (1.13, 78.24) | 2.5 (0.52, 11.99) | 3.71 (0.68, 20.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Ning, Y.; Lei, L.; Yuan, H.; Liu, H.; Luo, G.; Wei, M.; Li, Y. Cerebral Small Vessel Diseases and Outcomes for Acute Ischemic Stroke Patients after Endovascular Therapy. J. Clin. Med. 2022, 11, 6883. https://doi.org/10.3390/jcm11236883
Zhao Y, Ning Y, Lei L, Yuan H, Liu H, Luo G, Wei M, Li Y. Cerebral Small Vessel Diseases and Outcomes for Acute Ischemic Stroke Patients after Endovascular Therapy. Journal of Clinical Medicine. 2022; 11(23):6883. https://doi.org/10.3390/jcm11236883
Chicago/Turabian StyleZhao, Yixin, Yuye Ning, Lei Lei, Huijie Yuan, Hui Liu, Guogang Luo, Meng Wei, and Yongxin Li. 2022. "Cerebral Small Vessel Diseases and Outcomes for Acute Ischemic Stroke Patients after Endovascular Therapy" Journal of Clinical Medicine 11, no. 23: 6883. https://doi.org/10.3390/jcm11236883
APA StyleZhao, Y., Ning, Y., Lei, L., Yuan, H., Liu, H., Luo, G., Wei, M., & Li, Y. (2022). Cerebral Small Vessel Diseases and Outcomes for Acute Ischemic Stroke Patients after Endovascular Therapy. Journal of Clinical Medicine, 11(23), 6883. https://doi.org/10.3390/jcm11236883






