Exercise-Associated Hyponatremia in Marathon Runners
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Role of Sodium in Exercise-Associated Hyponatremia
3.2. Aetiology and Evidence of Exercise-Induced Hyponatremia in Marathon Runners
4. Discussion
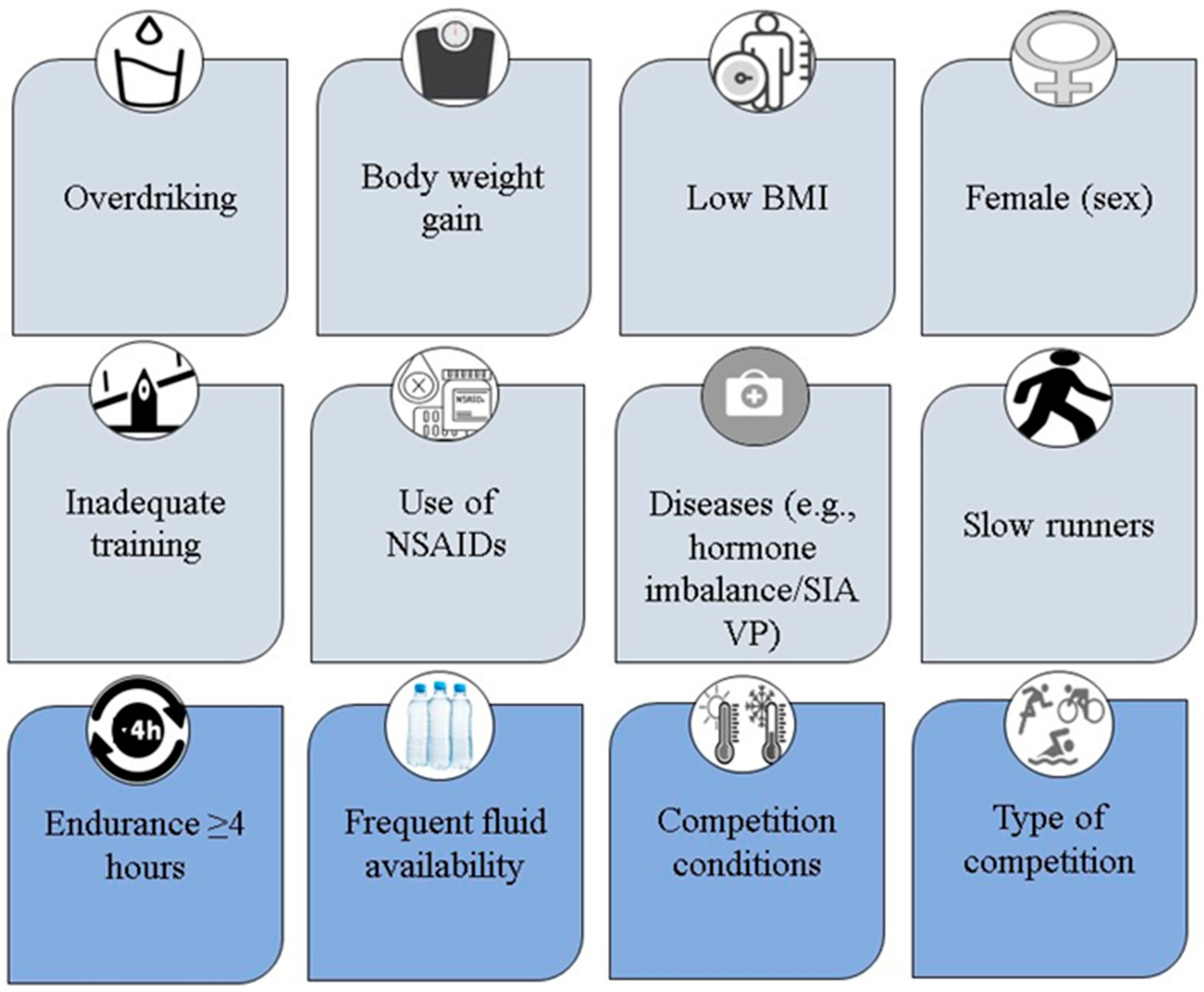
4.1. Risk Factors
4.2. Prevention and Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noakes, T.D.; Goodwin, N.; Rayner, B.L.; Branken, T.; Taylor, R.K. Water intoxication: A possible complication during endurance exercise. Med. Sci. Sport. Exerc. 1985, 17, 370–375. [Google Scholar] [CrossRef]
- Frizzell, R.T.; Lang, G.H.; Lowance, D.C.; Lathan, S.R. Hyponatremia and ultramarathon running. JAMA 1986, 255, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Rosner, M.H. Exercise-associated hyponatremia. Trans. Am. Clin. Climatol. Assoc. 2019, 130, 76–87. [Google Scholar] [PubMed]
- Petzold, A.; Keir, G.; Appleby, I. Marathon related death due to brainstem herniation in rehydration-related hyponatraemia: A case report. J. Med. Case Rep. 2007, 1, 186. [Google Scholar] [CrossRef] [PubMed]
- Buffington, M.A.; Abreo, K. Hyponatremia: A Review. J. Intensive Care Med. 2016, 31, 223–236. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Anley, C.; Schwartz, P.; Noakes, T. The treatment of symptomatic hyponatremia with hypertonic saline in an Ironman triathlete. Clin. J. Sport Med. 2007, 17, 68–69. [Google Scholar] [CrossRef]
- Scheer, V.; Hoffman, M.D. Exercise-Associated Hyponatremia: Practical Guide to its Recognition, Treatment and Avoidance during Prolonged Exercise. Dtsch. Z. Sportmed. 2018, 69, 311–318. [Google Scholar] [CrossRef]
- Whatmough, S.; Mears, S.; Kipps, C. Serum sodium changes in marathon participants who use NSAIDs. BMJ Open Sport Exerc. Med. 2018, 4, e000364. [Google Scholar] [CrossRef]
- Rosner, M.H.; Kirven, J. Exercise-associated hyponatremia. Clin. J. Am. Soc. Nephrol. 2007, 2, 151–161. [Google Scholar] [CrossRef]
- Namineni, N.; Potok, O.A.; Ix, J.H.; Ginsberg, C.; Negoianu, D.; Rifkin, D.E.; Garimella, P.S. Marathon Runners’ Knowledge and Strategies for Hydration. Clin. J. Sport Med. 2022, 32, 517–522. [Google Scholar] [CrossRef]
- Lewis, D.; Blow, A.; Tye, J.; Hew-Butler, T. Considering exercise-associated hyponatraemia as a continuum. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Hew-Butler, T.; Loi, V.; Pani, A.; Rosner, M.H. Exercise-Associated Hyponatremia: 2017 Update. Front. Med. 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Hiller, W.D.; O’Toole, M.L.; Fortess, E.E.; Laird, R.H.; Imbert, P.C.; Sisk, T.D. Medical and physiological considerations in triathlons. Am. J. Sport. Med. 1987, 15, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Reusser, M.; Sousa, C.V.; Villiger, E.; Alvero Cruz, J.R.; Hill, L.; Rosemann, T.; Nikolaidis, P.T.; Knechtle, B. Increased Participation and Decreased Performance in Recreational Master Athletes in “Berlin Marathon” 1974–2019. Front. Physiol. 2021, 12, 631237. [Google Scholar] [CrossRef]
- Vitti, A.; Nikolaidis, P.T.; Villiger, E.; Onywera, V.; Knechtle, B. The “New York City Marathon”: Participation and performance trends of 1.2M runners during half-century. Res. Sport. Med. 2020, 28, 121–137. [Google Scholar] [CrossRef]
