Efficacy of Autologous Intrauterine Infusion of Platelet-Rich Plasma in Patients with Unexplained Repeated Implantation Failures in Embryo Transfer: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Selection (Inclusion and Exclusion) Criteria
2.3. PRP Protocols
2.4. Statistical Analysis
3. Results
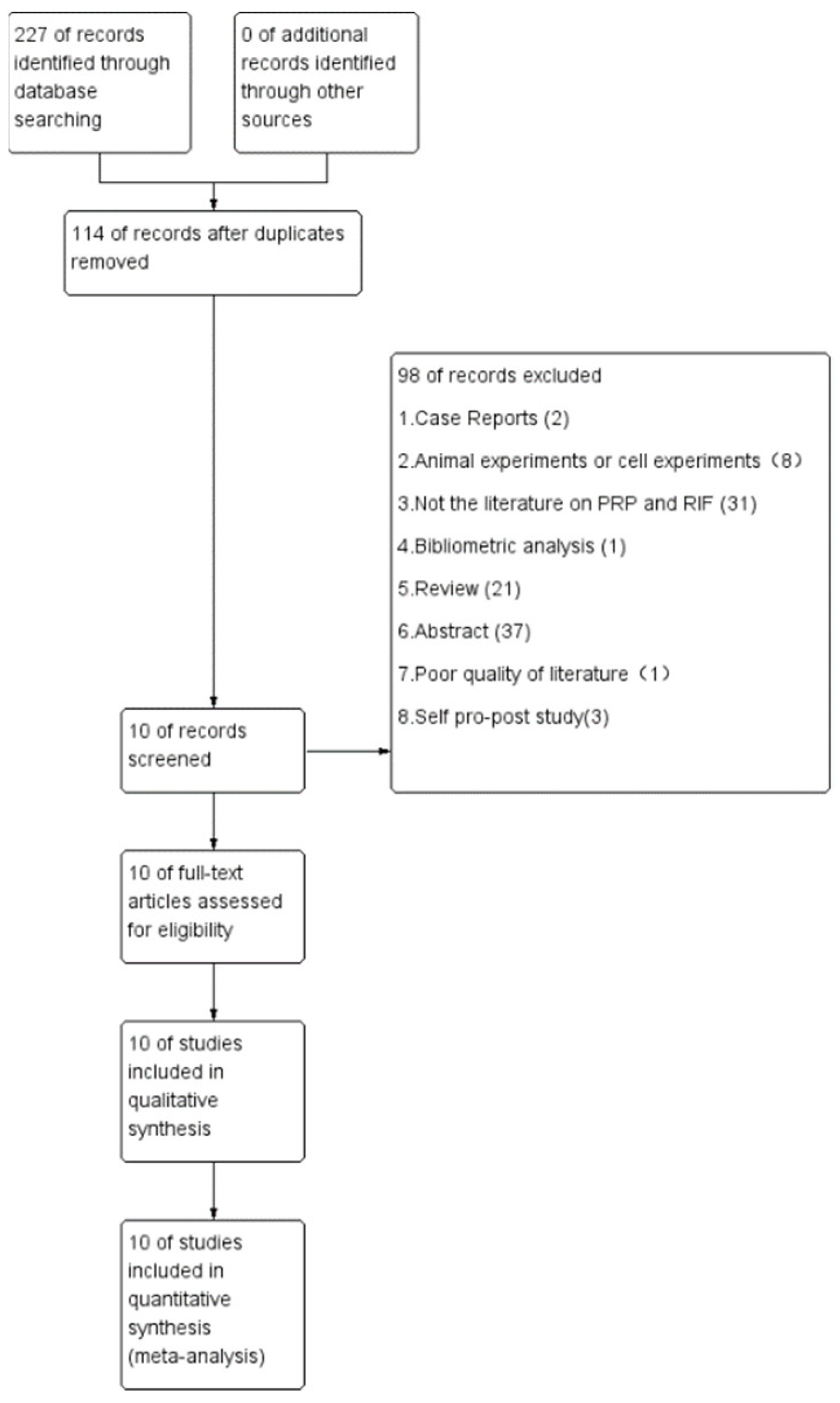
3.1. Summary of Literature Research and Description of Studies
3.2. Study Characteristics
3.3. Risk of Bias Assessment
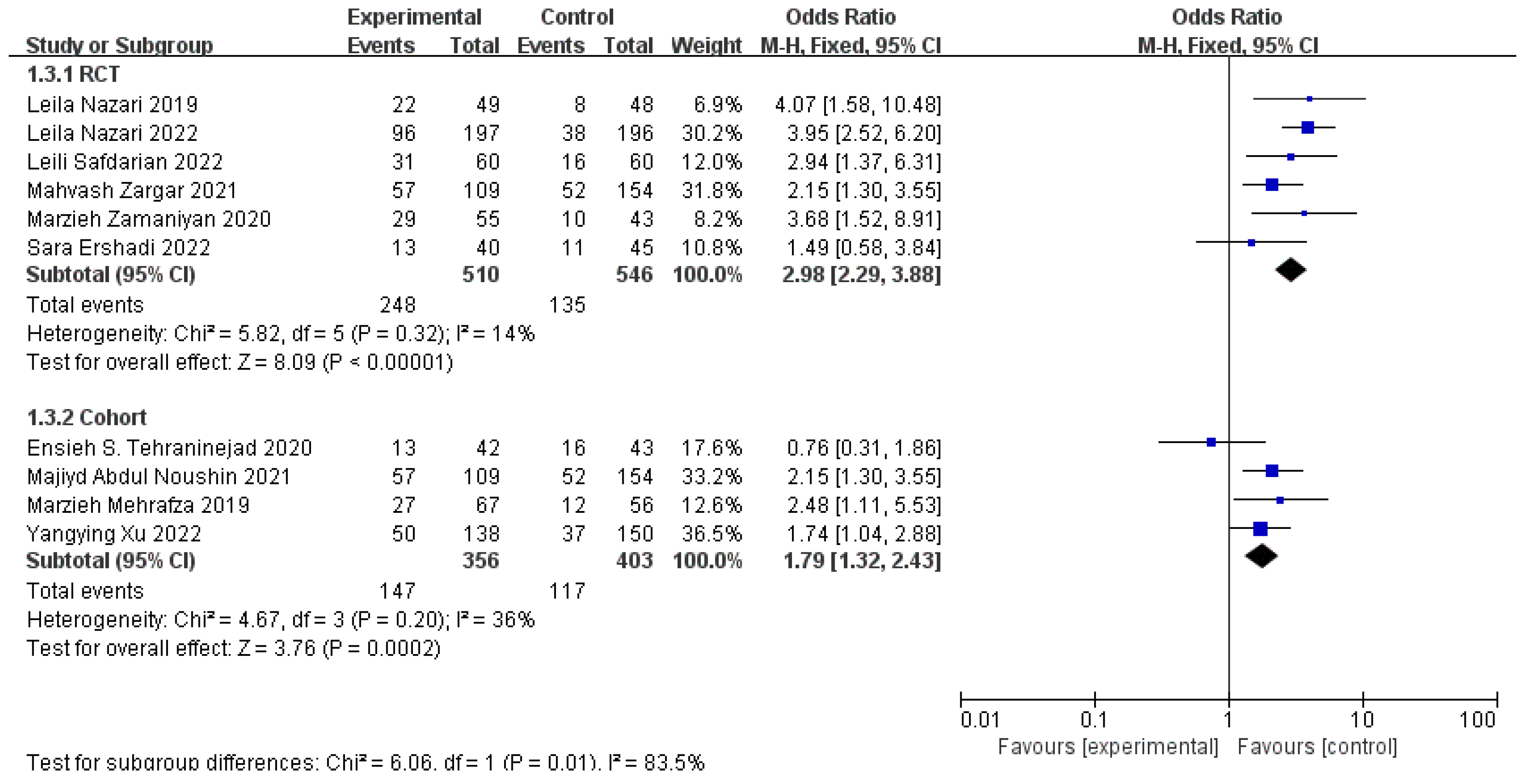
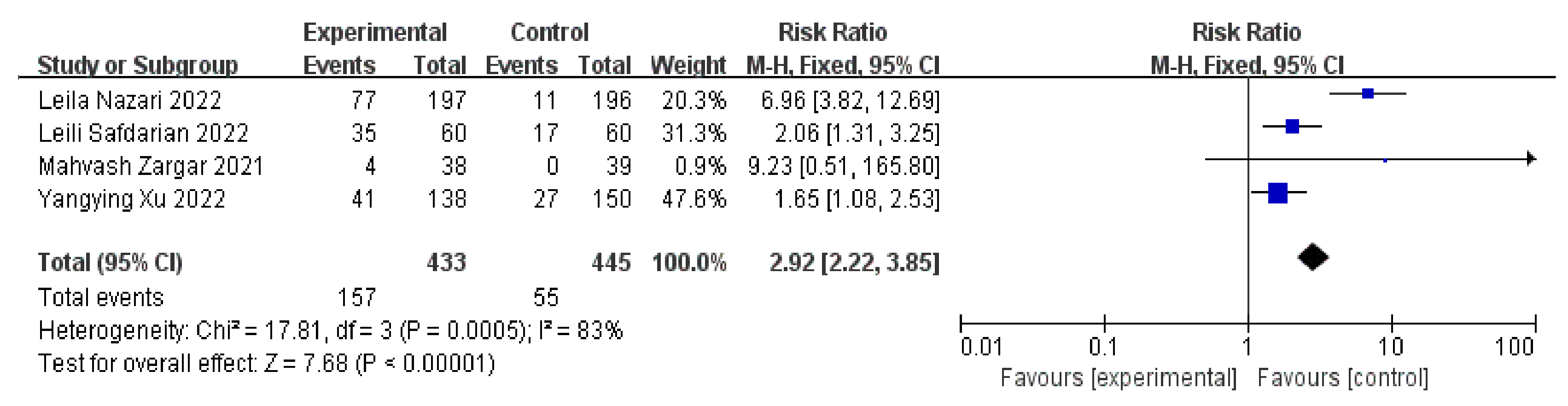
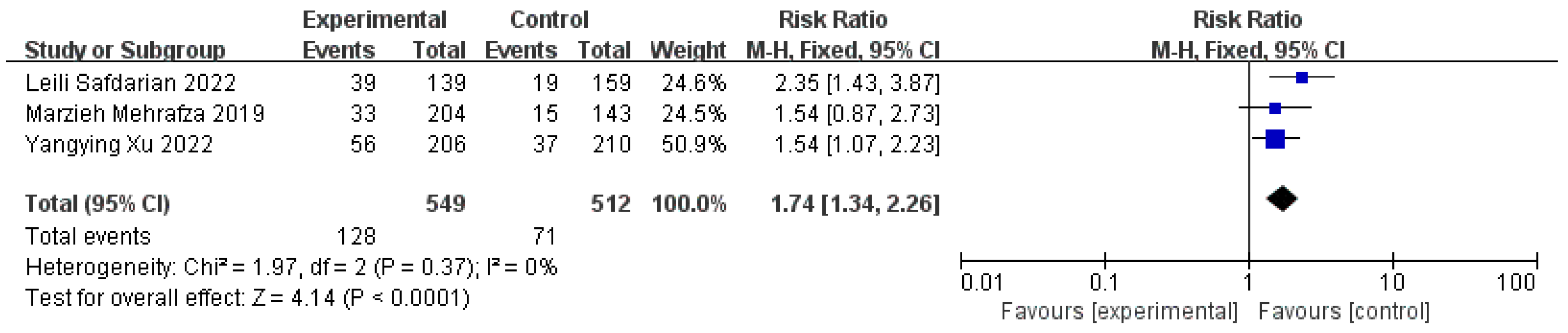
3.4. Clinical Pregnancy Rates
3.5. Live Birth Rates
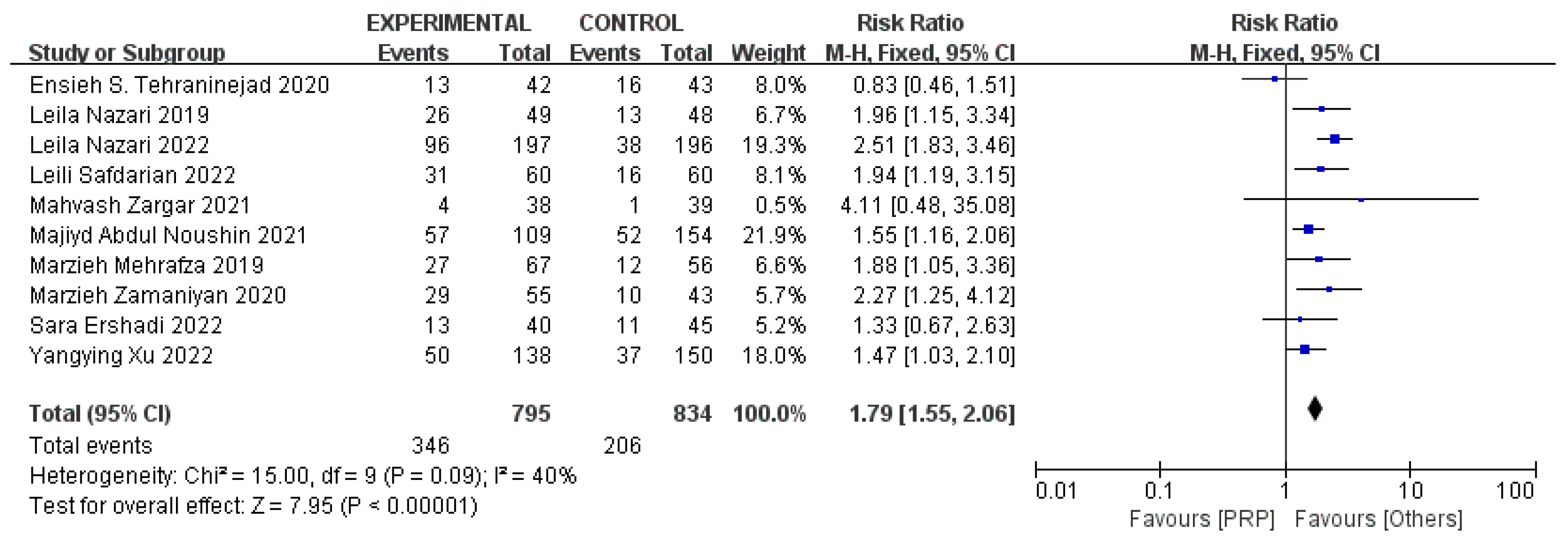
3.6. Positive Serum β-HCG Rates on 14 Days after ET and Implantation Rates
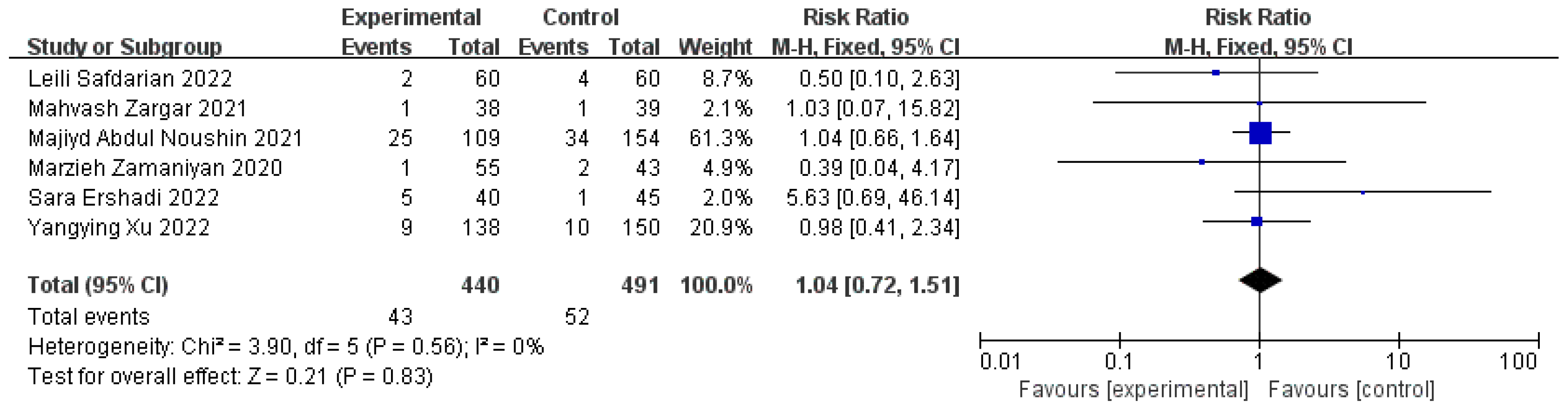
3.7. Miscarriage Rates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pourakbari, R.; Ahmadi, H.; Yousefi, M.; Aghebati-Maleki, L. Cell Therapy in Female Infertility-Related Diseases: Emphasis on Recurrent Miscarriage and Repeated Implantation Failure. Life Sci. 2020, 258, 118181. [Google Scholar] [CrossRef] [PubMed]
- Makrigiannakis, A.; Makrygiannakis, F.; Vrekoussis, T. Approaches to Improve Endometrial Receptivity in Case of Repeated Implantation Failures. Front. Cell Dev. Biol. 2021, 9, 613277. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hao, C.; Fang, J.; Liu, X.; Xue, P.; Miao, R. Intrauterine Perfusion of Autologous Platelet-Rich Plasma before Frozen-Thawed Embryo Transfer Improves the Clinical Pregnancy Rate of Women with Recurrent Implantation Failure. Front. Med. 2022, 9, 850002. