Placental Volume and Uterine Artery Doppler in Pregnancy Following In Vitro Fertilization: A Comprehensive Literature Review
Abstract
1. Introduction
2. Obstetric and Perinatal Outcomes Resulting from Ivf Pregnancies
2.1. The Role of Ovarian Stimulation
2.2. Differences between Fresh and Freeze and Thawed Embryo Transfer
2.3. Endometrial Preparation
3. Non-Invasive Parameters in the First Trimester of Placental Development In-Vitro Fertilization Pregnancies
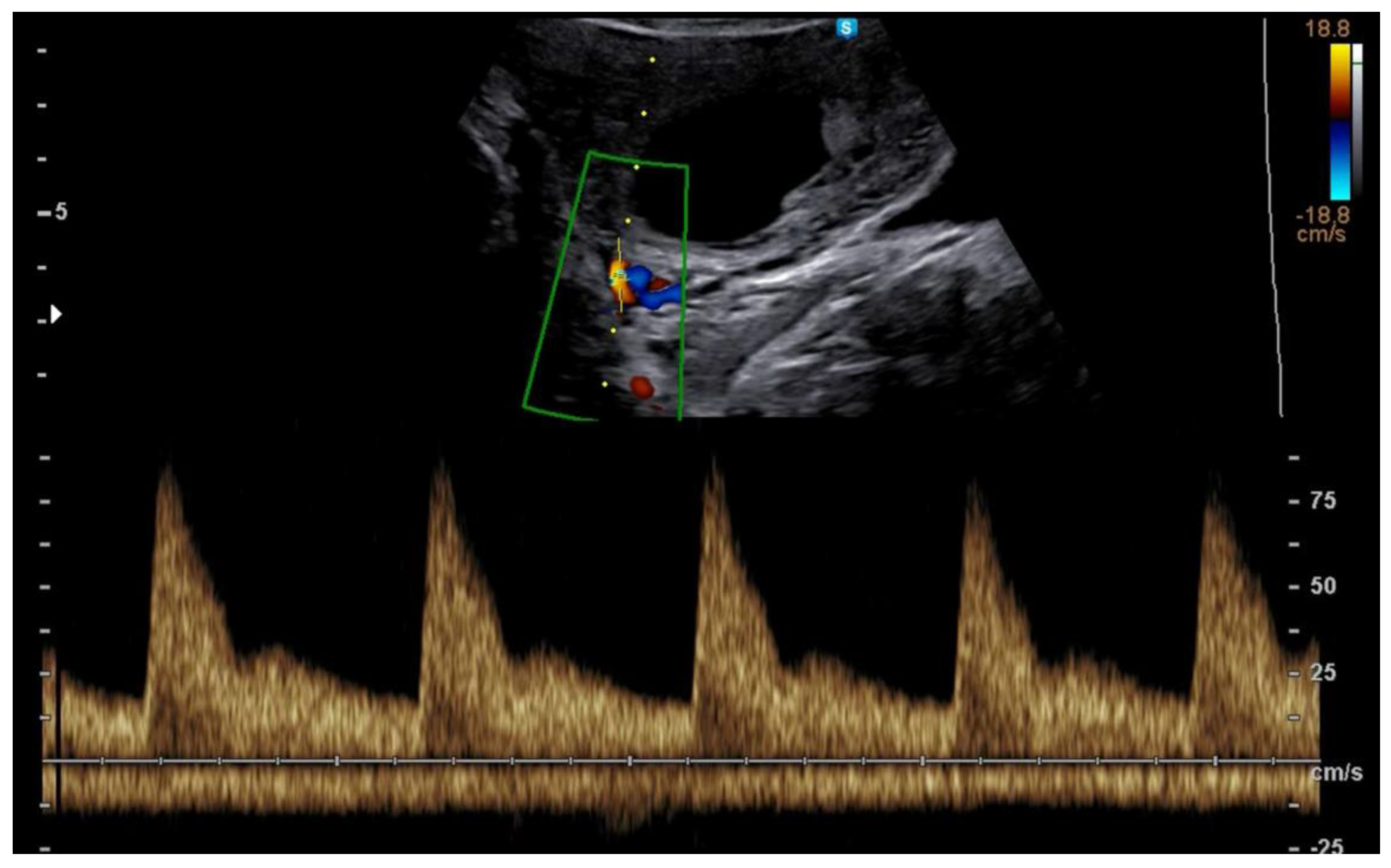
3.1. First Trimester Uterine Doppler
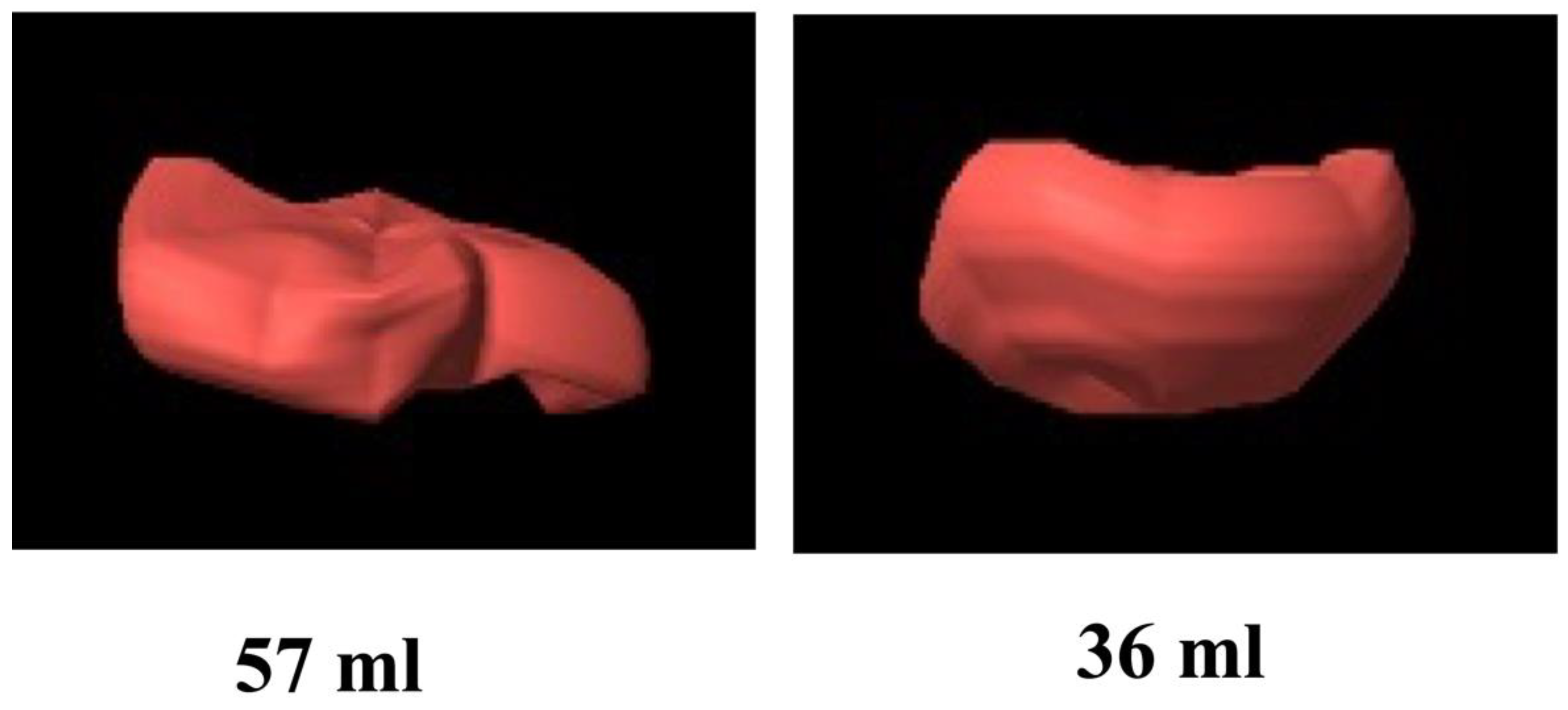
3.2. First Trimester 3D Placental Volume
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyer, S.; Chambers, G.M.; de Mouzon, J.; Nygren, K.G.; Zegers-Hochschild, F.; Mansour, R.; Ishihara, O.; Banker, M.; Adamson, G.D. International Committee for Monitoring Assisted Reproductive Technologies World Report: Assisted Reproductive Technology 2008, 2009 and 2010. Hum. Reprod. 2016, 31, 1588–1609. [Google Scholar] [CrossRef] [PubMed]
- Ministero Della Salute: Procreazione Medicalmente Assistita. Available online: https://www.salute.gov.it/portale/donna/dettaglioContenutiDonna.jsp?lingua=italiano&id=4570&area=Salute%20donna&menu=nascita (accessed on 16 September 2022).