- Mohseni, M.; Silvers, S.; McNeil, R.; Diehl, N.; Vadeboncoeur, T.; Taylor, W.; Shapiro, S.; Roth, J.; Mahoney, S. Prevalence of hyponatremia, renal dysfunction, and other electrolyte abnormalities among runners before and after completing a marathon or half marathon. Sport. Health 2011, 3, 145–151. [Google Scholar] [CrossRef]
- McCubbin, A.J. Modelling sodium requirements of athletes across a variety of exercise scenarios—Identifying when to test and target, or season to taste. Eur. J. Sport Sci. 2022, 1–9. [Google Scholar] [CrossRef]
- Fitzpatrick, D.; Walter, E.; Leckie, T.; Richardson, A.; Stacey, M.; Hunter, A.; Short, S.; Hill, N.; Woods, D.; Grimaldi, R.; et al. Association between collapse and serum creatinine and electrolyte concentrations in marathon runners: A 9-year retrospective study. Eur. J. Emerg. Med. Off. J. Eur. Soc. Emerg. Med. 2021, 28, 34–42. [Google Scholar] [CrossRef]
- Mente, A.; O’Donnell, M.; Yusuf, S. Sodium Intake and Health: What Should We Recommend Based on the Current Evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 338–342. [Google Scholar] [CrossRef] [PubMed]
- DeMars, M.M.; Perruso, C. MeSH and text-word search strategies: Precision, recall, and their implications for library instruction. J. Med. Libr. Assoc. JMLA 2022, 110, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulou, E.; Braconnier, P.; Burnier, M. New Insights on the Role of Sodium in the Physiological Regulation of Blood Pressure and Development of Hypertension. Front. Cardiovasc. Med. 2019, 6, 136. [Google Scholar] [CrossRef]
- Wojszel, Z.B. What Serum Sodium Concentration Is Suggestive for Underhydration in Geriatric Patients? Nutrients 2020, 12, 496. [Google Scholar] [CrossRef]
- Grider, M.H.; Jessu, R.; Kabir, R. Physiology, Action Potential. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538143/ (accessed on 4 November 2022).
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; The National Academies Press: Washington, DC, USA, 2005; p. 638. [Google Scholar]
- Joergensen, D.; Tazmini, K.; Jacobsen, D. Acute Dysnatremias—A dangerous and overlooked clinical problem. Scand. J. Trauma Resusc. Emerg. Med. 2019, 27, 58. [Google Scholar] [CrossRef]
- Au-Yeung, K.L.; Wu, W.C.; Yau, W.H.; Ho, H.F. A study of serum sodium level among Hong Kong runners. Clin. J. Sport Med. 2010, 20, 482–487. [Google Scholar] [CrossRef]
- Lüning, H.; Mangelus, C.; Carlström, E.; Nilson, F.; Börjesson, M. Incidence and characteristics of severe exercise-associated collapse at the world’s largest half-marathon. PLoS ONE 2019, 14, e0217465. [Google Scholar] [CrossRef]
- Hoorn, E.J.; Zietse, R. Diagnosis and Treatment of Hyponatremia: Compilation of the Guidelines. J. Am. Soc. Nephrol. JASN 2017, 28, 1340–1349. [Google Scholar] [CrossRef]
- Sahay, M.; Sahay, R. Hyponatremia: A practical approach. Indian J. Endocrinol. Metab. 2014, 18, 760–771. [Google Scholar] [CrossRef]
- Chen, J.S.; Sabir, S.; Al Khalili, Y. Physiology, Osmoregulation and Excretion. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541108/ (accessed on 4 November 2022).