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Salehpour, S.; Hosseini, S.; Sheibani, S.; Hosseinirad, H. The Effects of Autologous Platelet-Rich Plasma on Pregnancy Outcomes in Repeated Implantation Failure Patients Undergoing Frozen Embryo Transfer: A Randomized Controlled Trial. Reprod. Sci. 2022, 29, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Ershadi, S.; Noori, N.; Dashipoor, A.; Ghasemi, M.; Shamsa, N. Evaluation of the Effect of Intrauterine Injection of Platelet-Rich Plasma on the Pregnancy Rate of Patients with a History of Implantation Failure in the Fertilization Cycle. J. Fam. Med. Prim. Care 2022, 11, 2162–2166. [Google Scholar] [CrossRef]
- Zargar, M.; Pazhouhanfar, R.; Najafian, M.; Choghakabodi, P.M. Effects of Intrauterine Autologous Platelet-Rich Plasma Infusions on Outcomes in Women with Repetitive in Vitro Fertilization Failures: A Prospective Randomized Study. Clin. Exp. Obstetr. Gynecol. 2021, 48, 180–185. [Google Scholar] [CrossRef]
- Zhou, T.; Ni, T.; Li, Y.; Zhang, Q.; Yan, J.; Chen, Z.-J. Circfam120a Participates in Repeated Implantation Failure by Regulating Decidualization via the Mir-29/Abhd5 Axis. FASEB J. 2021, 35, e21872. [Google Scholar] [CrossRef]
- Coughlan, C.; Ledger, W.; Wang, Q.; Liu, F.; Demirol, A.; Gurgan, T.; Cutting, R.; Ong, K.; Sallam, H.; Li, T.C. Recurrent Implantation Failure: Definition and Management. Reprod. Biomed. Online 2014, 28, 14–38. [Google Scholar] [CrossRef]
