Nuclear Medicine and Radiological Imaging of Pancreatic Neuroendocrine Neoplasms: A Multidisciplinary Update
Abstract
1. Introduction
2. Conventional Nuclear Medicine Radiopharmaceuticals
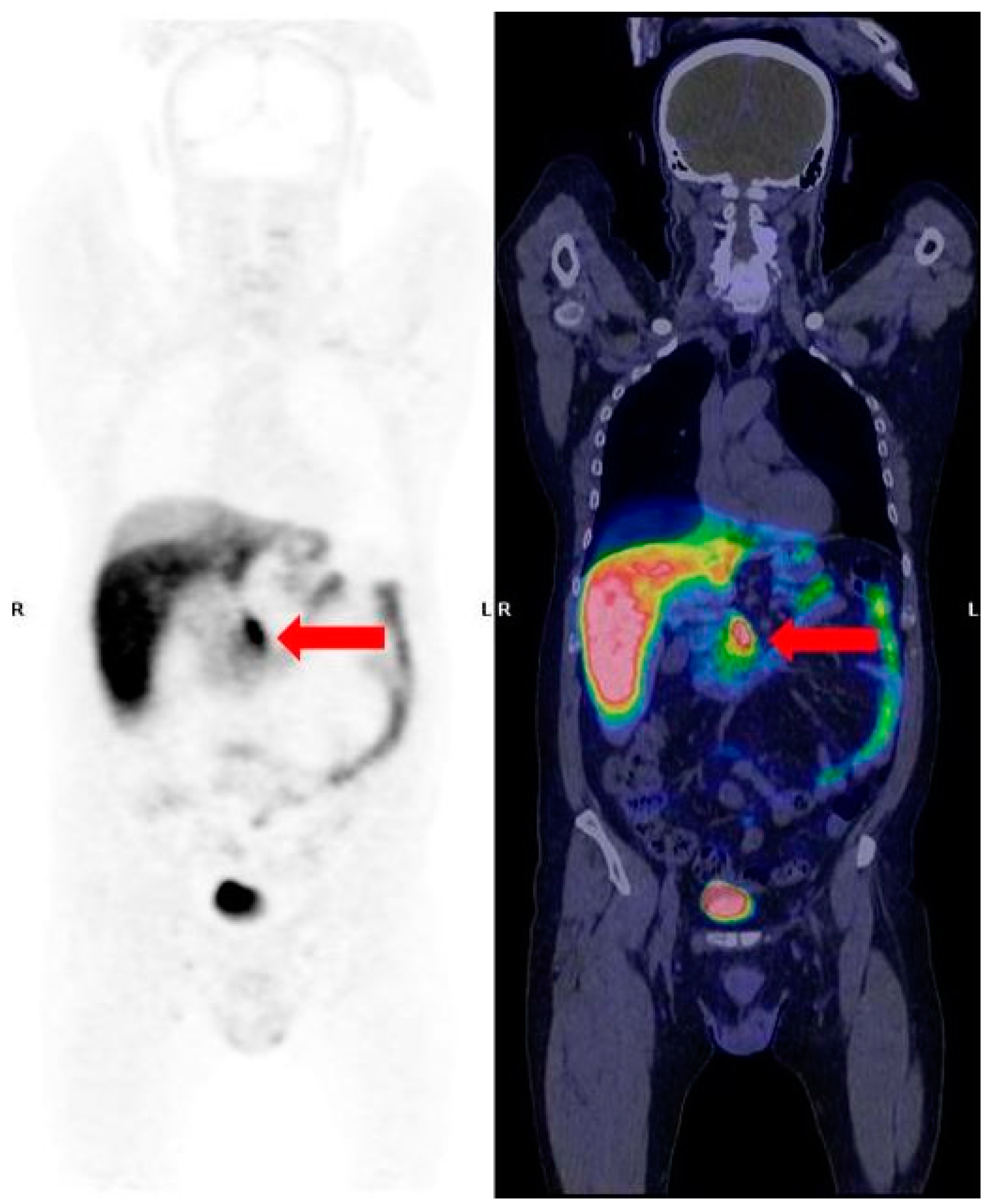
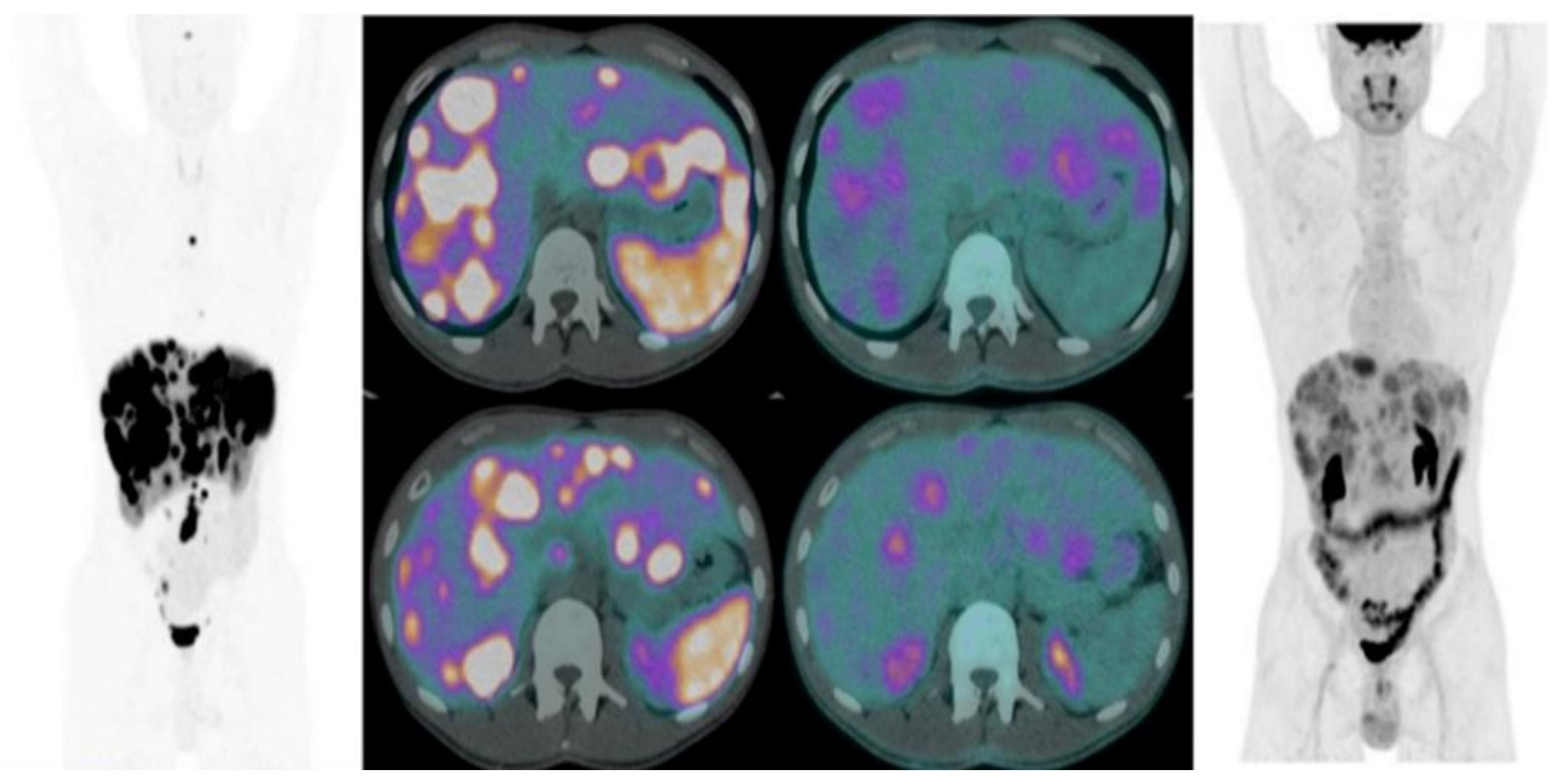
2.1. 68Ga-SSA PET/CT
2.2. 18F-FDG PET/CT
2.3. 68Ga-Exendin-4
2.4. 18F-DOPA
2.5. New Radiopharmaceuticals
3. Conventional Radiological Imaging
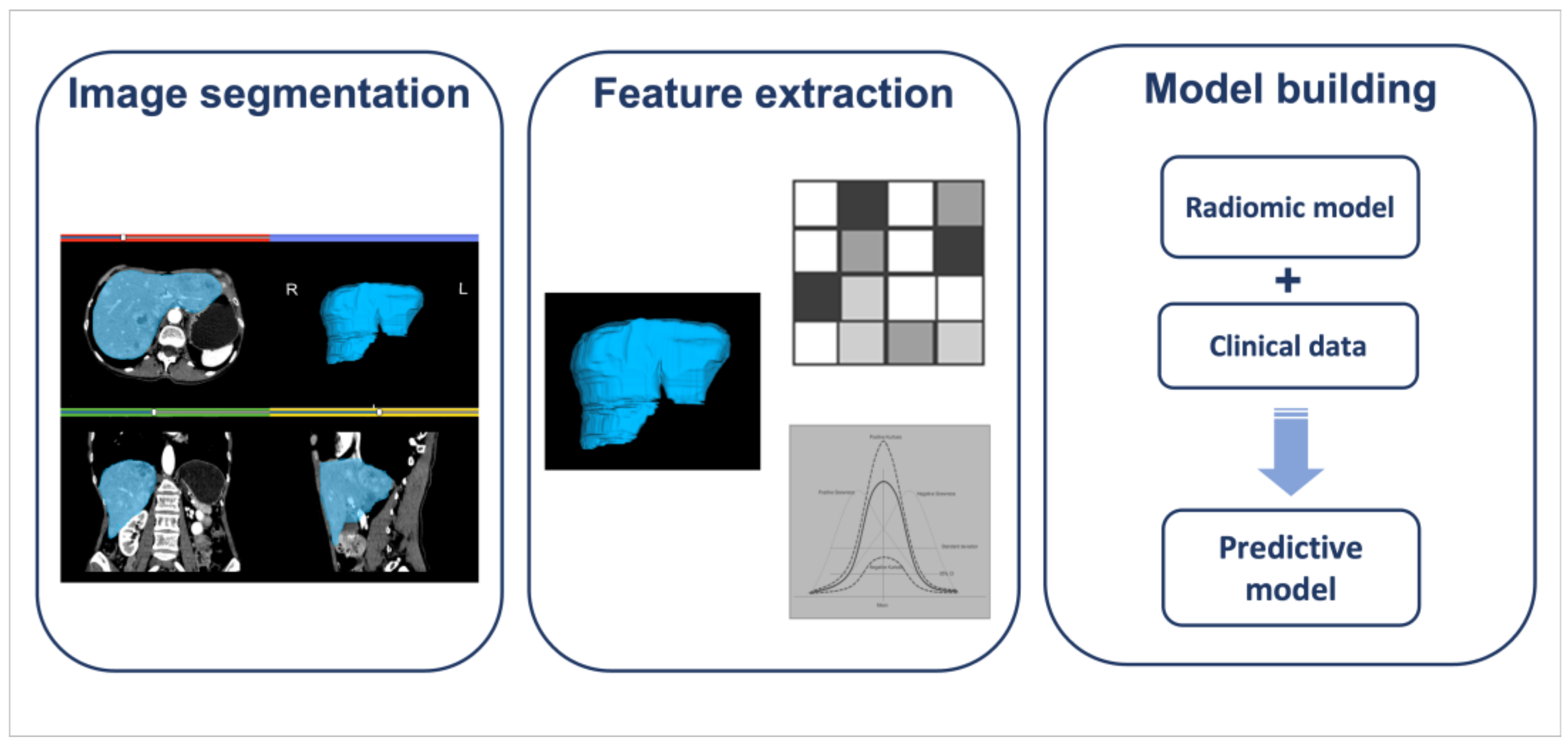
4. Radiomics
Radiomics and Nuclear Medicine
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Merola, E.; Pavel, M.E.; Rinke, A.; Kump, P.; Partelli, S.; Rinzivillo, M.; Rodriguez-Laval, V.; Pape, U.F.; Lipp, R.; et al. Stage IV Gastro-Entero-Pancreatic Neuroendocrine Neoplasms: A Risk Score to Predict Clinical Outcome. Oncologist 2017, 22, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, D.S.; Klöppel, G.; La Rosa, S.; Rindi, G. Classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours, 5th ed.; Digestive System Tumours; IARC: Lyon, France, 2019; pp. 16–19. [Google Scholar]
- Panzuto, F.; Pusceddu, S.; Faggiano, A.; Rinzivillo, M.; Brighi, N.; Prinzi, N.; Riccardi, F.; Iannicelli, E.; Maggio, I.; Femia, D.; et al. Prognostic impact of tumour burden in stage IV neuroendocrine neoplasia: A comparison between pancreatic and gastrointestinal localizations. Pancreatology 2019, 19, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.C.R.; Aalbersberg, E.A.; van der Velden, D.; Wilthagen, E.A.; Tesselaar, M.E.T.; Beets-Tan, R.G.H.; Maas, M. GEP-NET radiomics: A systematic review and radiomics quality score assessment. Eur. Radiol. 2022, 32, 7278–7294. [Google Scholar] [CrossRef] [PubMed]
- Reisine, T.; Bell, G.I. Molecular biology of somatostatin receptors. Endocr. Rev. 1995, 16, 427–442. [Google Scholar]
- Csaba, Z.; Dournaud, P. Cellular biology of somatostatin receptors. Neuropeptides 2001, 35, 1–23. [Google Scholar] [CrossRef]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur. J. Nucl. Med. 2001, 28, 836–846. [Google Scholar] [CrossRef]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in somatostatin research: Biological, chemical and therapeutic aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef]
- Krenning, E.P.; Bakker, W.H.; Breeman, W.A.; Koper, J.W.; Kooij, P.P.; Ausema, L.; Lameris, J.S.; Reubi, J.C.; Lamberts, S.W. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, 1, 242–244. [Google Scholar] [CrossRef]
- Heiman, M.L.; Murphy, W.A.; Coy, D.H. Differential binding of somatostatin agonists to somatostatin receptors in brain and adenohypophysis. Neuroendocrinology 1987, 45, 429–436. [Google Scholar] [CrossRef]
- Mikołajczak, R.; Maecke, H.R. Radiopharmaceuticals for somatostatin receptor imaging. Nucl. Med. Rev. Cent. East. Eur. 2016, 19, 126–132. [Google Scholar] [CrossRef]
- Virgolini, I.; Ambrosini, V.; Bomanji, J.B.; Baum, R.P.; Fanti, S.; Gabriel, M.; Papathanasiou, N.D.; Pepe, G.; Oyen, W.; De Cristoforo, C.; et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2004–2010. [Google Scholar] [CrossRef]
- Hofmann, M.; Maecke, H.; Börner, R.; Weckesser, E.; Schöffski, P.; Oei, L.; Schumacher, J.; Henze, M.; Heppeler, A.; Meyer, J.; et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: Preliminary data. Eur. J. Nucl. Med. 2001, 28, 1751–1757. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lau, W.F.; Hicks, R.J. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: Clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics 2015, 35, 500–516. [Google Scholar] [CrossRef]
- Wild, D.; Mäcke, H.R.; Waser, B.; Reubi, J.C.; Ginj, M.; Rasch, H.; Müller-Brand, J.; Hofmann, M. 68Ga-DOTANOC: A first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 724. [Google Scholar] [CrossRef]
- Poeppel, T.D.; Binse, I.; Petersenn, S.; Lahner, H.; Schott, M.; Antoch, G.; Brandau, W.; Bockisch, A.; Boy, C. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J. Nucl. Med. 2011, 52, 1864–1870. [Google Scholar] [CrossRef]