- Baylis, P.H. Osmoregulation and control of vasopressin secretion in healthy humans. Am. J. Physiol. 1987, 253, R671–R678. [Google Scholar] [CrossRef] [PubMed]
- Stanton, B.A. Molecular mechanisms of ANP inhibition of renal sodium transport. Can. J. Physiol. Pharmacol. 1991, 69, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Li, Z.; Lang, C.N.; Schmid, B.; Lang, F.K.; Grad, S.; Alini, M.; Richards, R.G.; Schmal, H.; Südkamp, N.; et al. The Tissue Renin-Angiotensin System and Its Role in the Pathogenesis of Major Human Diseases: Quo Vadis? Cells 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Cuzzo, B.; Padala, S.A.; Lappin, S.L. Physiology, Vasopressin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526069/ (accessed on 4 November 2022).
- Bürge, J.; Knechtle, B.; Knechtle, P.; Gnädinger, M.; Rüst, C.A.; Rosemann, T. Maintained serum sodium in male ultra-marathoners--the role of fluid intake, vasopressin, and aldosterone in fluid and electrolyte regulation. Horm. Metab. Res. = Horm.-Stoffwechs. = Horm. Et Metab. 2011, 43, 646–652. [Google Scholar] [CrossRef]
- Theis, S.R.; Khandhar, P.B. Pseudohyponatremia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553207/ (accessed on 4 November 2022).
- Heinrich, S.; Wagner, A.; Gross, P. Hyponatremia. Med. Klin. Intensivmed. Notf. 2013, 108, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Haack, K.K.; Pollack, B.P.; Millard-Stafford, M.; McCarty, N.A. Low abundance of sweat duct Cl- channel CFTR in both healthy and cystic fibrosis athletes with exceptionally salty sweat during exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R605–R615. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Rosner, M.H.; Fowkes-Godek, S.; Dugas, J.P.; Hoffman, M.D.; Lewis, D.P.; Maughan, R.J.; Miller, K.C.; Montain, S.J.; Rehrer, N.J.; et al. Statement of the 3rd International Exercise-Associated Hyponatremia Consensus Development Conference, Carlsbad, California, 2015. Br. J. Sport. Med. 2015, 49, 1432–1446. [Google Scholar] [CrossRef]
- Hew-Butler, T. Exercise-Associated Hyponatremia. Front. Horm. Res. 2019, 52, 178–189. [Google Scholar] [CrossRef]
- Young, M.; Sciurba, F.; Rinaldo, J. Delirium and pulmonary edema after completing a marathon. Am. Rev. Respir. Dis. 1987, 136, 737–739. [Google Scholar] [CrossRef]
- Nelson, P.B.; Robinson, A.G.; Kapoor, W.; Rinaldo, J. Hyponatremia in a marathoner. Physician Sportsmed. 1988, 16, 78–88. [Google Scholar] [CrossRef]
- Schrier, R.W.; Bansal, S. Diagnosis and management of hyponatremia in acute illness. Curr. Opin. Crit. Care 2008, 14, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D.; Sharwood, K.; Speedy, D.; Hew, T.; Reid, S.; Dugas, J.; Almond, C.; Wharam, P.; Weschler, L. Three independent biological mechanisms cause exercise-associated hyponatremia: Evidence from 2135 weighed competitive athletic performances. Proc. Natl. Acad. Sci. USA 2005, 102, 18550–18555. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Nikolaidis, P.T. Physiology and pathophysiology in ultra-marathon running. Front. Physiol. 2018, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.W.; Nio, A.Q.X.; Ang, W.H.; Johnson, C.; Aziz, A.R.; Lim, C.L.; Hew-Butler, T. First reported cases of exercise-associated hyponatremia in Asia. Int. J. Sport. Med. 2011, 32, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.J.; Verbalis, J.G.; Clement, S.; Mendelson, J.H.; Mello, N.K.; Adner, M.; Shirey, T.; Glowacki, J.; Lee-Lewandrowski, E.; Lewandrowski, K.B. Hyponatremia in Marathon Runners due to Inappropriate Arginine Vasopressin Secretion. Am. J. Med. 2007, 120, 461.e411–461.e417. [Google Scholar] [CrossRef]
- Kratz, A.; Wood, M.J.; Siegel, A.J.; Hiers, J.R.; Van Cott, E.M. Effects of marathon running on platelet activation markers: Direct evidence for in vivo platelet activation. Am. J. Clin. Pathol. 2006, 125, 296–300. [Google Scholar] [CrossRef]
- Sanchez, L.D.; Corwell, B.; Berkoff, D. Medical problems of marathon runners. Am. J. Emerg. Med. 2006, 24, 608–615. [Google Scholar] [CrossRef]
- Seedat, Y.K.; Aboo, N.; Naicker, S.; Parsoo, I. Acute renal failure in the “comrades Marathon” runners. Ren. Fail. 1989, 11, 209–212. [Google Scholar] [CrossRef]
- Hull, C.M.; Hopkins, C.L.; Purdy, N.J.; Lloyd, R.C.; Harris, J.A. A case of unprovoked venous thromboembolism in a marathon athlete presenting atypical sequelae: What are the chances? Scand. J. Med. Sci. Sport. 2015, 25, 699–705. [Google Scholar] [CrossRef]
- Clarkson, P.M. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sport. Med. 2007, 37, 361–363. [Google Scholar] [CrossRef]