- Yin, W.; Qi, X.; Zhang, Y.; Sheng, J.; Xu, Z.; Tao, S.; Xie, X.; Li, X.; Zhang, C. Advantages of Pure Platelet-Rich Plasma Compared with Leukocyte- and Platelet-Rich Plasma in Promoting Repair of Bone Defects. J. Transl. Med. 2016, 14, 73. [Google Scholar] [CrossRef]
- Wang, D.; Rodeo, S.A. Platelet-Rich Plasma in Orthopaedic Surgery: A Critical Analysis Review. JBJS Rev. 2017, 5, e7. [Google Scholar] [CrossRef]
- Scully, D.; Naseem, K.M.; Matsakas, A. Platelet Biology in Regenerative Medicine of Skeletal Muscle. Acta Physiol. 2018, 223, e13071. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, W.Y.; Lin, C.J.; Sun, Y.C.; Chang, P.Y.; Wang, H.Y.; Lu, J.J.; Yeh, W.L.; Chiueh, T.S. Sustained or Higher Levels of Growth Factors in Platelet-Rich Plasma During 7-Day Storage. Clin. Chim. Acta 2018, 483, 89–93. [Google Scholar] [CrossRef]
- Mijiritsky, E.; Assaf, H.D.; Peleg, O.; Shacham, M.; Cerroni, L.; Mangani, L. Use of Prp, Prf and Cgf in Periodontal Regeneration and Facial Rejuvenation—A Narrative Review. Biology 2021, 10, 317. [Google Scholar] [CrossRef]
- Raheem, O.A.; Natale, C.; Dick, B.; Reddy, A.G.; Yousif, A.; Khera, M.; Baum, N. Novel Treatments of Erectile Dysfunction: Review of the Current Literature. Sex. Med. Rev. 2021, 9, 123–132. [Google Scholar] [CrossRef]
- Gilat, R.; Haunschild, E.D.; Knapik, D.M.; Evuarherhe, A.; Parvaresh, K.C.; Cole, B.J. Hyaluronic Acid and Platelet-Rich Plasma for the Management of Knee Osteoarthritis. Int. Orthop. 2021, 45, 345–354. [Google Scholar] [CrossRef]
