Fetal Bradycardia Caused by Monogenic Disorders—A Review of the Literature
Abstract
1. Background
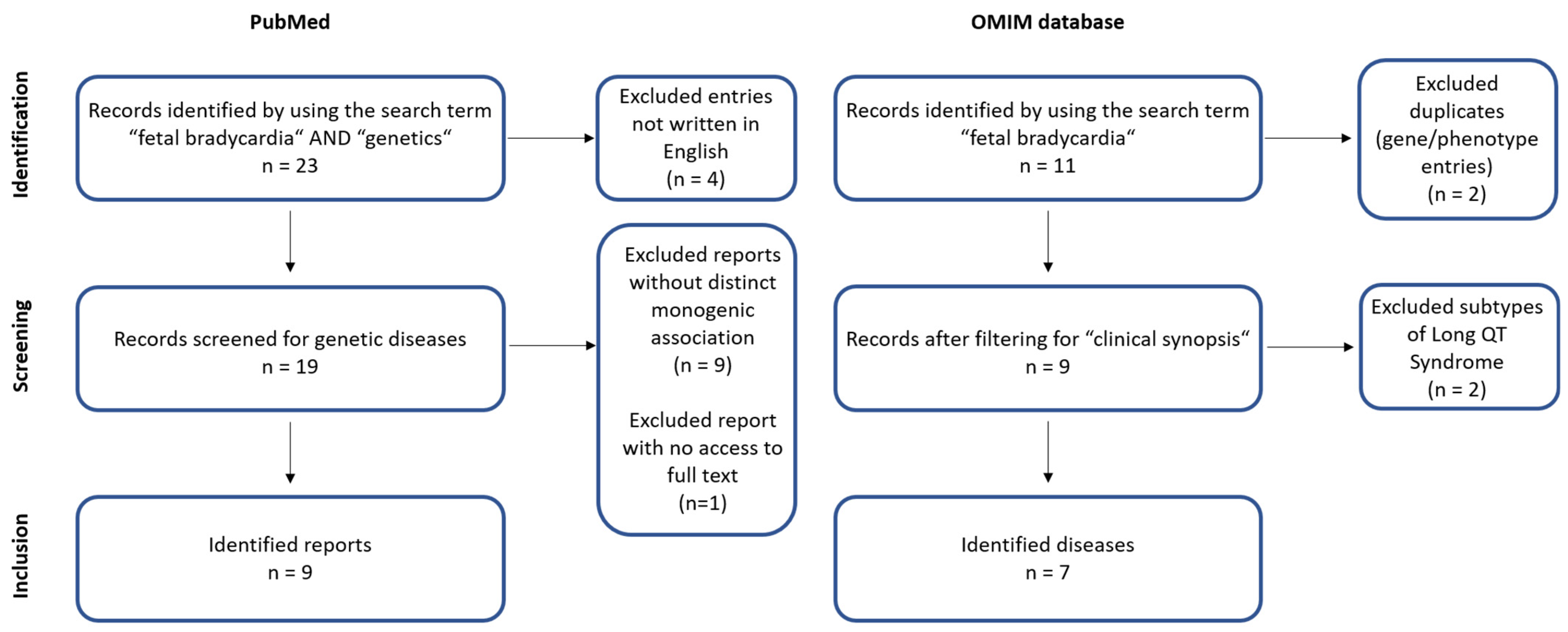
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macones, G.; Blackwell, S.; Moore, T.; Spong, C.; Hauth, J.; Hankins, G. Management of intrapartum fetal heart rate tracings: ACOG practice bulletin no. 116. Obstet. Gynecol. 2010, 116, 1232–1240. [Google Scholar]
- Widnes, C.; Flo, K.; Wilsgaard, T.; Kiserud, T.; Acharya, G. Sex differences in umbilical artery Doppler indices: A longitudinal study. Biol. Sex Differ. 2018, 9, 16. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Wakai, R.T.; Strasburger, J.F. Importance of Fetal Arrhythmias to the Neonatologist and Pediatrician. Neoreviews 2016, 17, e568–e578. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.W.; Snijders, R.; Geerts, L.; Spencer, K.; Nicolaides, K.H. Fetal heart rate in chromosomally abnormal fetuses. Ultrasound. Obstet. Gynecol. 2000, 16, 610–613. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Strasburger, J.F.; Cuneo, B.F.; Wakai, R.T. Diagnosis and treatment of fetal arrhythmia. Am. J. Perinatol. 2014, 31, 617–628. [Google Scholar] [CrossRef]
- Low, J.A.; Cox, M.J.; Karchmar, E.J.; McGrath, M.J.; Pancham, S.R.; Piercy, W.N. The prediction of intrapartum fetal metabolic acidosis by fetal heart rate monitoring. Am. J. Obstet. Gynecol. 1981, 139, 299–305. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Cuneo, B.F.; Etheridge, S.P.; Horigome, H.; Weng, H.Y.; Benson, D.W. Fetal heart rate predictors of long QT syndrome. Circulation 2012, 126, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.; Strasburger, J.F.; Cuneo, B.F.; Wakai, R.T. Complex and Novel Arrhythmias Precede Stillbirth in Fetuses With De Novo Long QT Syndrome. Circ. Arrhythm. Electrophysiol. 2020, 13, e008082. [Google Scholar] [CrossRef] [PubMed]
- Lupoglazoff, J.M.; Denjoy, I.; Villain, E.; Fressart, V.; Simon, F.; Bozio, A.; Berthet, M.; Benammar, N.; Hainque, B.; Guicheney, P. Long QT syndrome in neonates: Conduction disorders associated with HERG mutations and sinus bradycardia with KCNQ1 mutations. J. Am. Coll. Cardiol. 2004, 43, 826–830. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Strasburger, J.F.; Yu, S.; Horigome, H.; Hosono, T.; Kandori, A.; Wakai, R.T. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation 2013, 128, 2183–2191. [Google Scholar] [CrossRef]
- Vrtel, R.; Verhoef, S.; Bouman, K.; Maheshwar, M.M.; Nellist, M.; van Essen, A.J.; Bakker, P.L.; Hermans, C.J.; Bink-Boelkens, M.T.; van Elburg, R.M.; et al. Identification of a nonsense mutation at the 5’ end of the TSC2 gene in a family with a presumptive diagnosis of tuberous sclerosis complex. J. Med. Genet. 1996, 33, 47–51. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.A.; Fong, J.C.; Basson, C.T. Holt-Oram Syndrome. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Sun, H.; Liu, X.; Hao, X.; Wang, J.; Han, J.; Liang, M.; Zhang, H.; He, Y. Case Report: Biventricular Noncompaction Cardiomyopathy With Pulmonary Stenosis and Bradycardia in a Fetus With KCNH2 Mutation. Front. Genet. 2022, 24, 821226. [Google Scholar] [CrossRef] [PubMed]