- Schmidt, W.; Rojas, J.; Bö, D.; Bernal, H.; Garcia, S.; Garcia, O. Plasma-electrolytes in natives to hypoxia after marathon races at different altitudes. Med. Sci. Sport. Exerc. 1999, 31, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Dehydration during exercise: What are the real dangers? Clin. J. Sport Med. 1995, 5, 123–128. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, J.; Thompson, J.; Hanna, C.; Noakes, T.D.; Stewart, J.; Speedy, D. Sensitivity and specificity of clinical signs for assessment of dehydration in endurance athletes. Br. J. Sport. Med. 2010, 44, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Salinero, J.J.; Areces, F.; Ruiz-Vicente, D.; Gallo-Salazar, C.; Abián-Vicén, J.; Del Coso, J. Sweat sodium loss influences serum sodium concentration in a marathon. Scand. J. Med. Sci. Sport. 2017, 27, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ayus, J.C.; Varon, J.; Arieff, A.I. Hyponatremia, cerebral edema, and noncardiogenic pulmonary edema in marathon runners. Ann. Intern. Med. 2000, 132, 711–714. [Google Scholar] [CrossRef]
- Rehrer, N.J.; Beckers, E.J.; Brouns, F.; Hoor, F.T.; Saris, W.H.M. Effects of dehydration on gastric emptying and gastrointestinal distress while running. Med. Sci. Sport. Exerc. 1990, 22, 790–795. [Google Scholar] [CrossRef]
- Rehrer, N.J.; Brouns, F.; Beckers, E.J.; Frey, W.O.; Villiger, B.; Riddoch, C.J.; Menheere, P.P.C.A.; Saris, W.H.M. Physiological changes and gastro-intestinal symptoms as a result of ultra-endurance running. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 1–8. [Google Scholar] [CrossRef]
- Rehrer, N.J.; Janssen, G.M.E.; Brouns, F.; Saris, W.H.M. Fluid intake and gastrointestinal problems in runners competiting in a 25-km race and a marathon. Int. J. Sport. Med. 1989, 10, S22–S25. [Google Scholar] [CrossRef]
- Cohen, D.C.; Winstanley, A.; Engledow, A.; Windsor, A.C.; Skipworth, J.R. Marathon-induced ischemic colitis: Why running is not always good for you. Am. J. Emerg. Med. 2009, 27, 255.e255–255.e257. [Google Scholar] [CrossRef]
- Watelet, J.; Bigard, M.A. Gastrointestinal and liver disorders in athletes. Gastroenterol. Clin. Biol. 2005, 29, 522–532. [Google Scholar] [CrossRef]
- Lucas, W.; Schroy Iii, P.C. Reversible ischemic colitis in a high endurance athlete. Am. J. Gastroenterol. 1998, 93, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Smetanka, R.D.; Lambert, G.P.; Murray, R.; Eddy, D.; Horn, M.; Gisolfi, C.V. Intestinal permeability in runners in the 1996 Chicago marathon. Int. J. Sport Nutr. 1999, 9, 426–433. [Google Scholar] [CrossRef]
- Rüst, C.A.; Knechtle, B.; Knechtle, P.; Rosemann, T. Higher prevalence of exercise-associated hyponatremia in Triple Iron ultra-triathletes than reported for Ironman triathletes. Chin. J. Physiol. 2012, 55, 1–9. [Google Scholar]
- Hew, T.D.; Chorley, J.N.; Cianca, J.C.; Divine, J.G. The incidence, risk factors, and clinical manifestations of hyponatremia in marathon runners. Clin. J. Sport Med. 2003, 13, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chorley, J.; Cianca, J.; Divine, J. Risk factors for exercise-associated hyponatremia in non-elite marathon runners. Clin. J. Sport Med. 2007, 17, 471–477. [Google Scholar] [CrossRef]
- Almond, C.S.D.; Shin, A.Y.; Fortescue, E.B.; Mannix, R.C.; Wypij, D.; Binstadt, B.A.; Duncan, C.N.; Olson, D.P.; Salerno, A.E.; Newburger, J.W.; et al. Hyponatremia among runners in the Boston marathon. N. Engl. J. Med. 2005, 352, 1550–1556+1622. [Google Scholar] [CrossRef]
- Hsieh, M.; Roth, R.; Davis, D.L.; Larrabee, H.; Callaway, C.W. Hyponatremia in runners requiring on-site medical treatment at a single marathon. Med. Sci. Sport. Exerc. 2002, 34, 185–189. [Google Scholar] [CrossRef]
- Siegel, A.J.; D’Hemecourt, P.; Adner, M.M.; Shirey, T.; Brown, J.L.; Lewandrowski, K.B. Exertional dysnatremia in collapsed marathon runners: A critical role for point-of-care testing to guide appropriate therapy. Am. J. Clin. Pathol. 2009, 132, 336–340. [Google Scholar] [CrossRef]
- Mettler, S.; Rusch, C.; Frey, W.O.; Bestmann, L.; Wenk, C.; Colombani, P.C. Hyponatremia among runners in the Zurich marathon. Clin. J. Sport Med. 2008, 18, 344–349. [Google Scholar] [CrossRef]
- Glace, B.W.; Murphy, C.A.; McHugh, M.P. Food intake and electrolyte status of ultramarathoners competing in extreme heat. J. Am. Coll. Nutr. 2002, 21, 553–559. [Google Scholar] [CrossRef]
- Reid, S.A.; Speedy, D.B.; Thompson, J.M.D.; Noakes, T.D.; Mulligan, G.; Page, T.; Campbell, R.G.D.; Milne, C. Study of hematological and biochemical parameters in runners completing a standard marathon. Clin. J. Sport Med. 2004, 14, 344–353. [Google Scholar] [CrossRef]