- Hesseler, M.J.; Shyam, N. Platelet-Rich Plasma and Its Utility in Medical Dermatology: A Systematic Review. J. Am. Acad. Dermatol. 2019, 81, 834–846. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.; Wei, L.-N.; Pang, J.; Chen, J.; Liang, X. Autologous Platelet-Rich Plasma Infusion Improves Clinical Pregnancy Rate in Frozen Embryo Transfer Cycles for Women with Thin Endometrium. Medicine 2019, 98, e14062. [Google Scholar] [CrossRef]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of Intraovarian Injection of Autologous Platelet Rich Plasma on Ovarian Reserve and Ivf Outcome Parameters in Women with Primary Ovarian Insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Hsu, L.; Hsu, I.; Chiu, Y.-J.; Dorjee, S. Live Birth in Woman with Premature Ovarian Insufficiency Receiving Ovarian Administration of Platelet-Rich Plasma (Prp) in Combination with Gonadotropin: A Case Report. Front. Endocrinol. 2020, 11, 50. [Google Scholar] [CrossRef]
- Amable, P.R.; Carias, R.B.V.; Teixeira, M.V.T.; Pacheco, I.D.C.; Amaral, R.J.F.C.D.; Granjeiro, J.M.; Borojevic, R. Platelet-Rich Plasma Preparation for Regenerative Medicine: Optimization and Quantification of Cytokines and Growth Factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Sánchez-González, D.J.; Méndez-Bolaina, E.; Trejo-Bahena, N.I. Platelet-Rich Plasma Peptides: Key for Regeneration. Int. J. Pept. 2012, 2012, 532519. [Google Scholar] [CrossRef] [PubMed]
