Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Exclusion Criteria
2.5. Data Extraction
2.6. Risk-of-Bias Assessment
2.7. Statistical Analysis
3. Results
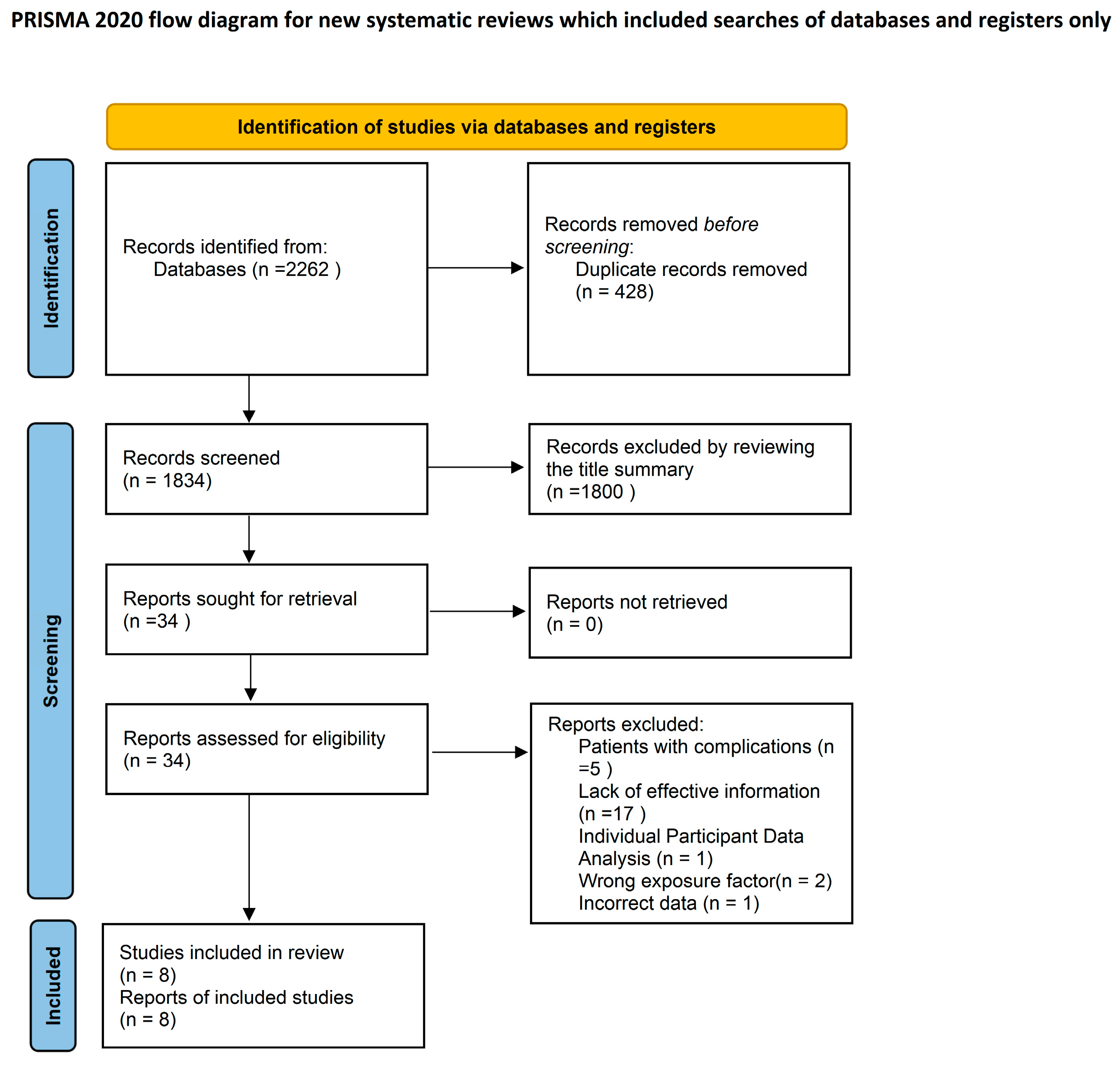
3.1. Literature Search