- Choux, C.; Ginod, P.; Barberet, J.; Rousseau, T.; Bruno, C.; Sagot, P.; Astruc, K.; Fauque, P. Placental volume and other first-trimester outcomes: Are there differences between fresh embryo transfer, frozen-thawed embryo transfer and natural conception? Reprod. Biomed. Online 2019, 38, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Gibson, K.A.; Wu, Y.W.; Croughan, M.S. Perinatal outcomes in singletons following in vitro fertilization: A meta-analysis. Obstet. Gynecol. 2004, 103, 551–563. [Google Scholar] [CrossRef]
- McDonald, S.D.; Han, Z.; Mulla, S.; Murphy, K.E.; Beyene, J.; Ohlsson, A.; Knowledge Synthesis Group. Preterm birth and low birth weight among in vitro fertilization singletons: A systematic review and meta-analyses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 146, 138–148. [Google Scholar] [CrossRef]
- Saito, K.; Kuwahara, A.; Ishikawa, T.; Morisaki, N.; Miyado, M.; Miyado, K.; Fukami, M.; Miyasaka, N.; Ishihara, O.; Irahara, M.; et al. Endometrial preparation methods for frozen-thawed embryo transfer are associated with altered risks of hypertensive disorders of pregnancy, placenta accreta, and gestational diabetes mellitus. Hum. Reprod. 2019, 34, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, C.; Tsioufis, C.; Michalopoulou, H.; Makris, T.; Papademetriou, V.; Stefanadis, C. Assisted reproductive technology and pregnancy-related hypertensive complications: A systematic review. J. Hum. Hypertens. 2013, 27, 148–157. [Google Scholar] [CrossRef]
- Wong, K.M.; Wely, M.; van Mol, F.; Repping, S.; Mastenbroek, S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Libr. Cochrane Rev. 2017, 3, CD011184. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Obstetric and perinatal outcomes in singleton pregnancies resulting from the transfer of frozen thawed versus fresh embryos generated through in vitro fertilization treatment: A systematic review and meta-analysis. Fertil. Steril. 2012, 98, 368–377. [Google Scholar] [CrossRef]
- Sutcliffe, A.G.; Ludwig, M. Outcome of assisted reproduction. Lancet 2007, 370, 351–359. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar] [CrossRef]
- Choux, C.; Carmignac, V.; Bruno, C.; Sagot, P.; Vaiman, D.; Fauque, P. The placenta: Phenotypic and epigenetic modifications induced by Assisted Reproductive Technologies throughout pregnancy. Clin. Epigenetics 2015, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- van der Hoorn, M.L.; Lashley, E.E.; Bianchi, D.W.; Claas, F.H.; Schonkeren, C.M.; Scherjon, S.A. Clinical and immunologic aspects of egg donation pregnancies: A systematic review. Hum. Reprod. Update 2010, 16, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Effendi, M.; Demers, S.; Giguère, Y.; Forest, J.C.; Brassard, N.; Girard, M.; Gouin, K.; Bujold, E. Association between first-trimester placental volume and birth weight. Placenta 2014, 35, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, W.; González-Dávila, E.; González Lorenzo, A.; Armas-González, M.; Padrón, E.; González-González, N.L. First trimester placental volume and vascular indices in pregnancies complicated by preeclampsia. Prenat. Diagn. 2015, 35, 1247–1254. [Google Scholar] [CrossRef]
- Boss, A.L.; Chamley, L.W.; James, J.L. Placental formation in early pregnancy: How is the centre of the placenta made? Hum. Reprod. Update 2018, 24, 750–760. [Google Scholar] [CrossRef]
- Brosens, I. Placental bed & maternal—Fetal disorders. Preface. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 247–248. [Google Scholar]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- Turco, M.Y.; Moffett, A. Development of the human placenta. Development 2019, 146, dev163428. [Google Scholar] [CrossRef]
- Smith, G.C. First-trimester determination of complications of late pregnancy. JAMA 2010, 303, 561–562. [Google Scholar] [CrossRef]
- Steegers, E.A.; von Dadelszen, P.; Duvekot, J.J.; Pijnenborg, R. Pre-eclampsia. Lancet 2010, 376, 631–644. [Google Scholar] [CrossRef]