- Bodei, L.; Ambrosini, V.; Herrmann, K.; Modlin, I. Current Concepts in (68)Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J. Nucl. Med. 2017, 58, 1718–1726. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R.; Giovinazzo, F.; Galiandro, F.; Annunziata, S.; Muoio, B.; Kroiss, A.S. PET with Different Radiopharmaceuticals in Neuroendocrine Neoplasms: An Umbrella Review of Published Meta-Analyses. Cancers 2021, 13, 5172. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.M.; Gu, X.; Ginader, T.; Breheny, P.; Sunderland, J.J. (68)Ga-DOTATOC Imaging of Neuroendocrine Tumors: A Systematic Review and Metaanalysis. J. Nucl. Med. 2017, 58, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovacs, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Putzer, D.; Gabriel, M.; Henninger, B.; Kendler, D.; Uprimny, C.; Dobrozemsky, G.; Decristoforo, C.; Bale, R.J.; Jaschke, W.; Virgolini, I.J. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J. Nucl. Med. 2009, 50, 1214–1221. [Google Scholar] [CrossRef]
- Sundin, A.; Arnold, R.; Baudin, E.; Cwikla, J.B.; Eriksson, B.; Fanti, S.; Fazio, N.; Giammarile, F.; Hicks, R.J.; Kjaer, A.; et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Radiological, nuclear medicine and hybrid imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar] [CrossRef]
- Sharma, P.; Arora, S.; Dhull, V.S.; Naswa, N.; Kumar, R.; Ammini, A.C.; Bal, C. Evaluation of (68)Ga-DOTANOC PET/CT imaging in a large exclusive population of pancreatic neuroendocrine tumors. Abdom. Imaging 2015, 40, 299–309. [Google Scholar] [CrossRef]
- Rufini, V.; Baum, R.P.; Castaldi, P.; Treglia, G.; De Gaetano, A.M.; Carreras, C.; Kaemmerer, D.; Hommann, M.; Hörsch, D.; Bonomo, L.; et al. Role of PET/CT in the functional imaging of endocrine pancreatic tumors. Abdom. Imaging 2012, 37, 1004–1020. [Google Scholar] [CrossRef]
- Wild, D.; Bomanji, J.B.; Benkert, P.; Maecke, H.; Ell, P.J.; Reubi, J.C.; Caplin, M.E. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J. Nucl. Med. 2013, 54, 364–372. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Bodei, L.; Nanni, C.; Castellucci, P.; Allegri, V.; Montini, G.C.; Tomassetti, P.; Paganelli, G.; Fanti, S. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J. Nucl. Med. 2010, 51, 669–673. [Google Scholar] [CrossRef]
- Naji, M.; Al-Nahhas, A. ⁶⁸Ga-labelled peptides in the management of neuroectodermal tumours. Eur. J. Nucl. Med. Mol. Imaging 2012, 39 (Suppl. S1), S61–S67. [Google Scholar] [CrossRef]
- Sharma, P.; Arora, S.; Karunanithi, S.; Khadgawat, R.; Durgapal, P.; Sharma, R.; Kandasamy, D.; Bal, C.; Kumar, R. Somatostatin receptor based PET/CT imaging with 68Ga-DOTA-Nal3-octreotide for localization of clinically and biochemically suspected insulinoma. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 69–76. [Google Scholar]
- Christ, E.; Antwi, K.; Fani, M.; Wild, D. Innovative imaging of insulinoma: The end of sampling? A review. Endocr. Relat. Cancer 2020, 27, R79–R92. [Google Scholar] [CrossRef]
- Prasad, V.; Sainz-Esteban, A.; Arsenic, R.; Plöckinger, U.; Denecke, T.; Pape, U.F.; Pascher, A.; Kühnen, P.; Pavel, M.; Blankenstein, O. Role of (68)Ga somatostatin receptor PET/CT in the detection of endogenous hyperinsulinaemic focus: An explorative study. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1593–1600. [Google Scholar] [CrossRef]
- Wild, D.; Christ, E.; Caplin, M.E.; Kurzawinski, T.R.; Forrer, F.; Brändle, M.; Seufert, J.; Weber, W.A.; Bomanji, J.; Perren, A.; et al. Glucagon-like peptide-1 versus somatostatin receptor targeting reveals 2 distinct forms of malignant insulinomas. J. Nucl. Med. 2011, 52, 1073–1078. [Google Scholar] [CrossRef]
- Veltroni, A.; Cosaro, E.; Spada, F.; Fazio, N.; Faggiano, A.; Colao, A.; Pusceddu, S.; Zatelli, M.C.; Campana, D.; Piovesan, A.; et al. Clinico-pathological features, treatments and survival of malignant insulinomas: A multicenter study. Eur. J. Endocrinol. 2020, 182, 439–446. [Google Scholar] [CrossRef]
- Pattison, D.A.; Hicks, R.J. Molecular imaging in the investigation of hypoglycaemic syndromes and their management. Endocr. Relat. Cancer 2017, 24, R203–R221. [Google Scholar] [CrossRef]