- Sharara, F.I.; Lelea, L.L.; Rahman, S.; Klebanoff, J.S.; Moawad, G.N. A Narrative Review of Platelet-Rich Plasma (Prp) in Reproductive Medicine. Review. J. Assist. Reprod. Genet. 2021, 38, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Tehraninejad, E.S.; Kashani, N.G.; Hosseini, A.; Tarafdari, A. Autologous Platelet-Rich Plasma Infusion Does Not Improve Pregnancy Outcomes in Frozen Embryo Transfer Cycles in Women with History of Repeated Implantation Failure without Thin Endometrium. J. Obstet. Gynaecol. Res. 2021, 47, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Nazari, L.; Salehpour, S.; Hosseini, M.S.; Moghanjoughi, P.H. The Effects of Autologous Platelet-Rich Plasma in Repeated Implantation Failure: A Randomized Controlled Trial. Hum. Fertil. 2020, 23, 209–213. [Google Scholar] [CrossRef]
- Mehrafza, M.; Kabodmehri, R.; Nikpouri, Z.; Pourseify, G.; Raoufi, A.; Eftekhari, A.; Samadnia, S.; Hosseini, A. Comparing the Impact of Autologous Platelet-Rich Plasma and Granulocyte Colony Stimulating Factor on Pregnancy Outcome in Patients with Repeated Implantation Failure. J. Reprod. Infertil. 2019, 20, 35–41. [Google Scholar]
- Safdarian, L.; Aleyasin, A.; Aghahoseini, M.; Lak, P.; Mosa, S.H.; Sarvi, F.; Mahdavi, A. Efficacy of the Intrauterine Infusion of Platelet-Rich Plasma on Pregnancy Outcomes in Patients with Repeated Implantation Failure: A Randomized Control Trial. Int. J. Women’s Health Reprod. Sci. 2022, 10, 38–44. [Google Scholar] [CrossRef]
- Zamaniyan, M.; Peyvandi, S.; Gorji, H.H.; Moradi, S.; Jamal, J.; Aghmashhadi, F.Y.P.; Mohammadi, M.H. Effect of Platelet-Rich Plasma on Pregnancy Outcomes in Infertile Women with Recurrent Implantation Failure: A Randomized Controlled Trial. Gynecol. Endocrinol. 2021, 37, 141–145. [Google Scholar] [CrossRef]
