Long-Term Follow-Up of Macular Perfusion Evaluated by Optical Coherence Tomography Angiography after Rhegmatogenous Retinal Detachment Surgery
Abstract
1. Introduction
2. Materials and Methods
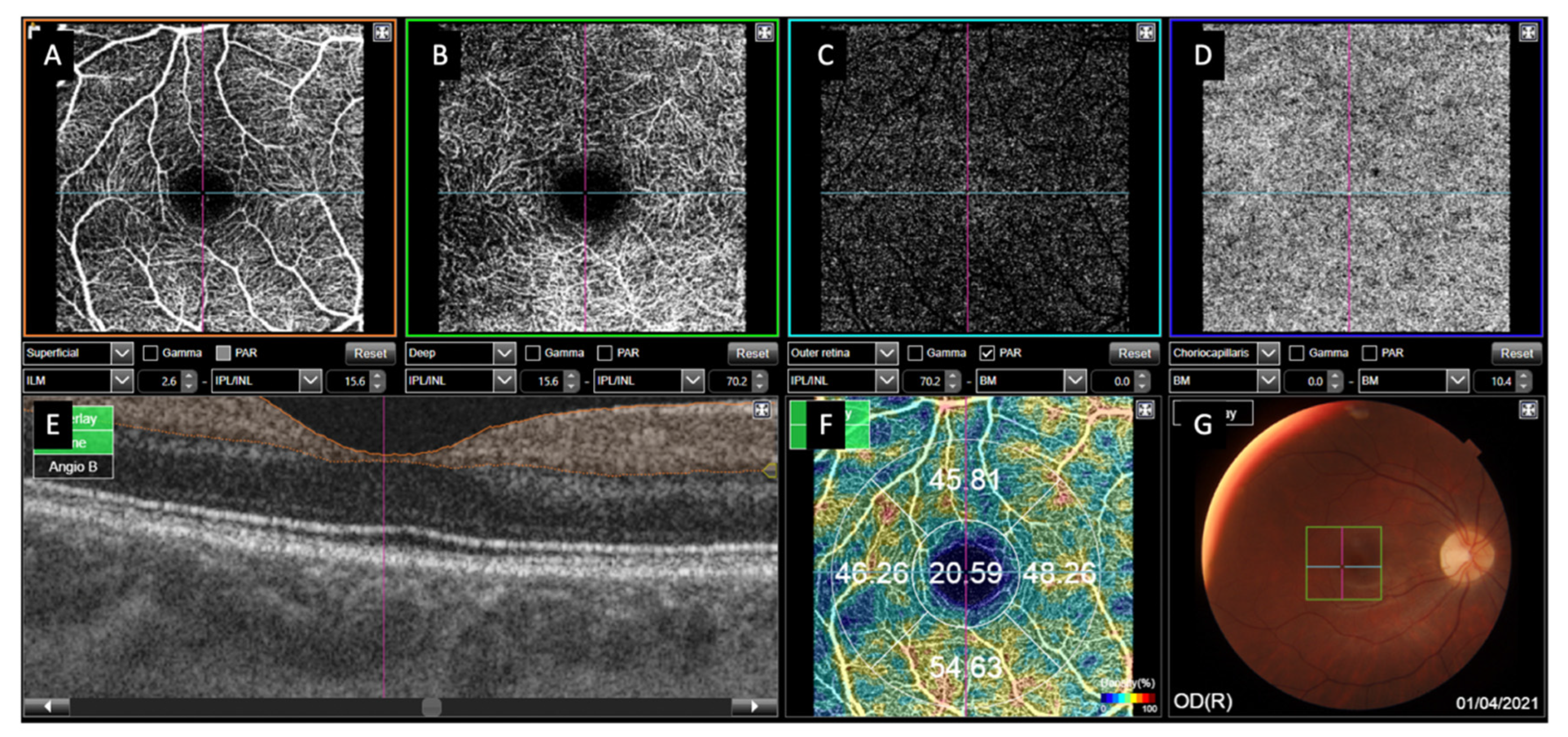
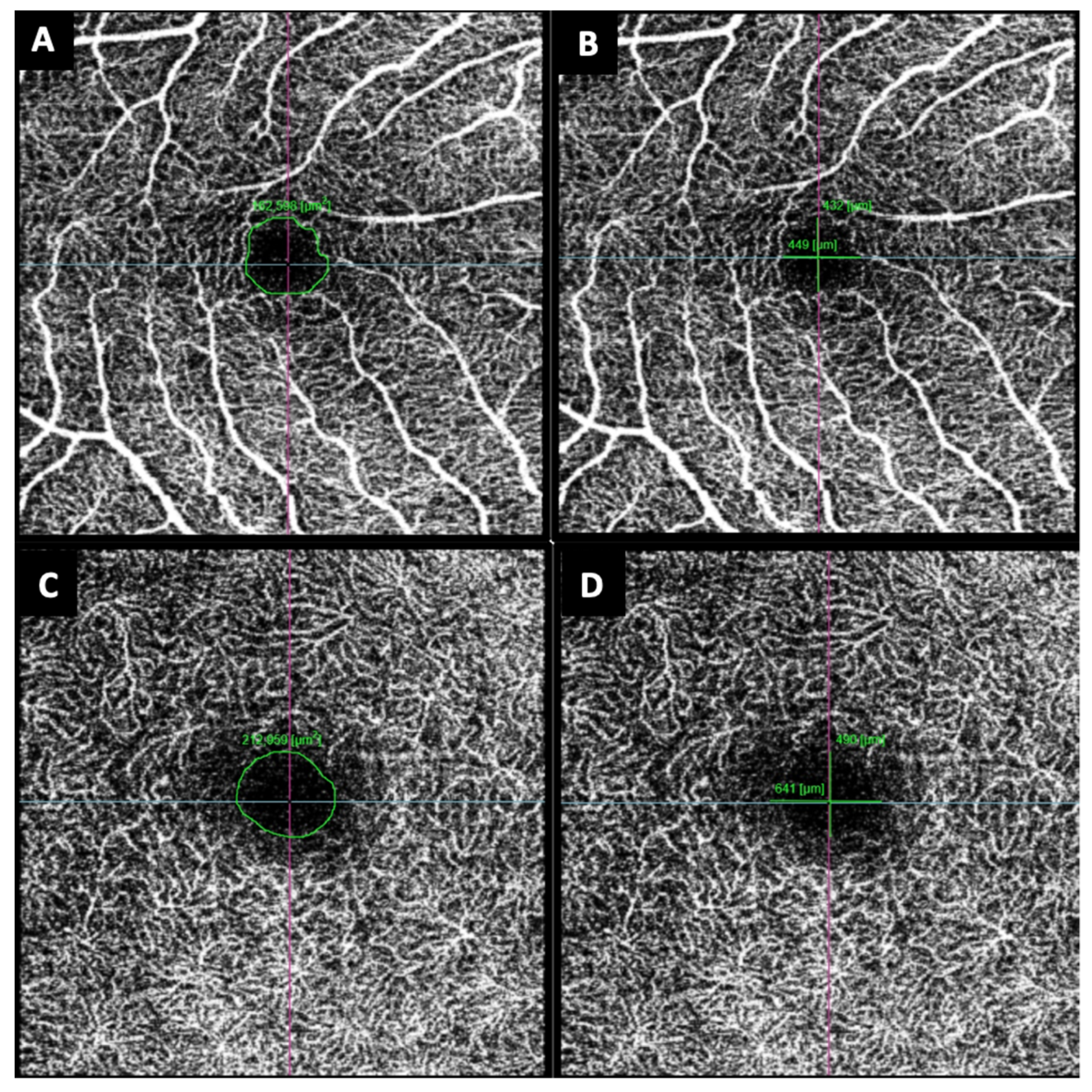
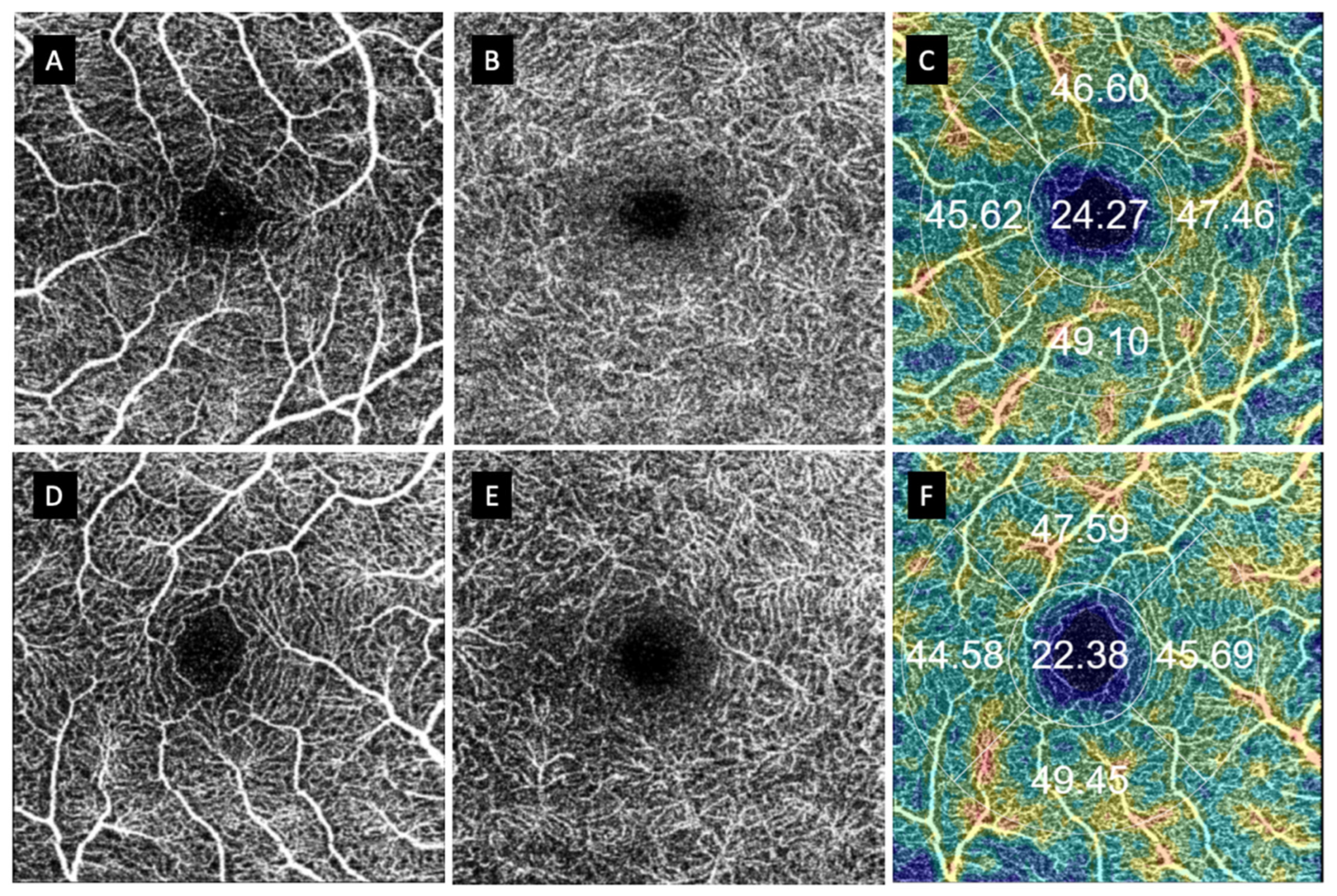
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bechrakis, N.E.; Dimmer, A. Rhegmatogenous retinal detachment: Epidemiology and risk factors. Der Ophthalmol. Z. Der Dtsch. Ophthalmol. Ges. 2018, 115, 163–178. [Google Scholar]
- Rim, T.H.; Kim, S.H.; Lim, K.H.; Choi, M.; Kim, H.Y.; Baek, S.H. Refractive Errors in Koreans: The Korea National Health and Nutrition Examination Survey 2008–2012. Korean J. Ophthalmol. 2016, 30, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, S.S.; Mikkelsen, K.L.; La Cour, M. Risk of pseudophakic retinal detachment in 202,226 patients using the fellow nonoperated eye as reference. Ophthalmology 2013, 120, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Daien, V.; Korobelnik, J.F.; Delcourt, C. French Medical-Administrative Database for Epidemiology and Safety in Ophthalmology (EPISAFE): The EPISAFE Collaboration Program in Cataract Surgery. Ophthalmic Res. 2017, 58, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Salicone, A.; Smiddy, W.E.; Venkatraman, A.; Feuer, W. Visual recovery after scleral buckling procedure for retinal detachment. Ophthalmology 2006, 113, 1734–1742. [Google Scholar] [CrossRef]
- Nork, T.M. Selective Loss of Blue Cones and Rods in Human Retinal Detachment. Arch. Ophthalmol. 1995, 113, 1066. [Google Scholar] [CrossRef]
- Delolme, M.P.; Dugas, B.; Nicot, F.; Muselier, A.; Bron, A.M.; Creuzot-Garcher, C. Anatomical and functional macular changes after rhegmatogenous retinal detachment with macula off. Am. J. Ophthalmol. 2012, 153, 128–136. [Google Scholar] [CrossRef]
- Wolfensberger, T.J.; Gonvers, M. Optical coherence tomography in the evaluation of incomplete visual acuity recovery after macula-off retinal detachments. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 85–89. [Google Scholar] [CrossRef]