- Malan, N.; Vangu, M.-D.-T. Normal Variants, Pitfalls and Artifacts in Ga-68 DOTATATE PET/CT Imaging. Front. Nucl. Med. 2022, 825486. [Google Scholar] [CrossRef]
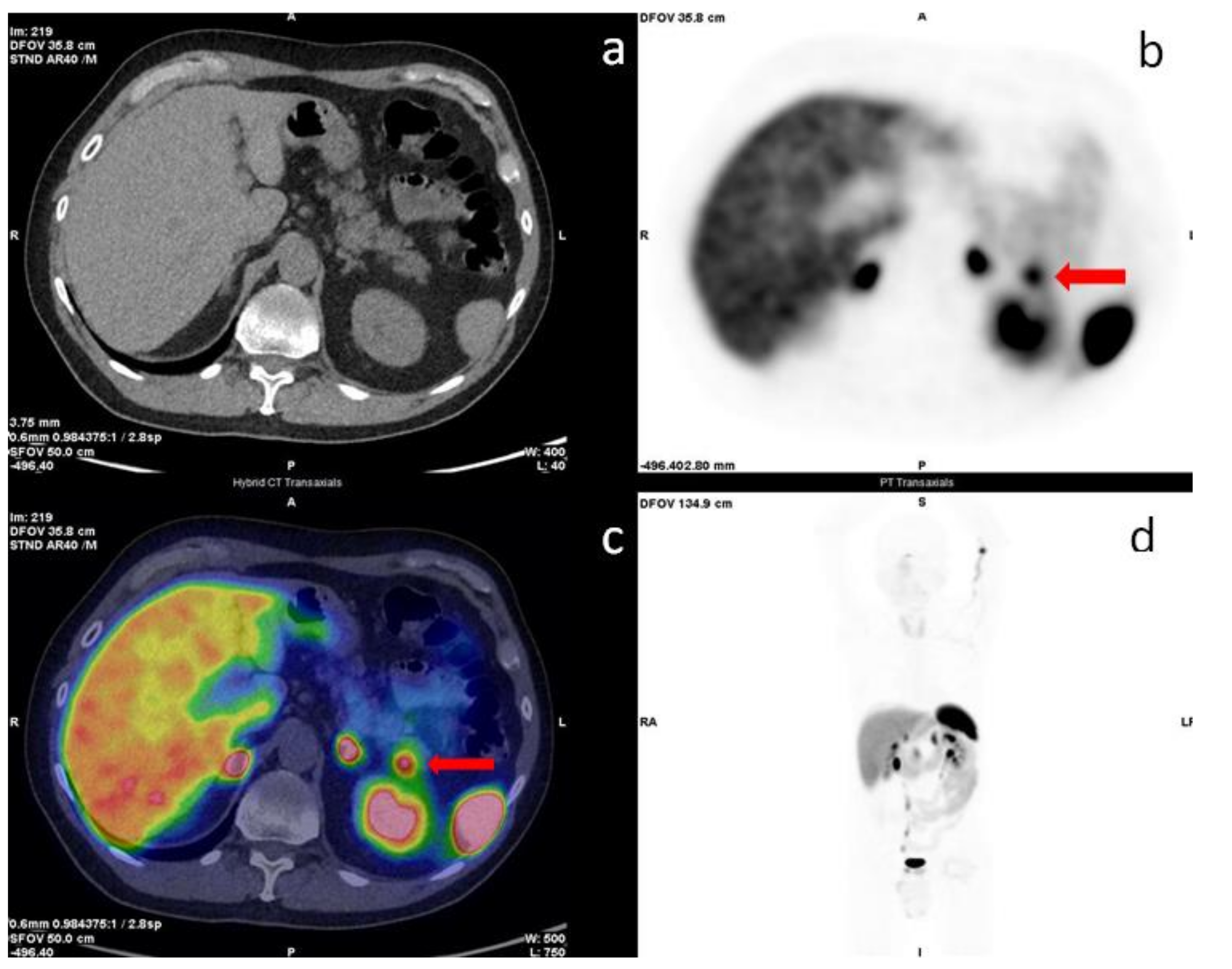
- Castellucci, P.; Pou Ucha, J.; Fuccio, C.; Rubello, D.; Ambrosini, V.; Montini, G.C.; Pettinato, V.; Malizia, C.; Lodi, F.; Fanti, S. Incidence of increased 68Ga-DOTANOC uptake in the pancreatic head in a large series of extrapancreatic NET patients studied with sequential PET/CT. J. Nucl. Med. 2011, 52, 886–890. [Google Scholar] [CrossRef]
- Krausz, Y.; Rubinstein, R.; Appelbaum, L.; Mishani, E.; Orevi, M.; Fraenkel, M.; Tshori, S.; Glaser, B.; Bocher, M.; Salmon, A.; et al. Ga-68 DOTA-NOC uptake in the pancreas: Pathological and physiological patterns. Clin. Nucl. Med. 2012, 37, 57–62. [Google Scholar] [CrossRef]
- Tabacchi, E.; Fortunati, E.; Argalia, G.; Zanoni, L.; Calabrò, D.; Telo, S.; Campana, D.; Lamberti, G.; Ricci, C.; Casadei, R.; et al. [68Ga]Ga-DOTANOC Uptake at Pancreatic Head/Uncinate Process: Is It a Persistent Diagnostic Pitfall Over Time? Cancers 2022, 14, 3541. [Google Scholar] [CrossRef] [PubMed]
- Halpert, B.; Gyorkey, F. Lesions observed in accessory spleens of 311 patients. Am. J. Clin. Pathol. 1959, 32, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, F.; Sacco, L.; Cerasari, S.; Bellato, V.; Cicconi, S.; Ciardi, A.; Muttillo, E.M.; Feola, T.; Caronna, R.; Chirletti, P. Intrapancreatic accessory spleen false positive to 68Ga-Dotatoc: Case report and literature review. World J. Surg. Oncol. 2019, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Bostancı, E.B.; Oter, V.; Okten, S.; Küçük, N.O.; Soydal, C.; Turhan, N.; Akoglu, M. Intra-pancreatic Accessory Spleen Mimicking Pancreatic Neuroendocrine Tumor on 68-Ga-Dotatate PET/CT. Arch. Iran. Med. 2016, 19, 816–819. [Google Scholar] [PubMed]
- Kawamoto, S.; Johnson, P.T.; Hall, H.; Cameron, J.L.; Hruban, R.H.; Fishman, E.K. Intrapancreatic accessory spleen: CT appearance and differential diagnosis. Abdom. Imaging 2012, 37, 812–827. [Google Scholar] [CrossRef]
- Al-Ibraheem, A.; Bundschuh, R.A.; Notni, J.; Buck, A.; Winter, A.; Wester, H.J.; Schwaiger, M.; Scheidhauer, K. Focal uptake of 68Ga-DOTATOC in the pancreas: Pathological or physiological correlate in patients with neuroendocrine tumours? Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2005–2013. [Google Scholar] [CrossRef]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Krenning, E.P.; Valkema, R.; Kwekkeboom, D.J.; de Herder, W.W.; van Eijck, C.H.; de Jong, M.; Reubi, J.C. Molecular imaging as in vivo molecular pathology for gastro- enteropancreatic neuroendocrine tumors: Implications for follow-up after therapy. J. Nucl. Med. 2005, 46, 76S–82S. [Google Scholar]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef]
- Abdulrezzak, U.; Kurt, Y.K.; Kula, M.; Tutus, A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT in the basis of volumetric parameters in neuroendocrine tumors. Nucl. Med. Commun. 2016, 37, 874–881. [Google Scholar] [CrossRef]
- Rinzivillo, M.; Partelli, S.; Prosperi, D.; Capurso, G.; Pizzichini, P.; Iannicelli, E.; Merola, E.; Muffatti, F.; Scopinaro, F.; Schillaci, O.; et al. Clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography in the diagnostic algorithm of advanced entero-pancreatic neuroendocrine neoplasms. Oncologist 2018, 23, 186–192. [Google Scholar] [CrossRef]
- Ijichi, H.; Shirabe, K.; Taketomi, A.; Yoshizumi, T.; Ikegami, T.; Mano, Y.; Aishima, S.; Abe, K.; Honda, H.; Maehara, Y. Clinical usefulness of 18 F-fluorodeoxyglucose positron emission tomography/computed tomography for patients with primary liver cancer with special reference to rare histological types, hepatocellular carcinoma with sarcomatous change and combined hepatocellular and cholangiocarcinoma. Hepatol. Res. 2013, 43, 481–487. [Google Scholar]
- Magi, L.; Prosperi, D.; Lamberti, G.; Marasco, M.; Ambrosini, V.; Rinzivillo, M.; Campana, D.; Gentiloni, G.; Annibale, B.; Signore, A.; et al. Role of 18-F -FDG PET/CT in the managment of G1 gastenteropancreatic neuroendocrine tumors. Endocrine 2022, 76, 484–490. [Google Scholar] [CrossRef]