- Noushin, M.A.; Ashraf, M.; Thunga, C.; Singh, S.; Singh, S.; Basheer, R.; Ashraf, R.; Jayaprakasan, K. A Comparative Evaluation of Subendometrial and Intrauterine Platelet-Rich Plasma Treatment for Women with Recurrent Implantation Failure. F & S Sci. 2021, 2, 295–302. [Google Scholar] [CrossRef]
- Arora, G.; Arora, S. Platelet-Rich Plasma-Where Do We Stand Today? A Critical Narrative Review and Analysis. Dermatol. Ther. 2021, 34, e14343. [Google Scholar] [CrossRef]
- Baba, K.; Yamazaki, Y.; Sone, Y.; Sugimoto, Y.; Moriyama, K.; Sugimoto, T.; Kumazawa, K.; Shimakura, Y.; Takeda, A. An In vitro Long-Term Study of Cryopreserved Umbilical Cord Blood-Derived Platelet-Rich Plasma Containing Growth Factors-Pdgf-Bb, Tgf-Β, and Vegf. J. Cranio-Maxillo-Facial Surg. 2019, 47, 668–675. [Google Scholar] [CrossRef]
- Lee, M.J.; Yoon, K.S.; Oh, S.; Shin, S.; Jo, C.H. Allogenic Pure Platelet-Rich Plasma Therapy for Adhesive Capsulitis: A Bed-to-Bench Study with Propensity Score Matching Using a Corticosteroid Control Group. Am. J. Sports Med. 2021, 49, 2309–2320. [Google Scholar] [CrossRef]
- Sun, Z.; Su, W.; Wang, L.; Cheng, Z.; Yang, F. Clinical Effect of Bushen Huoxue Method Combined with Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis and Its Effect on Il-1, Il-6, Vegf, and Pge-2. J. Healthc. Eng. 2022, 2022, 9491439. [Google Scholar] [CrossRef]