- Kipps, C.; Sharma, S.; Pedoe, D.T. The incidence of exercise-associated hyponatraemia in the London Marathon. Br. J. Sport. Med. 2011, 45, 14–19. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Haymes, E.M. Ad libitum fluid intakes and thermoregulatory responses of female distance runners in three environments. J. Sport. Sci. 2001, 19, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Magazanik, A.; Shapiro, Y.; Meytes, D.; Meytes, I. Enzyme blood levels and water balance during a marathon race. J. Appl. Physiol. 1974, 36, 214–217. [Google Scholar] [CrossRef]
- Roberts, W.O. A 12-yr profile of medical injury and illness for the Twin Cities Marathon. Med. Sci. Sport. Exerc. 2000, 32, 1549–1555. [Google Scholar] [CrossRef]
- Montain, S.J.; Cheuvront, S.N.; Sawka, M.N. Exercise associated hyponatraemia: Quantitative analysis to understand the aetiology. Br. J. Sport. Med. 2006, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Knechtle, P.; Rosemann, T. Low prevalence of exercise-associated hyponatremia in male 100 km ultra-marathon runners in Switzerland. Eur. J. Appl. Physiol. 2011, 111, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Stuempfle, K.J.; Lehmann, D.R.; Case, H.S.; Bailey, S.; Hughes, S.L.; McKenzie, J.; Evans, D. Hyponatremia in a cold weather ultraendurance race. Alsk. Med. 2002, 44, 51–55. [Google Scholar]
- Davis, D.P.; Videen, J.S.; Marino, A.; Vilke, G.M.; Dunford, J.V.; Van Camp, S.P.; Maharam, L.G. Exercise-associated hyponatremia in marathon runners: A two-year experience. J. Emerg. Med. 2001, 21, 47–57. [Google Scholar] [CrossRef]
- Wharam, P.C.; Speedy, D.B.; Noakes, T.D.; Thompson, J.M.; Reid, S.A.; Holtzhausen, L.M. NSAID use increases the risk of developing hyponatremia during an Ironman triathlon. Med. Sci. Sport. Exerc. 2006, 38, 618–622. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Stuempfle, K.J.; Rogers, I.R.; Weschler, L.B.; Hew-Butler, T. Hyponatremia in the 2009 161-km Western States Endurance Run. Int. J. Sport. Physiol. Perform. 2012, 7, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Scotney, B.; Reid, S. Body Weight, Serum Sodium Levels, and Renal Function in an Ultra-Distance Mountain Run. Clin. J. Sport Med. 2015, 25, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Hydration in the marathon: Using thirst to gauge safe fluid replacement. Sport. Med. 2007, 37, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Lebus, D.K.; Casazza, G.A.; Hoffman, M.D.; Van Loan, M.D. Can changes in body mass and total body water accurately predict hyponatremia after a 161-km running race? Clin. J. Sport Med. 2010, 20, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Knechtle, B.; Knechtle, P.; Rüst, C.A.; Rosemann, T.; Ward, S.A. Higher prevalence of exercise-associated hyponatremia in female than in male open-water ultra-endurance swimmers: The ‘Marathon-Swim’ in Lake Zurich. Eur. J. Appl. Physiol. 2012, 112, 1095–1106. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.H.; Hoogerwerf, M.D.; Maessen, M.F.H.; Seeger, J.P.H.; George, K.P.; Hopman, M.T.E.; Thijssen, D.H.J. Predictors of cardiac troponin release after a marathon. J. Sci. Med. Sport 2015, 18, 88–92. [Google Scholar] [CrossRef]
- Hew, T.D. Women hydrate more than men during a marathon race—Hyponatremia in the Houston marathon: A report on 60 cases. Clin. J. Sport Med. 2005, 15, 148–153. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Haymes, E.M. Thermoregulation and marathon running biological and environmental influences. Sport. Med. 2001, 31, 743–762. [Google Scholar] [CrossRef]
- Myhre, L.G.; Hartung, G.H.; Tucker, D.M. Plasma volume and blood metabolites in middle-aged runners during a warm-weather marathon. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 227–240. [Google Scholar] [CrossRef]
- Noakes, T.D.; Myburgh, K.H.; Plessis, J.D.; Lang, L.; Lambert, M.; Van Der Riet, C.; Schall, R. Metabolic rate, not percent dehydration, predicts rectal temperature in marathon runners. Med. Sci. Sport. Exerc. 1991, 23, 443–449. [Google Scholar] [CrossRef]
- Noakes, T.; Maharam, L.G. Fluid replacement during marathon running. Clin. J. Sport Med. 2003, 13, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Watson, P.; Shirreffs, S.M. Heat and cold: What does the environment do to the marathon runner? Sport. Med. 2007, 37, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Gallo-Salazar, C.; Puente, C.; Areces, F.; Salinero, J.J.; Del Coso, J. Interindividual variability in sweat electrolyte concentration in marathoners. J. Int. Soc. Sport. Nutr. 2016, 13, 31. [Google Scholar] [CrossRef]