- Cingarlini, S.; Ortolani, S.; Salgarello, M.; Butturini, G.; Malpaga, A.; Malfatti, V.; D’Onofrio, M.; Davì, M.V.; Vallerio, P.; Ruzzenente, A.; et al. Role of combined 68Ga-DOTA-TOC and 18F-FDG positron emission tomography/computed tomography in the diagnostic workup of pancreas neuroendocrine tumors. Pancreas 2017, 46, 42–47. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Tebaldi, S.; Zuffanter, M.; Salgarello, M.; Cingarlini, S.; D’onofrio, M.; Parisi, A.; Deiro, G.; Manfrin, E.; et al. Dual tracer (68Ga-DOTATOC and 18F-FDG) PET/CT scan and G1–G2 nonfunctioning pancreatic neuroendocrine tumors: A single center retrospective evaluation of 124 nonmetastatic resected cases. Neuroendocrinology 2022, 112, 143–152. [Google Scholar] [CrossRef]
- You, H.; Kandathil, A.; Beg, M.; De Blanche, L.; Kazmi, S.; Subramaniam, R.M. Ga-68-DOTATATE PET/CT and F-18 FDG PET/CT in the evaluation of lowand intermediate versus high-grade neuroendocrine tumors. Nucl. Med. Commun. 2020, 41, 1060–1065. [Google Scholar] [CrossRef]
- Evangelista, L.; Ravelli, I.; Bignotto, A.; Cecchin, D.; Zucchetta, P. Ga-68 DOTA-peptides and F-18 FDG PET/CT in patients with neuroendocrine tumor: A review. Clin. Imaging 2020, 67, 113–116. [Google Scholar] [CrossRef]
- Carideo, L.; Prosperi, D.; Panzuto, F.; Magi, L.; Pratesi, M.S.; Rinzivillo, M.; Annibale, B.; Signore, A. Role of Combined [68Ga] DOTA-SST Analogues and [18F] FDG PET/CT in the Management of GEP-NENs: A Systematic Review. J. Clin. Med. 2019, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.L.; Pavlakis, N.; Schembri, G.P.; Bernard, E.J.; Hsiao, E.; Hayes, A.; Bailey, D.L.; Pavlakis, N.; Schembri, G.P. Dual somatostatin receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: Proposal for a novel grading scheme with prognostic significance. Theranostics 2017, 7, 1149. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Ranade, R.; Ostwal, V.; Shrikhande, S.V.; Goel, M.; Basu, S. Performance of 177Lu-DOTA-TATE-based peptide receptor radionuclide therapy in metastatic gastroenteropencreatic neuroendocrine tumor: A multiparametric response evaluation correlating with primary tumor site- tumor proliferation index and dual tracer imaging characteristics. Nucl. Med. Commun. 2016, 37, 1030–1037. [Google Scholar] [PubMed]
- Zhang, J.; Liu, Q.; Singh, A.; Schuchardt, C.; Kulkarni, H.R.; Baum, R.P. Prognostic value of 18F-FDG PET/CT in a large cohort of patients with advanced metastatic neuroendocrine neoplasms treated with peptide receptor radionuclide therapy. J. Nucl. Med. 2020, 61, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Sansovini, M.; Severi, S.; Ianniello, A.; Nicolini, S.; Fantini, L.; Mezzenga, E.; Paganelli, G.; Severi, S.; Ianniello, A. Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-D OTATATE. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 490–499. [Google Scholar] [CrossRef]
- Alevroudis, E.; Spei, M.E.; Chatziioannou, S.N.; Tsoli, M.; Wallin, G.; Kaltsas, G.; Daskalakis, K. Clinical Utility of 18F-FDG PET in Neuroendocrine Tumors Prior to Peptide Receptor Radionuclide Therapy: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 1813. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Yao, J. Consensus on Molecular Imaging and Theranostics in Neuroendocrine Neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Christ, E.; Wild, D.; Ederer, S.; Béhé, M.; Nicolas, G.; Caplin, M.E.; Brändle, M.; Clerici, T.; Fischli, S.; Stettler, C.; et al. Glucagon-like peptide-1 receptor imaging for the localisation of insulinomas: A prospective multicentre imaging study. Lancet Diabetes Endocrinol. 2013, 1, 115–122. [Google Scholar] [CrossRef]
- Parihar, A.S.; Vadi, S.K.; Kumar, R.; Mittal, B.R.; Singh, H.; Bal, A.; Walia, R.; Shukla, J.; Sinha, S.K. 68Ga DOTA-Exendin PET/CT for Detection of Insulinoma in a Patient With Persistent Hyperinsulinemic Hypoglycemia. Clin. Nucl. Med. 2018, 43, e285–e286. [Google Scholar] [CrossRef]