- Oneto, P.; Etulain, J. Prp in Wound Healing Applications. Platelets 2021, 32, 189–199. [Google Scholar] [CrossRef]
- Zhang, S.; Li, P.; Yuan, Z.; Tan, J. Platelet-Rich Plasma Improves Therapeutic Effects of Menstrual Blood-Derived Stromal Cells in Rat Model of Intrauterine Adhesion. Stem Cell Res. Ther. 2019, 10, 61. [Google Scholar] [CrossRef]
- Crumley, E.; Koufogiannakis, D. Developing Evidence-Based Librarianship: Practical Steps for Implementation. Health Inf. Libr. J. 2002, 19, 61–70. [Google Scholar] [CrossRef]
- Liu, K.E.; Hartman, M.; Hartman, A.; Luo, Z.C.; Mahutte, N. The Impact of a Thin Endometrial Lining on Fresh and Frozen-Thaw Ivf Outcomes: An Analysis of over 40,000 Embryo Transfers. Hum. Reprod. 2018, 33, 1883–1888. [Google Scholar] [CrossRef]
- Von Wolff, M.; Fäh, M.; Roumet, M.; Mitter, V.; Stute, P.; Griesinger, G.; Schwartz, A.K. Thin Endometrium Is Also Associated with Lower Clinical Pregnancy Rate in Unstimulated Menstrual Cycles: A Study Based on Natural Cycle Ivf. Front. Endocrinol. 2018, 9, 776. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Yoon, S.S.S. Clinical Outcomes in High-Risk Pregnancies Due to Advanced Maternal Age. J. Women’s Health 2021, 30, 160–167. [Google Scholar] [CrossRef]
- Attali, E.; Yogev, Y. The Impact of Advanced Maternal Age on Pregnancy Outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 70, 2–9. [Google Scholar] [CrossRef]