- Pahnke, M.D.; Trinity, J.D.; Zachwieja, J.J.; Stofan, J.R.; Hiller, W.D.; Coyle, E.F. Serum sodium concentration changes are related to fluid balance and sweat sodium loss. Med. Sci. Sport. Exerc. 2010, 42, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Koenders, E.E.; Franken, C.P.G.; Cotter, J.D.; Thornton, S.N.; Rehrer, N.J. Restricting dietary sodium reduces plasma sodium response to exercise in the heat. Scand. J. Med. Sci. Sport. 2017, 27, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Myers, T.M. Case Study: Symptomatic Exercise-Associated Hyponatremia in an Endurance Runner Despite Sodium Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 603–606. [Google Scholar] [CrossRef]
- Hew-Butler, T.D.; Sharwood, K.; Collins, M.; Speedy, D.; Noakes, T. Sodium supplementation is not required to maintain serum sodium concentrations during an Ironman triathlon. Br. J. Sport. Med. 2006, 40, 255–259. [Google Scholar] [CrossRef]
- Del Coso, J.; González-Millán, C.; Salinero, J.J.; Abián-Vicén, J.; Areces, F.; Lledó, M.; Lara, B.; Gallo-Salazar, C.; Ruiz-Vicente, D. Effects of oral salt supplementation on physical performance during a half-ironman: A randomized controlled trial. Scand. J. Med. Sci. Sport. 2016, 26, 156–164. [Google Scholar] [CrossRef]
- Anastasiou, C.A.; Kavouras, S.A.; Arnaoutis, G.; Gioxari, A.; Kollia, M.; Botoula, E.; Sidossis, L.S. Sodium replacement and plasma sodium drop during exercise in the heat when fluid intake matches fluid loss. J. Athl. Train. 2009, 44, 117–123. [Google Scholar] [CrossRef]
- Twerenbold, R.; Knechtle, B.; Kakebeeke, T.H.; Eser, P.; Müller, G.; von Arx, P.; Knecht, H. Effects of different sodium concentrations in replacement fluids during prolonged exercise in women. Br. J. Sport. Med. 2003, 37, 300–303; discussion 303. [Google Scholar] [CrossRef]
- Barr, S.I.; Costill, D.L. Water: Can the endurance athlete get too much of a good thing? J. Am. Diet. Assoc. 1989, 89, 1629–1635. [Google Scholar] [CrossRef]
- Speedy, D.B.; Noakes, T.D.; Kimber, N.E.; Rogers, I.R.; Thompson, J.M.; Boswell, D.R.; Ross, J.J.; Campbell, R.G.; Gallagher, P.G.; Kuttner, J.A. Fluid balance during and after an ironman triathlon. Clin. J. Sport Med. 2001, 11, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Speedy, D.B.; Thompson, J.M.; Rodgers, I.; Collins, M.; Sharwood, K.; Noakes, T.D. Oral salt supplementation during ultradistance exercise. Clin. J. Sport Med. 2002, 12, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.E.; Rogers, I.R.; Hoffman, M.D.; Stuempfle, K.J.; Lewis, D.; Fogard, K.; Verbalis, J.G.; Hew-Butler, T. Efficacy of oral versus intravenous hypertonic saline in runners with hyponatremia. J. Sci. Med. Sport 2014, 17, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.R.; Hook, G.; Stuempfle, K.J.; Hoffman, M.D.; Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: A preliminary randomized trial. Clin. J. Sport Med. 2011, 21, 200–203. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Stuempfle, K.J. Sodium Supplementation and Exercise-Associated Hyponatremia during Prolonged Exercise. Med. Sci. Sport. Exerc. 2015, 47, 1781–1787. [Google Scholar] [CrossRef]
- Traiperm, N.; Gatterer, H.; Burtscher, M. Plasma electrolyte and hematological changes after marathon running in adolescents. Med. Sci. Sport. Exerc. 2013, 45, 1182–1187. [Google Scholar] [CrossRef]
- Eijsvogels, T.M.; Scholten, R.R.; van Duijnhoven, N.T.; Thijssen, D.H.; Hopman, M.T. Sex difference in fluid balance responses during prolonged exercise. Scand. J. Med. Sci. Sport. 2013, 23, 198–206. [Google Scholar] [CrossRef]
- Pastene, J.; Germain, M.; Allevard, A.M.; Gharib, C.; Lacour, J.R. Water balance during and after marathon running. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 49–55. [Google Scholar] [CrossRef]
- Hew-Butler, T. Arginine vasopressin, fluid balance and exercise: Is exercise-associated hyponatraemia a disorder of arginine vasopressin secretion? Sport. Med. 2010, 40, 459–479. [Google Scholar] [CrossRef]
- Demir, M.E.; Horoz, M.; Ulas, T.; Eren, M.A.; Ercan, Z. Nonsteroidal anti-inflammatory drug-induced severe hyponatremia. Medicina 2012, 48, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Levi, A.M.; Duran Rodriguez-Hervada, A.; Mendez-Bailon, M.; Marco-Martinez, J. Drug-induced hyponatremia: An updated review. Minerva Endocrinol. 2014, 39, 1–12. [Google Scholar] [PubMed]
- Ware, J.S.; Wain, L.V.; Channavajjhala, S.K.; Jackson, V.E.; Edwards, E.; Lu, R.; Siew, K.; Jia, W.; Shrine, N.; Kinnear, S.; et al. Phenotypic and pharmacogenetic evaluation of patients with thiazide-induced hyponatremia. J. Clin. Investig. 2017, 127, 3367–3374. [Google Scholar] [CrossRef]
- Milvy, P.; Colt, E.; Thornton, J. A high incidence of urolithiasis in male marathon runners. J. Sport. Med. Phys. Fit. 1981, 21, 295–298. [Google Scholar]
- Upadhyay, A.; Jaber, B.L.; Madias, N.E. Incidence and prevalence of hyponatremia. Am. J. Med. 2006, 119, S30–S35. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, J. Hyperthermia impairs brain, heart and muscle function in exercising humans. Sport. Med. 2007, 37, 371–373. [Google Scholar] [CrossRef]