- Reijnders, I.F.; Mulders, A.G.M.G.J.; Koster, M.P.H. Placental development and function in women with a history of placenta-related complications: A systematic review. Acta Obstet. Et Gynecol. Scand. 2018, 97, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Shetty, A.; Hamilton, M.; Bhattacharya, S.; Maheshwari, A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: A systematic review and meta-analysis. Hum. Reprod. Update 2012, 18, 485–503. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists Committee on Obstetric Practice Society for Maternal-Fetal Medicine. Committee opinion no 671: Perinatal risks associated with assisted reproductive technology. Obstet. Gynecol. 2016, 128, e61–e68. [Google Scholar] [CrossRef] [PubMed]
- Kawwass, J.F.; Badell, M.L. Maternal and fetal risk associated with assisted reproductive technology. Obstet. Gynecol. 2018, 132, 763–772. [Google Scholar] [CrossRef]
- Woo, I.; Hindoyan, R.; Landay, M.; Ho, J.; Ingles, S.A.; McGinnis, L.K.; Paulson, R.J.; Chung, K. Perinatal outcomes after natural conception versus in vitro fertilization (IVF) in gestational surrogates: A model to evaluate IVF treatment versus maternal effects. Fertil. Steri. 2017, 108, 993–998. [Google Scholar] [CrossRef]
- Palomba, S.; Homburg, R.; Santagni, S.; La Sala, G.B.; Orvieto, R. Risk of adverse pregnancy and perinatal outcomes after high technology infertility treatment: A comprehensive systematic review. Reprod. Biol. Endocrinol. 2016, 14, 76. [Google Scholar] [CrossRef]
- Fitzpatrick, K.E.; Tuffnell, D.; Kurinczuk, J.J.; Knight, M. Pregnancy at very advanced maternal age: A UK population-based cohort study. BJOG 2017, 124, 1097–1106. [Google Scholar] [CrossRef]
- Sandovici, I.; Hoelle, K.; Angiolini, E.; Constância, M. Placental adaptations to the maternal-fetal environment: Implications for fetal growth and developmental programming. Reprod. Biomed. Online 2012, 25, 68–89. [Google Scholar] [CrossRef]
- Qin, J.; Liu, X.; Sheng, X.; Wang, H.; Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancyoutcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil. Steril. 2016, 105, 73–85. [Google Scholar] [CrossRef]
- Chen, Z.J.; Shi, Y.; Sun, Y.; Zhang, B.; Liang, X.; Cao, Y.; Yang, J.; Liu, J.; Wei, D.; Weng, N.; et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2016, 375, 523–533. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Hudson, C.; Thomas, S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective rando—Mized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil. Steril. 2011, 96, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Badell, M.L.; Kawwass, J.F. The impact of endometrial preparation for frozen embryo transfer on maternal and neonatal outcomes: A review. Reprod. Biol. Endocrinol. 2022, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Kansal Kalra, S.; Ratcliffe, S.J.; Milman, L.; Gracia, C.R.; Coutifaris, C.; Barnhart, K.T. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil. Steril. 2011, 95, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Pinborg, A.; Henningsen, A.A.; Loft, A.; Malchau, S.S.; Forman, J.; Andersen, A.N. Large baby syndrome in singletons born after frozen embryo transfer (FET): Is it due to maternal factors or the cryotechnique? Hum. Reprod. 2014, 29, 618–627. [Google Scholar] [CrossRef]
- Maheshwari, A.; Pandey, S.; Raja, E.A.; Shetty, A.; Hamilton, M.; Bhattacharya, S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum. Reprod. Update 2018, 24, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Haahr, T.; Geber, S.; Esteves, S.C.; Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: A systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update. 2019, 25, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Ghobara, T.; Gelbaya, T.A.; Ayeleke, R.O. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst. Rev. 2017, 7, CD003414. [Google Scholar] [CrossRef]
- Ginstrom Ernstad, E.; Wennerholm, U.B.; Khatibi, A.; Petzold, M.; Bergh, C. Neonatal and maternal outcome after frozen embryotransfer: Increased risks in programmed cycles. Am. J. Obstet. Gynecol. 2019, 221, 126.e1–126.e18. [Google Scholar] [CrossRef]