- Benson, S.E.; Schlottmann, P.G.; Bunce, C.; Xing, W.; Charteris, D.G. Optical Coherence Tomography Analysis of the Macula after Scleral Buckle Surgery for Retinal Detachment. Ophthalmology 2007, 114, 108–112. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical coherence tomography angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Boned-Murillo, A.; Albertos-Arranz, H.; Diaz-Barreda, M.D.; Orduna-Hospital, E.; Sánchez-Cano, A.; Ferreras, A.; Cuenca, N.; Pinilla, I. Optical Coherence Tomography Angiography in Diabetic Patients: A Systematic Review. Biomedicines 2021, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Kutsyr, O.; Albertos-Arranz, H.; Ferández-Sánchez, L.; Martínez-Gil, N.; Noailles, A.; López-Garrido, J.A.; López-Gálvez, M.; et al. Interpretation of OCT and OCTA images from a histological approach: Clinical and experimental implications. Prog. Retin. Eye Res. 2020, 77, 100828. [Google Scholar] [CrossRef] [PubMed]
- Samara, W.A.; Decir, A.T.E.; Khoo, C.T.L.; Higgins, T.P.; Magrath, J.; Ferenczy, S.; Escudos, C. Correlation of Foveal Avascular Zone Size With Foveal Morphology in Normal Eyes Using Optical Coherence Tomography Angiography. Retina 2015, 35, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Dursun, M.E.; Erdem, S.; Karahan, M.; Ava, S.; Hazar, L.; Dursun, B.; Keklikci, U. Evaluation of parafoveal vascular density using optical coherence tomography angiography in patients with central serous chorioretinopathy. Lasers Med. Sci. 2022, 37, 1147–1154. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, Y.; Liao, R. Evaluation of macular microvasculature and foveal avascular zone in patients with retinal vein occlusion using optical coherence tomography angiography. Int. Ophthalmol. 2022, 42, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Murueta-Goyena, A.; Barrenechea, M.; Erramuzpe, A.; Teijeira-Portas, S.; Pengo, M.; Ayala, U.; Romero-Bascones, D.; Acera, M.; Del-Pino, R.; Gómez-Esteban, J.C.; et al. Foveal Remodeling of Retinal Microvasculature in Parkinson’s Disease. Front. Neurosci. 2021, 15, 708700. [Google Scholar] [CrossRef]
- Aly, L.; Strauß, E.M.; Feucht, N.; Weiß, I.; Berthele, A.; Mitsdoerffer, M.; Haass, C.; Hemmer, B.; Maier, M.; Korn, T.; et al. Optical coherence tomography angiography indicates subclinical retinal disease in neuromyelitis optica spectrum disorders. Mult. Scler. 2022, 28, 522–531. [Google Scholar] [CrossRef]