- Wacker-Gussmann, A.; Oberhoffer-Fritz, R.; Westphal, D.S.; Hessling, G.; Wakai, R.T.; Strasburger, J.F. The missense variant p.(Gly482Arg) in HCN4 is responsible for fetal tachy-bradycardia syndrome. HeartRhythm Case Rep. 2020, 6, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Desai, L.; Wakai, R.; Tsao, S.; Strasburger, J.; Gotteiner, N.; Patel, A. Fetal diagnosis of KCNQ1-variant long QT syndrome using fetal echocardiography and magnetocardiography. Pacing Clin. Electrophysiol. 2020, 43, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Gusic, M.; Schottmann, G.; Feichtinger, R.G.; Du, C.; Scholz, C.; Wagner, M.; Mayr, J.A.; Lee, C.Y.; Yepez, V.A.; Lorenz, N.; et al. Bi-Allelic UQCRFS1 Variants Are Associated with Mitochondrial Complex III Deficiency, Cardiomyopathy, and Alopecia Totalis. Am. J. Hum. Genet. 2020, 106, 102–111. [Google Scholar] [CrossRef]
- Sepp, R.; Hategan, L.; Bácsi, A.; Cseklye, J.; Környei, L.; Borbás, J.; Széll, M.; Forster, T.; Nagy, I.; Hegedus, Z. Timothy syndrome 1 genotype without syndactyly and major extracardiac manifestations. Am. J. Med. Genet. A 2017, 173, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Hoosien, M.; Ahearn, M.E.; Myerburg, R.J.; Pham, T.V.; Miller, T.E.; Smets, M.J.; Baumbach-Reardon, L.; Young, M.-L.; Farooq, A.; Bishopric, N.H. Dysfunctional potassium channel subunit interaction as a novel mechanism of long QT syndrome. Heart Rhythm. 2013, 10, 728–737. [Google Scholar] [CrossRef]
- Schneider, U.; Haueisen, J.; Loeff, M.; Bondarenko, N.; Schleussner, E. Prenatal diagnosis of a long QT syndrome by fetal magnetocardiography in an unshielded bedside environment. Prenat. Diagn. 2005, 25, 704–708. [Google Scholar] [CrossRef]
- Chang, C.-C.; Acharfi, S.; Wu, M.-H.; Chiang, F.-T.; Wang, J.-K.; Sung, T.-C.; Chahine, M. A novel SCN5A mutation manifests as a malignant form of long QT syndrome with perinatal onset of tachycardia/bradycardia. Cardiovasc. Res. 2004, 64, 268–278. [Google Scholar] [CrossRef]
- Tester, D.J.; McCormack, J.; Ackerman, M.J. Prenatal molecular genetic diagnosis of congenital long QT syndrome by strategic genotyping. Am. J. Cardiol. 2004, 93, 788–791. [Google Scholar] [CrossRef]
- Alders, M.; Bikker, H.; Christiaans, I. Long QT Syndrome. In GeneReviews((R)); Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Krause, U.; Gravenhorst, V.; Kriebel, T.; Ruschewski, W.; Paul, T. A rare association of long QT syndrome and syndactyly: Timothy syndrome (LQT 8). Clin. Res. Cardiol. 2011, 100, 1123–1127. [Google Scholar] [CrossRef]
- Brackley, K.J.; Farndon, P.A.; Weaver, J.B.; Dow, D.J.; Chapman, S.; Kilby, M.D. Prenatal diagnosis of tuberous sclerosis with intracerebral signs at 14 weeks’ gestation. Prenat. Diagn. 1999, 19, 575–579. [Google Scholar] [CrossRef]
- Regalado, J.J.; Rodriguez, M.M.; Ferrer, P.L. Infantile hypertrophic cardiomyopathy of glycogenosis type IX: Isolated cardiac phosphorylase kinase deficiency. Pediatr. Cardiol. 1999, 20, 304–307. [Google Scholar] [CrossRef]