- Conrad, K.P.; Baker, V.L. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductivetechnologies. Am. J. Phys. Regul. Integr. Comp. Phys. 2013, 304, R69–R72. [Google Scholar]
- von Versen-Höynck, F.; Schaub, A.M.; Chi, Y.Y.; Chiu, K.H.; Liu, J.; Lingis, M.; Stan Williams, R.; Rhoton-Vlasak, A.; Nichols, W.W.; Fleischmann, R.R.; et al. Increased Preeclampsia Risk and Reduced Aortic Compliance With In Vitro Fertilization Cycles in the Absence of a Corpus Luteum. Hypertension 2019, 73, 640–649. [Google Scholar] [CrossRef]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update. 2017, 23, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Li, S.; Zheng, L.; Gu, J.; Li, T.; Du, H.; Gao, C.; Ding, C.; Quan, S.; Zhou, C.; et al. Perinatal outcomes of singletons following vitrification versus slow-freezing of embryos: A multicenter cohort study using propensity score analysis. Hum. Reprod. 2019, 34, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The ‘Great Obstetrical Syndromes’ are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2010, 25, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Pietrolucci, M.E.; Mappa, I.; Bitsadze, V.; Khizroeva, J.; Makatsariya, A.; D’Antonio, F. Modeling Pulsatility Index nomograms from different maternal and fetal vessels by quantile regression at 24–40 weeks of gestation: A prospective cross-sectional study. J. Matern. Fetal. Neonatal. Med. 2022, 35, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, W.; Maiz, N.; Bonino, S.; Kaihura, C.; Nicolaides, K. Uterine artery Doppler at 11+0 to 13+6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2007, 30, 742–749. [Google Scholar] [CrossRef]
- Velauthar, L.; Plana, M.N.; Kalidindi, M.; Zamora, J.; Thilaganathan, B.; Illanes, S.E.; Khan, K.S.; Aquilina, J.; Thangaratinam, S. First-trimester uterine artery Doppler and adverse pregnancy outcome: A meta-analysis involving 55974 women. Ultrasound Obstet. Gynecol. 2014, 43, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Capponi, A.; Cavicchioni, O.; Vendola, M.; Arduini, D. First trimester uterine Doppler and three-dimensional ultrasound placental volume calculation in predicting preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 147–151. [Google Scholar]
- Carbone, I.F.; Cruz, J.J.; Sarquis, R.; Akolekar, R.; Nicolaides, K.H. Assisted conception and placental perfusion assessed by uterine artery Doppler at 11–13 weeks’ gestation. Hum. Reprod. 2011, 26, 1659–1664. [Google Scholar]
- Prefumo, F.; Fratelli, N.; Soares, S.C.; Thilaganathan, B. Uterine artery Doppler velocimetry at 11–14 weeks in singleton pregnancies conceived by assisted reproductive technology. Ultrasound Obstet. Gynecol. 2007, 29, 141–145. [Google Scholar] [CrossRef]
- Rizzo, G.; Aiello, E.; Pietrolucci, M.E.; Arduini, D. Are There Differences in Placental Volume and Uterine Artery Doppler in Pregnancies Resulting From the Transfer of Fresh Versus Frozen-Thawed Embryos Through In Vitro Fertilization. Reprod. Sci 2016, 23, 1381–1386. [Google Scholar] [CrossRef]
- Nelissen, E.C.; Dumoulin, J.C.; Busato, F.; Ponger, L.; Eijssen, L.M.; Evers, J.L.; Tost, J.; van Montfoort, A.P. Altered gene expression in human placentas after IVF/ICSI. Hum. Reprod. 2014, 29, 2821–2831. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gundogan, F.; Bianchi, D.W.; Scherjon, S.A.; Roberts, D.J. Placental pathology in egg donor pregnancies. Fertil. Steril. 2010, 93, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.I.; Farina, A.; Gaeta, G.; Sigismondi, C.; Spinillo, S.; Casiero, D.; Pozzoni, M.; Vigano, P.; Papaleo, E.; Candiani, M. Uterine artery Doppler in singleton pregnancies conceived after in-vitro fertilization or intracytoplasmic sperm injection with fresh vs. frozen blastocyst transfer: Longitudinal cohort study. Ultrasound Obstet. Gynecol. 2020, 56, 603–610. [Google Scholar] [CrossRef] [PubMed]