- Cuthbertson, D.J.; Banks, M.; Khoo, B.; Antwi, K.; Christ, E.; Campbell, F.; Raraty, M.; Wild, D. Application of Ga(68)-DOTA-exendin-4 PET/CT to localize an occult insulinoma. Clin. Endocrinol. 2016, 84, 789–791. [Google Scholar] [CrossRef]
- Antwi, K.; Hepprich, M.; Müller, N.A.; Reubi, J.C.; Fani, M.; Rottenburger, C.; Nicolas, G.; Kaul, F.; Christ, E.R.; Wild, D. Pitfalls in the Detection of InsulinomasWith Glucagon-Like Peptide-1 Receptor Imaging. Clin. Nucl. Med. 2020, 45, e386–e392. [Google Scholar] [CrossRef]
- Bongetti, E.; Lee, M.H.; Pattison, D.A.; Hicks, R.J.; Norris, R.; Sachithanandan, N.; MacIsaac, R.J. Diagnostic challenges in a patient with an occult insulinoma: 68 Ga-DOTA-exendin-4 PET/CT and 68Ga-DOTATATE PET/CT. Clin. Case Rep. 2018, 6, 719–722. [Google Scholar] [CrossRef]
- Velikyan, I.; Bulenga, T.N.; Selvaraju, R.; Lubberink, M.; Espes, D.; Rosenström, U.; Eriksson, O. Dosimetry of [(177)Lu]-DO3A-VS-Cys(40)-Exendin-4—Impact on the feasibility of insulinoma internal radiotherapy. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 109–126. [Google Scholar]
- Jansen, T.J.P.; van Lith, S.A.M.; Boss, M.; Brom, M.; Joosten, L.; Béhé, M.; Buitinga, M.; Gotthardt, M. Exendin–4 analogs in insulinoma theranostics. J. Label. Compd. Radiopharm. 2019, 62, 656–672. [Google Scholar] [CrossRef]
- Montravers, F.; Grahek, D.; Kerrou, K.; Ruszniewski, P.; de Beco, V.; Aide, N.; Gutman, F.; Grangé, J.; Lotz, J.; Talbot, J.N. Can fluorodihydroxyphenylalanine PET replace somatostatin receptor scintigraphy in patients with digestive endocrine tumors? J. Nucl. Med. 2006, 47, 1455–1462. [Google Scholar]
- Ambrosini, V.; Tomassetti, P.; Castellucci, P.; Campana, D.; Montini, G.; Rubello, D.; Nanni, C.; Rizzello, A.; Franchi, R.; Fanti, S. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1431–1438. [Google Scholar] [CrossRef]
- Piccardo, A.; Fiz, F.; Bottoni, G.; Ugolini, M.; Noordzij, W.; Trimboli, P. Head-to-Head comparison between 18 F-DOPA PET/CT and 68 Ga- DOTA peptides PET/CT in detecting intestinal neuroendocrine tumours: A systematic review and meta-analysis. Clin. Endocrinol. 2021, 95, 595–605. [Google Scholar] [CrossRef]
- Santhanam, P.; Taieb, D. Role of (18) F-FDOPA PET/CT imaging in endocrinology. Clin. Endocrinol. 2014, 81, 789–798. [Google Scholar] [CrossRef]
- Somme, F.; Montaz-Rosset, M.; Averous, G.; Deur, J.; Goichot, B.; Bachellier, P.; Addeo, P.; Imperiale, A. Solid pseudopapillary tumour should be part of differential diagnosis of focal pancreatic lesions with increased 18F-FDOPA uptake. Clin. Endocrinol. 2020, 93, 78–81. [Google Scholar] [CrossRef]
- Koopmans, K.P.; de Vries, E.G.; Kema, I.P.; Elsinga, P.H.; Neels, O.C.; Sluiter, W.J.; van der Horst-Schrivers, A.N.; Jager, P.L. Staging of carcinoid tumours with 18F-DOPA PET: A prospective, diagnostic accuracy study. Lancet Oncol. 2006, 7, 728–734. [Google Scholar] [CrossRef]
- Helali, M.; Addeo, P.; Heimburger, C.; Detour, J.; Goichot, B.; Bachellier, P.; Namer, I.J.; Taïeb, D.; Imperiale, A. Carbidopa-assisted 18F- fluorodihydroxyphenylalanine PET/CT for the localization and staging of non-functioning neuroendocrine pancreatic tumors. Ann. Nucl. Med. 2016, 30, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Veenstra, E.B.; Brouwers, A.H.; de Groot, D.J.A.; Hofland, J.; Walenkamp, A.M.E.; Brabnder, T.; Zandee, W.T.; Noordzij, W. Comparison of [18F]DOPA and [68Ga]DOTA-TOC as a PET imaging tracer before peptide receptor radionuclide therapy. Eur. J. Hybrid Imaging 2022, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Rottenburger, C.; Nicolas, G.; Wild, D. Gastroenteropancreatic neuroendocrine tumours (GEP-NET) Imaging and staging. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Fani, M.; Braun, F.; Waser, B.; Beetschen, K.; Cescato, R.; Erchegyi, J.; Rivier, J.E.; Weber, W.A.; Maecke, H.R.; Reubi, J.C. Unexpected sensitivity of sst2 antagonists to N-terminal radiometal modifications. J. Nucl. Med. 2012, 53, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, G.P.; Beykan, S.; Bouterfa, H.; Kaufmann, J.; Bauman, A.; Lassmann, M.; Reubi, J.C.; Rivier, J.E.F.; Maecke, H.R.; Fani, M.; et al. Safety, Biodistribution, and Radiation Dosimetry of 68Ga-OPS202 in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase I Imaging Study. J. Nucl. Med. 2018, 59, 909–914. [Google Scholar] [CrossRef]