- Schramm, T.; Predel, H.G. Volume and electrolyte disturbances in endurance sport. Der Internist 2006, 47, 1145–1150. [Google Scholar] [CrossRef]
- Knechtle, B.; Chlíbková, D.; Papadopoulou, S.; Mantzorou, M.; Rosemann, T.; Nikolaidis, P.T. Exercise-associated hyponatremia in endurance and ultra-endurance performance–aspects of sex, race location, ambient temperature, sports discipline, and length of performance: A narrative review. Medicina 2019, 55, 537. [Google Scholar] [CrossRef]
- Adams, W.C.; Fox, R.H.; Fry, A.J.; MacDonald, I.C. Thermoregulation during marathon running in cool, moderate, and hot environments. J. Appl. Physiol. 1975, 38, 1030–1037. [Google Scholar] [CrossRef]
- Maughan, R.J. Distance running in hot environments: A thermal challenge to the elite runner. Scand. J. Med. Sci. Sport. 2010, 20, 95–102. [Google Scholar] [CrossRef]
- Martin, D.E. Strategies for optimising marathon performance in the heat. Sport. Med. 2007, 37, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Treff, B.; Sandholtet, T.; Maassen, N.; Shushakov, V.; Kaesebieter, J.; Maassen, M. Carbohydrate supplementation stabilises plasma sodium during training with high intensity. Eur. J. Appl. Physiol. 2016, 116, 1841–1853. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; Costill, D.L.; Fink, W.J. Fluid replacement during prolonged exercise: Effects of water, saline, or no fluid. Med. Sci. Sport. Exerc. 1991, 23, 811–817. [Google Scholar] [CrossRef]
- O’Neal, E.K.; Wingo, J.E.; Richardson, M.T.; Leeper, J.D.; Neggers, Y.H.; Bishop, P.A. Half-marathon and full-marathon runners’ hydration practices and perceptions. J. Athl. Train. 2011, 46, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Tzortziou Brown, V.; Malliaras, P.; Perry, M.; Kipps, C. Hydration strategies of runners in the London Marathon. Clin. J. Sport Med. 2012, 22, 152–156. [Google Scholar] [CrossRef]
- Leggett, T.; Williams, J.; Daly, C.; Kipps, C.; Twycross-Lewis, R. Intended Hydration Strategies and Knowledge of Exercise-Associated Hyponatraemia in Marathon Runners: A Questionnaire-Based Study. J. Athl. Train. 2018, 53, 696–702. [Google Scholar] [CrossRef]
- Giersch, G.E.W.; Charkoudian, N.; Stearns, R.L.; Casa, D.J. Fluid Balance and Hydration Considerations for Women: Review and Future Directions. Sport. Med. 2020, 50, 253–261. [Google Scholar] [CrossRef]
- Gutiérrez-Vargas, R.; Martín-Rodríguez, S.; Sánchez-Ureña, B.; Rodríguez-Montero, A.; Salas-Cabrera, J.; Gutiérrez-Vargas, J.C.; Simunic, B.; Rojas-Valverde, D. Biochemical and Muscle Mechanical Postmarathon Changes in Hot and Humid Conditions. J. Strength Cond. Res. 2020, 34, 847–856. [Google Scholar] [CrossRef]
- Meinders, A.J.; Meinders, A.E. Hyponatraenmia during a long-distance run: Due to excessive fluid intake. Ned. Tijdschr. Geneeskd. 2007, 151, 581–587. [Google Scholar]
- Poettgen, Ironman Projekt 2010 Nahrungs-Sowie Flüssigkeitsaufnahme und Gastrointestinale Probleme Während Langer Ausdauerwettkämpfe Triathlon Band 23, Edition Czwalina. Available online: http://www.klaus-poettgen.de/2012-poettgen-gastrointestinale-Probleme-waehrend-langer-Ausdauerwettkaempfe-ironman-triathlon.pdf (accessed on 4 November 2022).
- Holland, J.J.; Skinner, T.L.; Irwin, C.G.; Leveritt, M.D.; Goulet, E.D.B. The Influence of Drinking Fluid on Endurance Cycling Performance: A Meta-Analysis. Sport. Med. 2017, 47, 2269–2284. [Google Scholar] [CrossRef]
- Speedy, D.B.; Rogers, I.R.; Noakes, T.D.; Thompson, J.M.; Guirey, J.; Safih, S.; Boswell, D.R. Diagnosis and prevention of hyponatremia at an ultradistance triathlon. Clin. J. Sport Med. 2000, 10, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Montain, S.J.; Sawka, M.N. Fluid replacement and performance during the marathon. Sport. Med. 2007, 37, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Pyne, S. Intravenous fluids post marathon: When and why? Sport. Med. 2007, 37, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Siegel, A.J.; Verbalis, J.G.; Adner, M.M.; Shirey, T.; Lee-Lewandrowski, E.; Lewandrowski, K.B. Sodium status of collapsed marathon runners. Arch. Pathol. Lab. Med. 2005, 129, 227–230. [Google Scholar] [CrossRef]
- Siegel, A.J. Hypertonic (3%) sodium chloride for emergent treatment of exercise-associated hypotonic encephalopathy. Sport. Med. 2007, 37, 459–462. [Google Scholar] [CrossRef]
- Elsaesser, T.F.; Pang, P.S.; Malik, S.; Chiampas, G.T. Large-volume hypertonic saline therapy in endurance athlete with exercise-associated hyponatremic encephalopathy. J. Emerg. Med. 2013, 44, 1132–1135. [Google Scholar] [CrossRef]