- Panagiotopoulou, N.; Karavolos, S.; Choudhary, M. Endometrial Injury Prior to Assisted Reproductive Techniques for Recurrent Implantation Failure: A Systematic Literature Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 193, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sar-Shalom Nahshon, C.; Sagi-Dain, L.; Wiener-Megnazi, Z.; Dirnfeld, M. The Impact of Intentional Endometrial Injury on Reproductive Outcomes: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2019, 25, 95–113. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Kang, Y.; Wang, Q.; Yan, L. Efficacy of Autologous Intrauterine Infusion of Platelet-Rich Plasma in Patients with Unexplained Repeated Implantation Failures in Embryo Transfer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6753. https://doi.org/10.3390/jcm11226753
Li M, Kang Y, Wang Q, Yan L. Efficacy of Autologous Intrauterine Infusion of Platelet-Rich Plasma in Patients with Unexplained Repeated Implantation Failures in Embryo Transfer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(22):6753. https://doi.org/10.3390/jcm11226753
Chicago/Turabian StyleLi, Muzi, Yan Kang, Qianfei Wang, and Lei Yan. 2022. "Efficacy of Autologous Intrauterine Infusion of Platelet-Rich Plasma in Patients with Unexplained Repeated Implantation Failures in Embryo Transfer: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 22: 6753. https://doi.org/10.3390/jcm11226753
APA StyleLi, M., Kang, Y., Wang, Q., & Yan, L. (2022). Efficacy of Autologous Intrauterine Infusion of Platelet-Rich Plasma in Patients with Unexplained Repeated Implantation Failures in Embryo Transfer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(22), 6753. https://doi.org/10.3390/jcm11226753