- Nicolas, G.P.; Schreiter, N.; Kaul, F.; Uiters, J.; Bouterfa, H.; Kaufmann, J.; Erlanger, T.E.; Cathomas, R.; Christ, E.; Fani, M.; et al. Sensitivity Comparison of 68Ga-OPS202 and 68Ga-DOTATOC PET/CT in Patients with Gastroenteropancreatic Neuroendocrine Tumors: A Prospective Phase II Imaging Study. J. Nucl. Med. 2018, 59, 915–921. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, Y.; Wang, X.; Yao, S.; Bai, C.; Zhao, H.; Jia, R.; Xu, J.; Huo, L. Head-to-Head Comparison of 68Ga-DOTA-JR11 and 68Ga-DOTATATE PET/CT in Patients with Metastatic, Well-Differentiated Neuroendocrine Tumors: A Prospective Study. J. Nucl. Med. 2020, 61, 897–903. [Google Scholar] [CrossRef]
- Krebs, S.; Pandit-Taskar, N.; Reidy, D.; Beattie, B.J.; Lyashchenko, S.K.; Lewis, J.S.; Bodei, L.; Weber, W.A.; O’Donoghue, J.A. Biodistribution and radiation dose estimates for 68Ga-DOTA-JR11 in patients with metastatic neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 677–685. [Google Scholar] [CrossRef]
- Bodei, L.; Weber, W.A. Somatostatin Receptor Imaging of Neuroendocrine Tumors: From Agonists to Antagonists. J. Nucl. Med. 2018, 59, 907–908. [Google Scholar] [CrossRef]
- Reidy-Lagunes, D.; Pandit-Taskar, N.; O’Donoghue, J.A.; Krebs, S.; Staton, K.D.; Lyashchenko, S.K.; Lewis, J.S.; Raj, N.; Gönen, M.; Lohrmann, C.; et al. Phase I Trial of Well-Differentiated Neuroendocrine Tumors (NETs) with Radiolabeled Somatostatin Antagonist 177Lu-Satoreotide Tetraxetan. Clin. Cancer Res. 2019, 25, 6939–6947. [Google Scholar] [CrossRef]
- Ilhan, H.; Lindner, S.; Todica, A.; Cyran, C.C.; Tiling, R.; Auernhammer, C.J.; Spitzweg, C.; Boeck, S.; Unterrainer, M.; Gildehaus, F.J.; et al. Biodistribution and first clinical results of 18F-SiFAlin-TATE PET: A novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 870–880. [Google Scholar] [CrossRef]
- Elin, P.; Frederik, C.; Térence, T.; Koole, M.; Kim, S.; Jeroen, D.; Van Cutsem, E.; Verslype, C.; Van Laere, K.; Bormans, G.; et al. [18 F] AlF-NOTA-octreotide PET imaging: Biodistribution, dosimetry and first comparison with [68Ga] Ga-DOTATATE in neuroendocrine tumour patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3033–3046. [Google Scholar]
- Chiti, G.; Grazzini, G.; Cozzi, D.; Danti, G.; Matteuzzi, B.; Granata, V.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Imaging of pancreatic neuroendocrine neoplasms. Int. J. Environ. Res. Public Health 2021, 18, 8895. [Google Scholar] [CrossRef]
- Tamm, E.P.; Bhosale, P.; Lee, J.H.; Rohren, E.M. State-of-the-art imaging of pancreatic neuroendocrine tumors. Surg. Oncol. Clin. 2016, 25, 375–400. [Google Scholar] [CrossRef]
- Manta, R.; Nardi, E.; Pagano, N.; Ricci, C.; Sica, M.; Castellani, D.; Bertani, H.; Piccoli, M.; Mullineris, B.; Tringali, A.; et al. Pre-operative diagnosis of pancreatic neuroendocrine tumors with endoscopic ultrasonography and computed tomography in a large series. J. Gastrointest. Liver Dis. 2016, 25, 317–321. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Okamura, Y.; Uemura, S.; Sugiura, T.; Ito, T.; Ashida, R.; Kato, Y.; Ohgi, K.; Yamada, M.; Sasaki, K.; et al. Vascularity and tumor size are significant predictors for recurrence after resection of a pancreatic neuroendocrine tumor. Ann. Surg. Oncol. 2017, 24, 2363–2370. [Google Scholar] [CrossRef]
- Kim, J.H.; Eun, H.W.; Kim, Y.J.; Lee, J.M.; Han, J.K.; Choi, B.I. Pancreatic neuroendocrine tumour (PNET): Staging accuracy of MDCT and its diagnostic performance for the differentiation of PNET with uncommon CT findings from pancreatic adenocarcinoma. Eur. Radiol. 2016, 26, 1338–1347. [Google Scholar] [CrossRef]
- Dromain, C.; de Baere, T.; Lumbroso, J.; Caillet, H.; Laplanche, A.; Boige, V.; Ducreux, M.; Duvillard, P.; Elias, D.; Schlumberger, M.; et al. Detection of liver metastases from endocrine tumors: A prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J. Clin. Oncol. 2005, 23, 70–78. [Google Scholar] [CrossRef]