- Friederich, M.W.; Timal, S.; Powell, C.A.; Dallabona, C.; Kurolap, A.; Palacios-Zambrano, S.; Bratkovic, D.; Derks, T.G.J.; Bick, D.; Bouman, K.; et al. Pathogenic variants in glutamyl-tRNA(Gln) amidotransferase subunits cause a lethal mitochondrial cardiomyopathy disorder. Nat. Commun. 2018, 9, 4065. [Google Scholar] [CrossRef]
- Muru, K.; Kalev, I.; Teek, R.; Sonajalg, M.; Kuuse, K.; Reimand, T.; Ounap, K. A Boy with Holt-Oram Syndrome Caused by Novel Mutation c.1304delT in the TBX5 Gene. Mol. Syndromol. 2011, 1, 307–310. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Lahrouchi, N.; Tadros, R.; Crotti, L.; Mizusawa, Y.; Postema, P.G.; Beekman, L.; Walsh, R.; Hasegawa, K.; Barc, J.; Ernsting, M.; et al. Transethnic Genome-Wide Association Study Provides Insights in the Genetic Architecture and Heritability of Long QT Syndrome. Circulation 2020, 142, 324–338. [Google Scholar] [CrossRef]
- Xie, T.; Wang, B.; Nolte, I.M.; van der Most, P.J.; Oldehinkel, A.J.; Hartman, C.A.; Snieder, H. Genetic Risk Scores for Complex Disease Traits in Youth. Circ. Genom. Precis. Med. 2020, 13, e002775. [Google Scholar] [CrossRef] [PubMed]
- Jervell, A.; Lange-Nielsen, F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am. Heart J. 1957, 54, 59–68. [Google Scholar] [CrossRef]
- Bauer, R.; Timothy, K.W.; Golden, A. Update on the Molecular Genetics of Timothy Syndrome. Front. Pediatr. 2021, 9, 668546. [Google Scholar] [CrossRef]
- Schweizer, P.A.; Schroter, J.; Greiner, S.; Haas, J.; Yampolsky, P.; Mereles, D.; Buss, S.J.; Seyler, C.; Bruehl, C.; Draguhn, A.; et al. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J. Am. Coll. Cardiol. 2014, 64, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Milano, A.; Vermeer, A.M.; Lodder, E.M.; Barc, J.; Verkerk, A.O.; Postma, A.V.; van der Bilt, I.A.; Baars, M.J.; van Haelst, P.L.; Caliskan, K.; et al. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J. Am. Coll. Cardiol. 2014, 64, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef] [PubMed]
- Strasburger, J.F.; Wakai, R.T. Fetal cardiac arrhythmia detection and in utero therapy. Nat. Rev. Cardiol. 2010, 7, 277–290. [Google Scholar] [CrossRef]
- Miyake, A.; Sakaguchi, H.; Miyazaki, A.; Miyoshi, T.; Aiba, T.; Shiraishi, I. Successful prenatal management of ventricular tachycardia and second-degree atrioventricular block in fetal long QT syndrome. HeartRhythm Case Rep. 2017, 3, 53–57. [Google Scholar] [CrossRef][Green Version]
- Austin, S.L.; Chiou, A.; Sun, B.; Case, L.E.; Govendrageloo, K.; Hansen, P.; Kishnani, P.S. Alglucosidase alfa enzyme replacement therapy as a therapeutic approach for a patient presenting with a PRKAG2 mutation. Mol. Genet. Metab. 2017, 120, 96–100. [Google Scholar] [CrossRef]
| Name | Type | Reference |
|---|---|---|
| Case Report: Biventricular Noncompaction Cardiomyopathy With Pulmonary Stenosis and Bradycardia in a Fetus With KCNH2 Mutation | Case report | [13] |
| The missense variant p.(Gly482Arg) in HCN4 is responsible for fetal tachy-bradycardia syndrome | Case report | [14] |