- Monteiro-Henriques, I.; Rocha-Sousa, A.; Barbosa-Breda, J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta Ophthalmologica 2022, 100, e1–e15. [Google Scholar] [CrossRef]
- Díaz-Barreda, M.D.; Bartolomé-Sesé, I.; Boned-Murillo, A.; Ferreras, A.; Orduna-Hospital, E.; Ascaso, J.; Pinilla, I. Microperimetry-Assessed Functional Alterations and OCT-Changes in Patients after Retinal Detachment Surgery Using Pars Plana Vitrectomy and SF6 Tamponade. Diagnostics 2021, 11, 1157. [Google Scholar] [CrossRef]
- Chen, F.K.; Menghini, M.; Hansen, A.; Mackey, D.A.; Constable, I.J.; Sampson, D.M. Intrasession Repeatability and Interocular Symmetry of Foveal Avascular Zone and Retinal Vessel Density in OCT Angiography. Trans. Vis. Sci. Tech. 2018, 7, 6. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Shoji, T.; Kanno, J.; Ibuki, H.; Ozaki, K.; Ishii, H.; Ichikawa, Y.; Kimura, I.; Shinoda, K. Evaluation of microvascular changes in the macular area of eyes with rhegmatogenous retinal detachment without macular involvement using swept-source optical coherence tomography angiography. Clin. Ophthalmol. 2018, 12, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.M.; Yoon, Y.S.; Woo, J.E.; Min, J.K. Foveal Avascular Zone Area Changes Analyzed Using OCT Angiography after Successful Rhegmatogenous Retinal Detachment Repair. Curr. Eye Res. 2018, 43, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Resch, M.D.; Balogh, A.; Lászik, G.; Nagy, Z.Z.; Papp, A. Association between retinal vessel density and postoperative time after primary repair of rhegmatogenous retinal detachment. PLoS ONE 2021, 16, e0258126. [Google Scholar] [CrossRef] [PubMed]
- Barca, F.; Bacherini, D.; Dragotto, F.; Tártaro, R.; Lenzetti, C.; Finocchio, L.; Virgili, G.; Caporossi, T.; Giansanti, F.; Savastano, A.; et al. OCT Angiography Findings in Macula-ON and Macula-OFF Rhegmatogenous Retinal Detachment: A Prospective Study. J. Clin. Med. 2020, 9, 3982. [Google Scholar] [CrossRef]
- Bonfiglio, V.; Ortisi, E.; Scollo, D.; Reibaldi, M.; Ruso, A.; Pizzo, A.; Faro, G.; Macchi, I.; Fallico, M.; Toro, M.; et al. Vascular changes after vitrectomy for rhegmatogenous retinal detachment: Optical coherence tomography angiography study. Acta Ophthalmol. 2020, 98, e563–e569. [Google Scholar] [CrossRef]