- Hayoz, R.; Vietti-Violi, N.; Duran, R.; Knebel, J.F.; Ledoux, J.B.; Dromain, C. The combination of hepatobiliary phase with Gd-EOB-DTPA and DWI is highly accurate for the detection and characterization of liver metastases from neuroendocrine tumor. Eur. Radiol. 2020, 30, 6593–6602. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Jagannathan, J.P.; Braschi-Amirfarzan, M.; Qin, L.; Balthazar, P.; Ramaiya, N.H.; Shinagare, A.B. Value of hepatocellular phase imaging after intravenous gadoxetate disodium for assessing hepatic metastases from gastroenteropancreatic neuroendocrine tumors: Comparison with other MRI pulse sequences and with extracellular agent. Abdom. Radiol. 2018, 43, 2329–2339. [Google Scholar] [CrossRef]
- Canellas, R.; Lo, G.; Bhowmik, S.; Ferrone, C.; Sahani, D. Pancreatic neuroendocrine tumor: Correlations between MRI features, tumor biology, and clinical outcome after surgery. J. Magn. Reson. Imaging 2018, 47, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Landolfi, F.; Nicolai, M.; Lucertini, E.; Tarallo, M.; et al. Radiomics in oncology, part 1: Technical principles and gastrointestinal application in CT and MRI. Cancers 2021, 13, 2522. [Google Scholar] [CrossRef] [PubMed]
- Bodalal, Z.; Trebeschi, S.; Nguyen-Kim, T.D.; Schats, W.; Beets-Tan, R. Radiogenomics: Bridging imaging and genomics. Abdom. Radiol. 2019, 44, 1960–1984. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Polici, M.; Zerunian, M.; Pucciarelli, F.; Guido, G.; Polidori, T.; Landolfi, F.; Nicolai, M.; Lucertini, E.; Tarallo, M.; et al. Radiomics in oncology, part 2: Thoracic, genito-urinary, breast, neurological, hematologic and musculoskeletal applications. Cancers 2021, 13, 2681. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhuge, X.; Wang, Z.; Wang, Q.; Sun, K.; Feng, Z.; Chen, X. Textural analysis on contrast-enhanced CT in pancreatic neuroendocrine neoplasms: Association with WHO grade. Abdom. Radiol. 2019, 44, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zhao, Z.; Jiang, H.; Fang, X.; Li, J.; Cao, K.; Ma, C.; Guo, S.; Wang, L.; Jin, G.; et al. Noncontrast radiomics approach for predicting grades of nonfunctional pancreatic neuroendocrine tumors. J. Magn. Reson. Imaging 2020, 52, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Jiang, H.; Ma, C.; Wang, L.; Zheng, J.; Jin, G.; Lu, J. CT-based radiomics score for distinguishing between grade 1 and grade 2 nonfunctioning pancreatic neuroendocrine tumors. Am. J. Roentgenol. 2020, 215, 852–863. [Google Scholar] [CrossRef]
- Gu, D.; Hu, Y.; Ding, H.; Wei, J.; Chen, K.; Liu, H.; Zeng, M.; Tian, J. CT radiomics may predict the grade of pancreatic neuroendocrine tumors: A multicenter study. Eur. Radiol. 2019, 29, 6880–6890. [Google Scholar] [CrossRef]
- He, M.; Liu, Z.; Lin, Y.; Wan, J.; Li, J.; Xu, K.; Wang, Y.; Jin, Z.; Tian, J.; Xue, H. Differentiation of atypical non-functional pancreatic neuroendocrine tumor and pancreatic ductal adenocarcinoma using CT based radiomics. Eur. J. Radiol. 2019, 117, 102–111. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef]
- Martini, I.; Polici, M.; Zerunian, M.; Panzuto, F.; Rinzivillo, M.; Landolfi, F.; Magi, L.; Caruso, D.; Eid, M.; Annibale, B.; et al. CT texture analysis of liver metastases in PNETs versus NPNETs: Correlation with histopathological findings. Eur. J. Radiol. 2020, 124, 108812. [Google Scholar] [CrossRef]
- Cook, G.J.; O’Brien, M.E.; Siddique, M.; Chicklore, S.; Loi, H.Y.; Sharma, B.; Punwani, R.; Bassett, P.; Goh, V.; Chua, S. Non–small cell lung cancer treated with erlotinib: Heterogeneity of 18F-FDG uptake at PET—Association with treatment response and prognosis. Radiology 2015, 276, 883–893. [Google Scholar] [CrossRef]
- Tixier, F.; Le Rest, C.C.; Hatt, M.; Albarghach, N.; Pradier, O.; Metges, J.P.; Corcos, L.; Visvikis, D. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 2011, 52, 369–378. [Google Scholar] [CrossRef]