- Goudie, A.M.; Tunstall-Pedoe, D.S.; Kerins, M.; Terris, J. Exercise-associated hyponatraemia after a marathon: Case series. J. R. Soc. Med. 2006, 99, 363–367. [Google Scholar] [CrossRef]
- O’Connor, R.E. Exercise-induced hyponatremia: Causes, risks, prevention, and management. Clevel. Clin. J. Med. 2006, 73, S13–S18. [Google Scholar] [CrossRef]
- Hew-Butler, T.; Sharwood, K.; Boulter, J.; Collins, M.; Tucker, R.; Dugas, J.; Shave, R.; George, K.; Cable, T.; Verbalis, J.G.; et al. Dysnatremia predicts a delayed recovery in collapsed ultramarathon runners. Clin. J. Sport Med. 2007, 17, 289–296. [Google Scholar] [CrossRef]
- Kenefick, R.W.; Sawka, M.N. Heat exhaustion and dehydration as causes of marathon collapse. Sport. Med. 2007, 37, 378–381. [Google Scholar] [CrossRef]
- Jain, V.; Ochoa, M.; Jiang, H.; Rahimi, R.; Ziaie, B. A mass-customizable dermal patch with discrete colorimetric indicators for personalized sweat rate quantification. Microsyst. Nanoeng. 2019, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Crouse, B.; Beattie, K. Marathon medical services: Strategies to reduce runner morbidity. Med. Sci. Sport. Exerc. 1996, 28, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.B.; Milsten, A.M.; Cushman, J.T. Injury patterns and levels of care at a marathon. Prehospital Disaster Med. 2008, 23, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chiampas, G.T.; Goyal, A.V. Innovative Operations Measures and Nutritional Support for Mass Endurance Events. Sport. Med. 2015, 45, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.P. Premarathon Evaluations: Is There a Role for Runner Prerace Medical Screening and Education to Reduce the Risk of Medical Complications? Curr. Sport. Med. Rep. 2017, 16, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Mydlík, M.; Derzsiová, K.; Bohus, B. Renal function abnormalities after marathon run and 16-kilometre long-distance run. Przegla̧d Lek. 2012, 69, 1–4. [Google Scholar]
- Murray, B. The role of salt and glucose replacement drinks in the marathon. Sport. Med. 2007, 37, 358–360. [Google Scholar] [CrossRef]
- Roberts, W.O. Exercise-associated collapse care matrix in the marathon. Sport. Med. 2007, 37, 431–433. [Google Scholar] [CrossRef]
- Siegel, A.J. Fatal water intoxication and cardiac arrest in runners during marathons: Prevention and treatment based on validated clinical paradigms. Am. J. Med. 2015, 128, 1070–1075. [Google Scholar] [CrossRef]
| EAH Form | Definition |
|---|---|
| Pseudohyponatremia | Plasma’s lipid, protein, and glucose content are increased, whereas the sodium content of plasma water is within the normal range (135–145 mmol/L) |
| Euvolemic hyponatremia | Total body volume increased, whereas total sodium is normal (135–145 mmol/L) |
| Hypovolemic hyponatremia | Total body volume decreased and serum sodium < 135 mmol/L |
| Hypervolemic hyponatremia | Total body volume increased and serum sodium < 135 mmol/L |
| Biochemical hyponatremia | Serum sodium ranges from 129 to 134.9 mmol/L |
| Clinically significant hyponatremia | Serum sodium < 129 mmol/L |
| Study (Year) | Prevalence of EAH |
|---|---|
| Hew et al., 2003 [69] | <1% |
| Chorley et al., 2007 [70] | 22% |
| Almond et al., 2005 [71] | 13% |
| Hsieh et al., 2002 [72] | 5.6% |
| Siegel et al., 2009 [73] | 4.8% |
| Mettler et al., 2008 [74] | 3% |
| Author (Year) | Overhydrated | Euhydrated | Dehydrated | Correlation between Body Weight and Serum Sodium | |||
|---|---|---|---|---|---|---|---|
| Biochemical hyponatremia | Clinically noticeable hyponatremia | Biochemical hyponatremia | Clinically noticeable hyponatremia | Biochemical hyponatremia | Clinically noticeable hyponatremia | ||
| Noakes et al., 2005 [47] | 44 | 25 | 41 | 6 | 38 | 0 | inverse |
| Glace et al., 2022 [75] | 1 | 0 | 0 | 0 | 0 | 0 | inverse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingert, M.; Nikolaidis, P.T.; Weiss, K.; Thuany, M.; Chlíbková, D.; Knechtle, B. Exercise-Associated Hyponatremia in Marathon Runners. J. Clin. Med. 2022, 11, 6775. https://doi.org/10.3390/jcm11226775
Klingert M, Nikolaidis PT, Weiss K, Thuany M, Chlíbková D, Knechtle B. Exercise-Associated Hyponatremia in Marathon Runners. Journal of Clinical Medicine. 2022; 11(22):6775. https://doi.org/10.3390/jcm11226775
Chicago/Turabian StyleKlingert, Mark, Pantelis T. Nikolaidis, Katja Weiss, Mabliny Thuany, Daniela Chlíbková, and Beat Knechtle. 2022. "Exercise-Associated Hyponatremia in Marathon Runners" Journal of Clinical Medicine 11, no. 22: 6775. https://doi.org/10.3390/jcm11226775
APA StyleKlingert, M., Nikolaidis, P. T., Weiss, K., Thuany, M., Chlíbková, D., & Knechtle, B. (2022). Exercise-Associated Hyponatremia in Marathon Runners. Journal of Clinical Medicine, 11(22), 6775. https://doi.org/10.3390/jcm11226775