- Nam, S.H.; Kim, K.; Kim, E.S.; Kim, D.G.; Yu, S.Y. Longitudinal Microvascular Changes on Optical Coherence Tomographic Angiography after Macula-Off Rhegmatogenous Retinal Detachment Repair Surgery. Ophthalmologica 2020, 244, 34–41. [Google Scholar] [CrossRef]
- Tsen, C.L.; Sheu, S.J.; Chen, S.C.; Wu, T.T. Imaging analysis with optical coherence tomography angiography after primary repair of macula-off rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1847–1855. [Google Scholar] [CrossRef]
- Piccolino, C. Vascular Changes in Rhegmatogenous Retinal Detachment. Ophthalmologica 1983, 86, 17–24. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, P.; Liu, H.; Jia, H.; Yu, Y.; Wang, F.; Wang, H.; Sol, X. Peripapillary Vessel Density in Eyes with Rhegmatogenous Retinal Detachment after Pars Plana Vitrectomy. J. Ophthalmol. 2021, 2021, 6621820. [Google Scholar] [CrossRef]
- Agarwal, A.; Aggarwal, K.; Akella, M.; Agrawal, R.; Khandelwal, N.; Bansal, R.; Sinh, R.; Gupta, V. Fractal Dimension and Optical Coherence Tomography Angiography Features of the Central Macula After Repair of Rhegmatogenous Retinal Detachments. Retina 2019, 39, 2167–2177. [Google Scholar] [CrossRef]
- Matthew-Mckay, K.; Vingopoulos, F.; Wang, J.C.; Papakostas, T.D.; Silverman, R.F.; Marmalidou, A.; Lains, I.; Elliot, D.; Vvvas, D.G.; Kim, L.; et al. Retinal Microvasculature Changes After Repair of Macula-off Retinal Detachment Assessed with Optical Coherence Tomography Angiography. Clin. Ophthalmol. 2020, 14, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Hassanpoor, N.; Milani, A.; Kordestani, A.; Niyousha, M. Analysis of retinal layers’ thickness and vascular density after successful scleral buckle surgery. J. Curr. Ophthalmol. 2021, 33, 304. [Google Scholar] [PubMed]
- Wang, H.; Xu, X.; Sun, X.; Ma, Y.; Sun, T. Macular perfusion changes assessed with optical coherence tomography angiography after vitrectomy for rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya-Yaprak, A.; Küçük, M.F.; Yaprak, L.; Erol, M.K. Change in retinal and choroidal microvascular structures after rhegmatogenous retinal detachment surgery and effects on visual recovery. J. Français d’Ophtalmologie 2021, 44, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Krawitz, B.D.; Mo, S.; Geyman, L.S.; Agemy, S.A.; Scripsema, S.K.; García, P.M.; Chui, T.Y.P.; Rosen, R.B. Acircularity index and axis ratio of the foveal avascular zone in diabetic eyes and healthy controls measured by optical coherence tomography angiography. Vis. Res. 2017, 139, 177–186. [Google Scholar] [CrossRef]