- Liberini, V.; Rampado, O.; Gallio, E.; De Santi, B.; Ceci, F.; Dionisi, B.; Thuillier, P.; Ciuffreda, L.; Piovesan, A.; Fioroni, F.; et al. (68)Ga-DOTATOC PET/CT-Based Radiomic Analysis and PRRT Outcome: A Preliminary Evaluation Based on an Exploratory Radiomic Analysis on Two Patients. Front. Med. 2020, 7, 601853. [Google Scholar] [CrossRef]
- Werner, R.A.; Ilhan, H.; Lehner, S.; Papp, L.; Zsótér, N.; Schatka, I.; Muegge, D.O.; Javadi, M.S.; Higuchi, T.; Buck, A.K.; et al. Pre-therapy Somatostatin Receptor-Based Heterogeneity Predicts Overall Survival in Pancreatic Neuroendocrine Tumor Patients Undergoing Peptide Receptor Radionuclide Therapy. Mol. Imaging Biol. 2019, 21, 582–590. [Google Scholar] [CrossRef]
- Werner, R.A.; Lapa, C.; Ilhan, H.; Higuchi, T.; Buck, A.K.; Lehner, S.; Bartenstein, P.; Bengel, F.; Schatka, I.; Muegge, D.O.; et al. Survival prediction in patients undergoing radionuclide therapy based on intratumoral somatostatin-receptor heterogeneity. Oncotarget 2017, 8, 7039–7049. [Google Scholar] [CrossRef]
- Wetz, C.; Apostolova, I.; Steffen, I.G.; Hofheinz, F.; Furth, C.; Kupitz, D.; Ruf, J.; Venerito, M.; Klose, S.; Amthauer, H. Predictive value of asphericity in pretherapeutic [111In]DTPA-octreotide SPECT/CT for response to peptide receptor radionuclide therapy with [177Lu]DOTATATE. Mol. Imaging Biol. 2017, 19, 437–445. [Google Scholar] [CrossRef]
- Fonti, R.; Panico, M.; Pellegrino, S.; Pulcrano, A.; Vastarella, L.A.; Hakkak, A.; Giuliano, M.; Palmieri, G.; De Placido, S.; Del Vecchio, S. Heterogeneity of SSTR2 expression assessed by 68Ga-DOTATOC PET/CT using coefficient of variation in patients with neuroendocrine tumors. J. Nucl. Med. 2022, 63, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Onner, H.; Abdulrezzak, U.; Tutus, A. Could the skewness and kurtosis texture parameters of lesions obtained from pretreatment Ga-68 DOTA-TATE PET/CT images predict receptor radionuclide therapy response in patients with gastroenteropancreatic neuroendocrine tumors? Nucl. Med. Commun. 2020, 41, 1034–1039. [Google Scholar] [CrossRef]
- Weber, M.; Kessler, L.; Schaarschmidt, B.M.; Fendler, W.P.; Lahner, H.; Antoch, G.; Umutlu, L.; Herrmann, K.; Rischpler, C. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from (68)Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC Cancer 2020, 20, 326. [Google Scholar] [CrossRef]
- Laudicella, R.; Comelli, A.; Liberini, V.; Vento, A.; Stefano, A.; Spataro, A.; Crocè, L.; Baldari, S.; Bambaci, M.; Deandreis, D.; et al. [68Ga] DOTATOC PET/CT Radiomics to Predict the Response in GEP-NETs Undergoing [177Lu] DOTATOC PRRT: The “Theragnomics” Concept. Cancers 2022, 14, 984. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi, D.; Gentiloni Silveri, G.; Panzuto, F.; Faggiano, A.; Russo, V.M.; Caruso, D.; Polici, M.; Lauri, C.; Filice, A.; Laghi, A.; et al. Nuclear Medicine and Radiological Imaging of Pancreatic Neuroendocrine Neoplasms: A Multidisciplinary Update. J. Clin. Med. 2022, 11, 6836. https://doi.org/10.3390/jcm11226836
Prosperi D, Gentiloni Silveri G, Panzuto F, Faggiano A, Russo VM, Caruso D, Polici M, Lauri C, Filice A, Laghi A, et al. Nuclear Medicine and Radiological Imaging of Pancreatic Neuroendocrine Neoplasms: A Multidisciplinary Update. Journal of Clinical Medicine. 2022; 11(22):6836. https://doi.org/10.3390/jcm11226836
Chicago/Turabian StyleProsperi, Daniela, Guido Gentiloni Silveri, Francesco Panzuto, Antongiulio Faggiano, Vincenzo Marcello Russo, Damiano Caruso, Michela Polici, Chiara Lauri, Angelina Filice, Andrea Laghi, and et al. 2022. "Nuclear Medicine and Radiological Imaging of Pancreatic Neuroendocrine Neoplasms: A Multidisciplinary Update" Journal of Clinical Medicine 11, no. 22: 6836. https://doi.org/10.3390/jcm11226836
APA StyleProsperi, D., Gentiloni Silveri, G., Panzuto, F., Faggiano, A., Russo, V. M., Caruso, D., Polici, M., Lauri, C., Filice, A., Laghi, A., & Signore, A. (2022). Nuclear Medicine and Radiological Imaging of Pancreatic Neuroendocrine Neoplasms: A Multidisciplinary Update. Journal of Clinical Medicine, 11(22), 6836. https://doi.org/10.3390/jcm11226836