| Fetal diagnosis of KCNQ1-variant long QT syndrome using fetal echocardiography and magnetocardiography | Case report | [15] |
| Bi-Allelic UQCRFS1 Variants Are Associated with Mitochondrial Complex III Deficiency, Cardiomyopathy, and Alopecia Totalis | Case report | [16] |
| Timothy syndrome 1 genotype without syndactyly and major extracardiac manifestations | Review | [17] |
| Dysfunctional potassium channel subunit interaction as a novel mechanism of long QT syndrome | Original article | [18] |
| Prenatal diagnosis of a long QT syndrome by fetal magnetocardiography in an unshielded bedside environment | Case report | [19] |
| A novel SCN5A mutation manifests as a malignant form of long QT syndrome with perinatal onset of tachycardia/bradycardia | Case report | [20] |
| Prenatal molecular genetic diagnosis of congenital long QT syndrome by strategic genotyping | Case report | [21] |
| Primary/Secondary Bradycardia | Associated Disease | Gene(s) | Inheritance | Further Prenatal Manifestations |
|---|---|---|---|---|
| Primary | Long QT Syndrome | KCNQ1, KCNH2, SCN5A * | AD, AR | AV block, prolonged QTc [22], syndactyly in Timothy Syndrome [23] |
| Sick Sinus Syndrome | HCN4, SCN5A | AD, AR | atrial flutter, prolonged QTc [14] | |
| Short QT Syndrome | KCNQ1, KCNH2, KCNJ2 | AD | not reported | |
| Holt Oram Syndrome | TBX5 | AD | structural heart defects (e.g., VSD), skeletal abnormalities (e.g., upper-limb malformations) [12] | |
| Tuberous sclerosis | TSC1, TSC2 | AD | neuronal migration disorder [24], cardiac rhabdomyosarcoma [11] | |
| Secondary | Lethal congenital glycogen storage disease of heart | PRKAG2 | AD | hypertrophic cardiomyopathy [25] |
| Combined oxidative phosphorylation deficiency, type 41 | GATB | AR | cardiomegaly, fetal hydrops [26] | |
| Familial erythrocytosis, type 2 | VHL | AR | not reported | |
| Nuclear mitochondrial complex III deficiency, type 10 | UQCRFS1 | AR | IUGR [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Westphal, D.S.; Hauser, M.; Beckmann, B.-M.; Wolf, C.M.; Hessling, G.; Oberhoffer-Fritz, R.; Wacker-Gussmann, A. Fetal Bradycardia Caused by Monogenic Disorders—A Review of the Literature. J. Clin. Med. 2022, 11, 6880. https://doi.org/10.3390/jcm11236880
Westphal DS, Hauser M, Beckmann B-M, Wolf CM, Hessling G, Oberhoffer-Fritz R, Wacker-Gussmann A. Fetal Bradycardia Caused by Monogenic Disorders—A Review of the Literature. Journal of Clinical Medicine. 2022; 11(23):6880. https://doi.org/10.3390/jcm11236880
Chicago/Turabian StyleWestphal, Dominik S., Michael Hauser, Britt-Maria Beckmann, Cordula M. Wolf, Gabriele Hessling, Renate Oberhoffer-Fritz, and Annette Wacker-Gussmann. 2022. "Fetal Bradycardia Caused by Monogenic Disorders—A Review of the Literature" Journal of Clinical Medicine 11, no. 23: 6880. https://doi.org/10.3390/jcm11236880
APA StyleWestphal, D. S., Hauser, M., Beckmann, B.-M., Wolf, C. M., Hessling, G., Oberhoffer-Fritz, R., & Wacker-Gussmann, A. (2022). Fetal Bradycardia Caused by Monogenic Disorders—A Review of the Literature. Journal of Clinical Medicine, 11(23), 6880. https://doi.org/10.3390/jcm11236880