- Hirata, A.; Nakada, H.; Mine, K.; Masumoto, M.; Sato, T.; Hayashi, K. Relationship between the morphology of the foveal avascular zone and the degree of aniseikonia before and after vitrectomy in patients with unilateral epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 507–515. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.Y.; Lee, S.Y.; Jeong, J.H.; Lee, K.E. Foveal Microvascular Structures in eyes with Silicone oil tamponade for Rhegmatogenous Retinal Detachment: A Swept-source optical coherence tomography Angiography Study. Sci. Rep. 2020, 10, 2555. [Google Scholar] [CrossRef]
- Ya, M.; Qing, Z.X.; Yan, P.X.; Ya, M.; Qing, Z.X.; Yan, P.X. Macular Perfusion Changes and Ganglion Cell Complex Loss in Patients with Silicone Oil-related Visual Loss. Biomed. Environ. Sci. 2020, 33, 151–157. [Google Scholar]
- Xiang, W.; Wei, Y.; Chi, W.; Zhang, Z.; Zhong, L.; Liu, R.J.; Zhang, S. Effect of silicone oil on macular capillary vessel density and thickness. Exp. Ther. Med. 2020, 19, 729–734. [Google Scholar] [CrossRef]
- Bayraktar, Z.; Pehlivanoglu, S.; Hagverdiyeva, S.; Albayrak, S.; Karakaya, M.; Bayraktar, Ş. Longitudinal evaluation of retinal thickness and OCTA parameters before and following silicone oil removal in eyes with macula-on and macula-off retinal detachments. Int. Ophthalmol. 2022, 42, 1963–1973. [Google Scholar] [CrossRef]
- Maqsood, S.; Elalfy, M.; Abdou-Hannon, A.; Hegazy, S.M.; Elborgy, E.S. Functional and Structural Outcomes at the Foveal Avascular Zone with Optical Coherence Tomography Following Macula off Retinal Detachment Repair. Clin. Ophthalmol. 2020, 14, 3261–3270. [Google Scholar] [CrossRef] [PubMed]

| Sex | Male | Female | |
|---|---|---|---|
| n = 57 | 42 (73.7%) | 15 (26.3%) | |
| Studied RRD eye | Right eye | Left eye | |
| n = 60 | 26 (43.3%) | 34 (56.7%) | |
| Mean age ± SD (years) | 59.14 ± 9.52 | ||
| Refractive error (D) | −2.89 ± 4.01 | ||
| Axial length (mm) | 25.74 ± 3.45 | ||
| Duration of symptoms (days) | 4.75 ± 2.55 | ||
| Lens status (%) | Phakic | Pseudophakic | |