- van Duijn, L.; Rousian, M.; Reijnders, I.F.; Willemsen, S.P.; Baart, E.B.; Laven, J.S.E.; Steegers-Theunissen, R.P.M. The influence of frozen-thawed and fresh embryo transfer on utero-placental (vascular) development: The Rotterdam Periconception cohort. Hum. Reprod. 2021, 36, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.; Lehmann, H.; Mantovani, E.; Thilaganathan, B.; Khalil, A. Correlation between central and uterine hemodynamics in hypertensive disorders of pregnancy. Ultrasound Obstet. Gynecol. 2019, 54, 58–63. [Google Scholar] [CrossRef]
- Arakaki, T.; Hasegawa, J.; Nakamura, M.; Hamada, S.; Muramoto, M.; Takita, H.; Ichizuka, K.; Sekizawa, A. Prediction of early- and late- onset pregnancy-induced hypertension using placental volume on three-dimensional ultrasound and uterine artery Doppler. Ultrasound Obstet. Gyneco. 2015, 45, 539–543. [Google Scholar] [CrossRef]
- Schuchter, K.; Metzenbauer, M.; Hafner, E.; Philipp, K. Uterine artery Doppler and placental volume in the first trimester in the prediction of pregnancy complications. Ultrasound Obstet. Gynecol. 2001, 18, 590–592. [Google Scholar] [CrossRef]
- Papastefanou, I.; Chrelias, C.; Siristatidis, C.; Kappou, D.; Eleftheriades, M.; Kassanos, D. Placental volume at 11 to 14 gestational weeks in pregnancies complicated with fetal growth restriction and preeclampsia. Prenat. Diagn. 2018, 38, 928–935. [Google Scholar] [CrossRef]
- Rifouna, M.S.; Reus, A.D.; Koning, A.H.; van der Spek, P.J.; Exalto, N.; Steegers, E.A.; Laven, J.S. First trimester trophoblast and placental bed vascular volume measurements in IVF or IVF/ICSI pregnancies. Hum. Reprod. 2014, 29, 2644–2649. [Google Scholar] [CrossRef]
- Rizzo, G.; Aiello, E.; Pietrolucci, M.E.; Arduini, D. Placental volume and uterine artery Doppler evaluation at 11 + 0 to 13 + 6 weeks’ gestation in pregnancies conceived with in-vitro fertilization: Comparison between autologous and donor oocyte recipients. Ultrasound Obstet. Gynecol. 2016, 47, 726–731. [Google Scholar] [CrossRef]
- Churchill, S.J.; Wang, E.T.; Akhlaghpour, M.; Goldstein, E.H.; Eschevarria, D.; Greene, N.; Macer, M.; Zore, T.; Williams, J., 3rd; Pisarska, M.D. Mode of conception does not appear to affect placental volume in the first trimester. Fertil. Steril. 2017, 107, 1341–1347.e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sundheimer, L.W.; Chan, J.L.; Buttle, R.; DiPentino, R.; Muramoto, O.; Castellano, K.; Wang, E.T.; Williams, J., 3rd; Pisarska, M.D. Mode of conception does not affect fetal or placental growth parameters or ratios in early gestation or at delivery. J. Assist. Reprod. Genet. 2018, 35, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Lacconi, V.; Rizzo, G.; De Lorenzo, A.; Massimiani, M. Placental Dysfunction in Assisted Reproductive Pregnancies: Perinatal, Neonatal and Adult Life Outcomes. Int. J. Mol. Sci. 2022, 23, 659. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resta, S.; Scandella, G.; Mappa, I.; Pietrolucci, M.E.; Maqina, P.; Rizzo, G. Placental Volume and Uterine Artery Doppler in Pregnancy Following In Vitro Fertilization: A Comprehensive Literature Review. J. Clin. Med. 2022, 11, 5793. https://doi.org/10.3390/jcm11195793
Resta S, Scandella G, Mappa I, Pietrolucci ME, Maqina P, Rizzo G. Placental Volume and Uterine Artery Doppler in Pregnancy Following In Vitro Fertilization: A Comprehensive Literature Review. Journal of Clinical Medicine. 2022; 11(19):5793. https://doi.org/10.3390/jcm11195793
Chicago/Turabian StyleResta, Serena, Gaia Scandella, Ilenia Mappa, Maria Elena Pietrolucci, Pavjola Maqina, and Giuseppe Rizzo. 2022. "Placental Volume and Uterine Artery Doppler in Pregnancy Following In Vitro Fertilization: A Comprehensive Literature Review" Journal of Clinical Medicine 11, no. 19: 5793. https://doi.org/10.3390/jcm11195793
APA StyleResta, S., Scandella, G., Mappa, I., Pietrolucci, M. E., Maqina, P., & Rizzo, G. (2022). Placental Volume and Uterine Artery Doppler in Pregnancy Following In Vitro Fertilization: A Comprehensive Literature Review. Journal of Clinical Medicine, 11(19), 5793. https://doi.org/10.3390/jcm11195793