| 40 (66.7%) | 20 (33.3%) | ||
| Macula-on | Macula-off | Fellow eye | |
| VA (log MAR) | 0.143 ± 0.25 | 0.203 ± 0.16 | 0.063 ± 0.14 |
| Refractive error | −2.34 ± 3.01 | −4.37 ± 5.18 | −2.18 ± 0.49 |
| Axial length (mm) | 25.43 ± 1.48 | 26.03 ± 4.35 | 25.70 ± 3.48 |
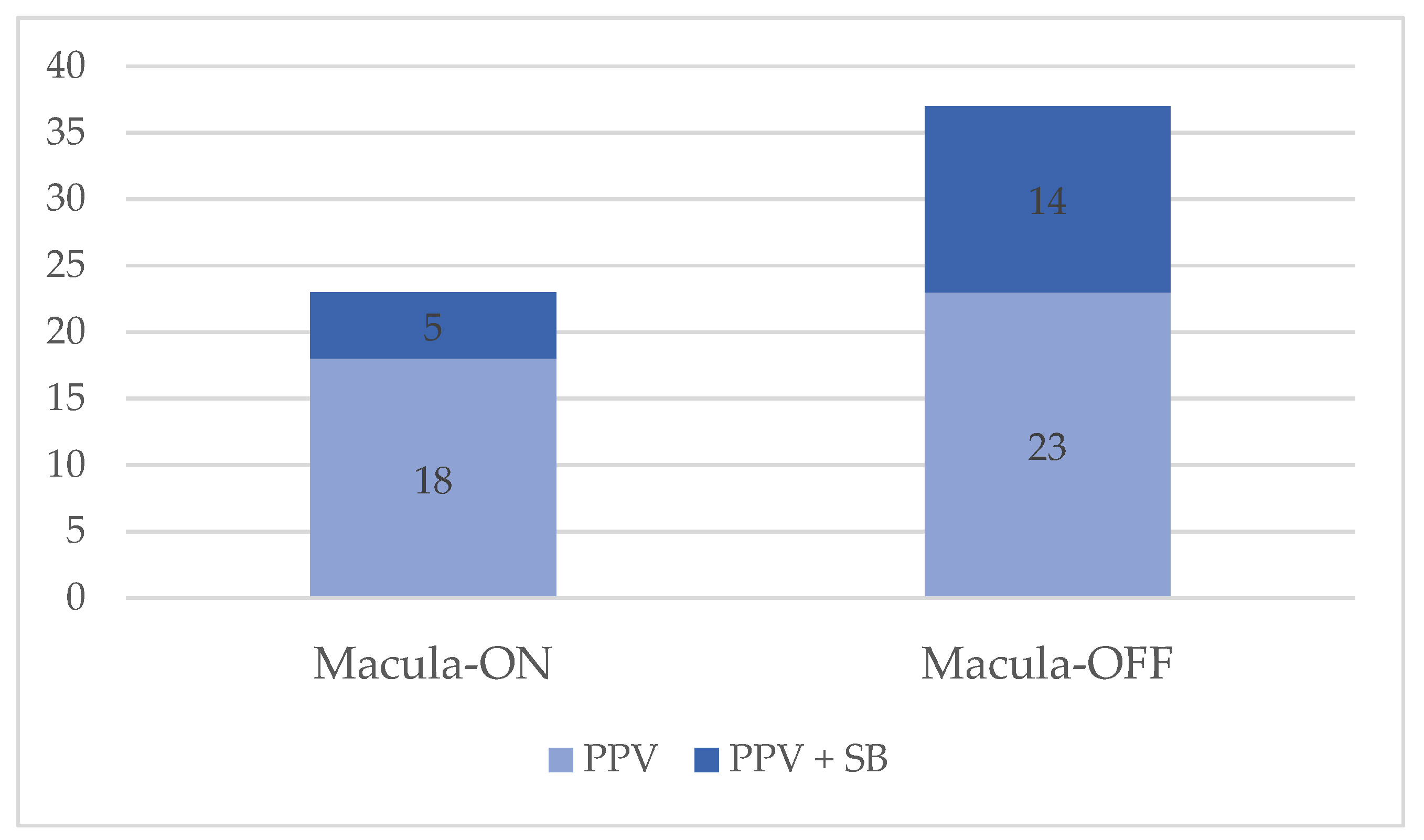
| Macula-On RRD PPV (n = 18) | Macula-On RRD PPV +SB (n = 5) | Macula-Off RRD PPV (n = 23) | Macula-Off RRD PPV + SB (n = 14) | Fellow Eye (n = 52) | P1 (ON PPV vs. PPV + SB) | P2 (ON PPV vs. Fellow Eye) | P3 (ON PPV + SB vs. Fellow Eye) | P4 (OFF PPV vs. PPV + SB) | P5 (OFF PPV vs. Fellow Eye) | P6 (OFF PPV + SB vs. Fellow Eye) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean central VD | |||||||||||
| SCP | 20.57 ± 3.69 | 20.94 ± 2.47 | 22.94 ± 4.48 | 26.73 ± 7.9 | 21.08 ± 4.74 | 0.419 | 0.685 | 0.950 | 0.070 | 0.116 | 0.001 |
| DCP | 24.23 ± 5.91 | 21.34 ± 2.26 | 26.44 ± 5.92 | 29.57 ± 9.76 | 21.25 ± 5.65 | 0.304 | 0.067 | 0.973 | 0.230 | <0.001 | <0.01 |
| CC | 48.77 ± 3.27 | 51.12 ± 3.03 | 48.26 ± 4.89 | 47.98 ± 4.33 | 49.58 ± 3.55 | 0.164 | 0.401 | 0.351 | 0.860 | 0.194 | 0.159 |
| Mean superior VD | |||||||||||
| SCP | 48.16 ± 3.89 | 47.39 ± 3.33 | 46.39 ± 4.26 | 45.18 ± 4.66 | 49.3 ± 4.01 | 0.345 | 0.782 | 0.309 | 0.421 | 0.006 | 0.002 |
| DCP | 50.49 ± 4.85 | 49.27 ± 3.38 | 48.01 ± 3.99 | 48.10 ± 3.91 | 51.06 ± 4.21 | 0.484 | 0.981 | 0.364 | 0.951 | 0.005 | 0.021 |
| CC | 51.26 ± 2.35 | 50.79 ± 1.84 | 51.13 ± 3.67 | 52.22 ± 2.52 | 52.38 ± 2.75 | 0.341 | 0.130 | 0.212 | 0.339 | 0.109 | 0.843 |
| Mean temporal VD | |||||||||||
| SCP | 47.18 ± 4.19 | 46.07 ± 1.33 | 46.24 ± 2.98 | 45.28 ± 3.03 | 47.04 ± 3.14 | 0.286 | 0.885 | 0.499 | 0.352 | 0.308 | 0.066 |
| DCP | 48.16 ± 3.46 | 48.53 ± 2.34 | 48.77 ± 3.96 | 45.32 ± 8.7 | 47.5 ± 3.54 | 0.974 | 0.275 | 0.530 | 0.107 | 0.170 | 0.155 |
| CC | 53.14 ± 1.94 | 52.49 ± 1.54 | 53.87 ± 2.40 | 51.99 ± 2.51 | 53.57 ± 2.41 | 0.499 | 0.500 | 0.333 | 0.030 | 0.616 | 0.036 |
| Mean nasal VD | |||||||||||
| SCP | 45.92 ± 3.11 | 46.08 ± 2.67 | 45.37 ± 3.5 | 45.64 ± 3.94 | 46.55 ± 2.96 | 0.460 | 0.449 | 0.735 | 0.830 | 0.139 | 0.350 |
| DCP | 49.63 ± 3.63 | 48.03 ± 4.43 | 49.49 ± 5.07 | 48.86 ± 3.38 | 49.08 ± 4.09 | 0.389 | 0.579 | 0.587 | 0.683 | 0.716 | 0.848 |
| CC | 52.81 ± 2.36 | 51.99 ± 3.01 | 52.86 ± 3.68 | 53.94 ± 2.17 | 53.10 ± 2.50 | 0.528 | 0.667 | 0.357 | 0.326 | 0.744 | 0.255 |
| Mean inferior VD | |||||||||||
| SCP | 49.39 ± 3.65 | 49.23 ± 3.86 | 48.44 ± 6.55 | 46.41 ± 3.91 | 50.23 ± 3.99 | 0.466 | 0.435 | 0.594 | 0.304 | 0.148 | 0.002 |
| DCP | 53.38 ± 3.43 | 52.17 ± 4.51 | 50.69 ± 5.43 | 49.48 ± 4.25 | 52.01 ± 4.03 | 0.467 | 0.159 | 0.937 | 0.483 | 0.245 | 0.043 |
| CC | 53.65 ± 2.46 | 53.68 ± 1.61 | 52.58 ± 2.74 | 52.90 ± 2.83 | 53.24 ± 3.07 | 0.981 | 0.612 | 0.757 | 0.732 | 0.377 | 0.711 |
| FAZ SCP Area | FAZ SCP Horizontal Diameter | FAZ SCP Vertical Diameter | FAZ DCP Area | FAZ DCP Horizontal Diameter | FAZ DCP Vertical Diameter | |
|---|---|---|---|---|---|---|
| Macula-on RRD (n = 23) | 252.77 ± 88.11 | 499.22 ± 123.664 | 505.48 ± 119.591 | 201.255 ± 72.43 | 448.30 ± 124.832 | 487.30 ± 141.027 |
| Macula-off RRD (n = 37) | 210.183 ± 171.254 | 403.11 ± 168.168 | 400.81 ± 176.891 | 170.501 ± 103.475 | 365.54 ± 150.155 | 392.11 ± 156.984 |
| Control eye (n = 52) | 244.204 ± 98.910 | 473.25 ± 132.170 | 468.62 ± 147.188 | 207.26 ± 76.42 | 443.37 ± 110.114 | 476.37 ± 117.212 |
| P1 (on vs. off) | 0.063 | 0.021 | 0.015 | 0.218 | 0.031 | 0.021 |
| P2 (on vs. control) | 0.765 | 0.426 | 0.295 | 0.751 | 0.864 | 0.727 |
| P3 (off vs. control) | 0.053 | 0.030 | 0.05 | 0.057 | 0.006 | 0.005 |
| Macula-on RRD treated with PPV (n = 18) | 261.57 ± 90.47 | 508.72 ± 130.835 | 504.72 ± 116.695 | 208.65 ± 72.67 | 444.61 ± 132.913 | 487.67 ± 148.558 |
| Macula-on RRD treated with PPV + SB (n = 5) | 221.097 ± 79.43 | 465.00 ± 97.414 | 508.20 ± 144.131 | 174.60 ± 72.64 | 461.60 ± 101.704 | 486.00 ± 124.856 |
| Macula-off RRD treated with PPV (n = 23) | 238.56 ± 191.85 | 425.52 ± 154.78 | 427.87 ± 170.508 | 191.75 ± 100.012 | 387.87 ± 103.778 | 427.74 ± 145.772 |
| Macula-off RRD treated with PPV + SB (n = 14) | 244.204 ± 123.109 | 366.29 ± 188.210 | 356.36 ± 184.462 | 135.58 ± 103.012 | 328.86 ± 204.645 | 333.57 ± 162.320 |
| P1 (on PPV vs. on PPV + SB) | 0.333 | 0.497 | 0.956 | 0.376 | 0.795 | 0.982 |
| P2 (on PPV vs. control) | 0.528 | 0.329 | 0.350 | 0.946 | 0.969 | 0.744 |
| P3 (on PPV + SB vs. control) | 0.574 | 0.893 | 0.567 | 0.364 | 0.724 | 0.862 |
| P4 (off PPV vs. off PPV + SB) | 0.210 | 0.305 | 0.238 | 0.110 | 0.252 | 0.076 |
| P5 (off PPV vs. control) | 0.280 | 0.176 | 0.296 | 0.465 | 0.044 | 0.129 |
| P6 (off PPV + SB vs. control) | 0.030 | 0.017 | 0.019 | 0.005 | 0.006 | <0.0001 |
| Patient Number | Difference in SCP Diameter | Difference in DCP Diameter | |
|---|---|---|---|
| Macula-on RRD treated with PPV | 18 | 47.20 ± 50.08 | 55.20 ± 36.45 |
| Macula-on RRD treated with PPV + SB | 5 | 63.22 ± 59.85 | 107.50 ± 77.22 |
| Macula-off RRD treated with PPV | 23 | 39.30 ± 29.63 | 104.91 ± 89.97 |
| Macula-off RRD treated with PPV + SB | 14 | 74.07 ± 75.54 | 84.85 ± 69.17 |
| Control eye | 52 | 57.51 ± 47.67 | 66.23 ± 55.27 |
| P1 (on PPV vs. on PPV +SB) | 0.478 | 0.205 | |
| P2 (on PPV vs. control) | 0.154 | 0.605 | |
| P3 (on PPV + SB vs. control) | 0.632 | 0.902 | |
| P4 (off PPV vs. off PPV + SB) | 0.621 | 0.442 | |
| P5 (off PPV vs. control) | 0.925 | 0.036 | |
| P6 (off PPV + SB vs. control) | 0.154 | 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolomé-Sesé, I.; Díaz-Barreda, M.D.; Orduna-Hospital, E.; Boned-Murillo, A.; Ascaso, F.J.; Pinilla, I. Long-Term Follow-Up of Macular Perfusion Evaluated by Optical Coherence Tomography Angiography after Rhegmatogenous Retinal Detachment Surgery. J. Clin. Med. 2022, 11, 6725. https://doi.org/10.3390/jcm11226725
Bartolomé-Sesé I, Díaz-Barreda MD, Orduna-Hospital E, Boned-Murillo A, Ascaso FJ, Pinilla I. Long-Term Follow-Up of Macular Perfusion Evaluated by Optical Coherence Tomography Angiography after Rhegmatogenous Retinal Detachment Surgery. Journal of Clinical Medicine. 2022; 11(22):6725. https://doi.org/10.3390/jcm11226725
Chicago/Turabian StyleBartolomé-Sesé, Isabel, María D. Díaz-Barreda, Elvira Orduna-Hospital, Ana Boned-Murillo, Francisco J. Ascaso, and Isabel Pinilla. 2022. "Long-Term Follow-Up of Macular Perfusion Evaluated by Optical Coherence Tomography Angiography after Rhegmatogenous Retinal Detachment Surgery" Journal of Clinical Medicine 11, no. 22: 6725. https://doi.org/10.3390/jcm11226725
APA StyleBartolomé-Sesé, I., Díaz-Barreda, M. D., Orduna-Hospital, E., Boned-Murillo, A., Ascaso, F. J., & Pinilla, I. (2022). Long-Term Follow-Up of Macular Perfusion Evaluated by Optical Coherence Tomography Angiography after Rhegmatogenous Retinal Detachment Surgery. Journal of Clinical Medicine, 11(22), 6725. https://doi.org/10.3390/jcm11226725