3.2. Study Characteristics
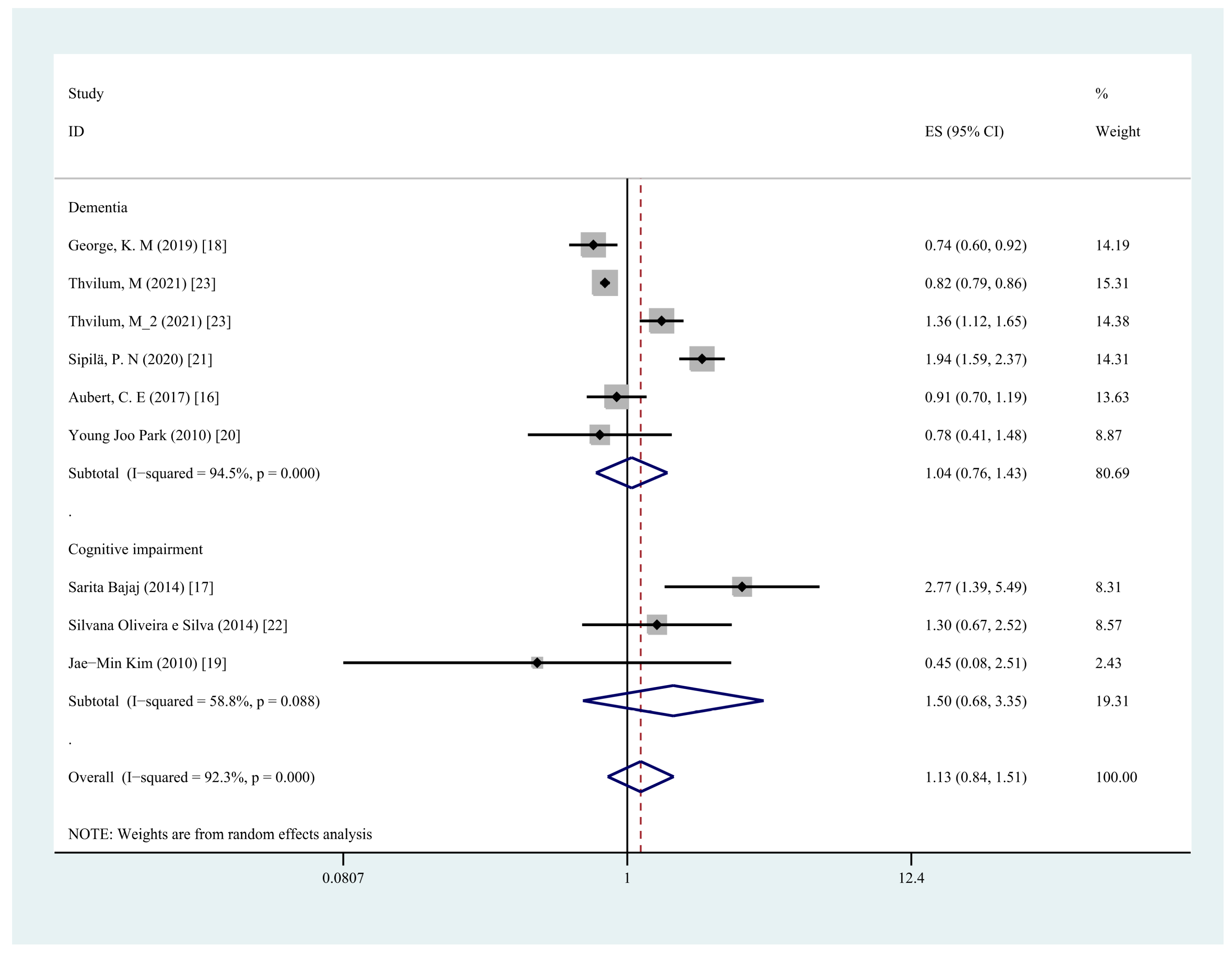
3.3. Any Hypothyroidism and Risk of Cognitive Dysfunction
3.4. Any Hypothyroidism and Risk of Dementia
3.5. Any Hypothyroidism and Risk of Cognitive Impairment
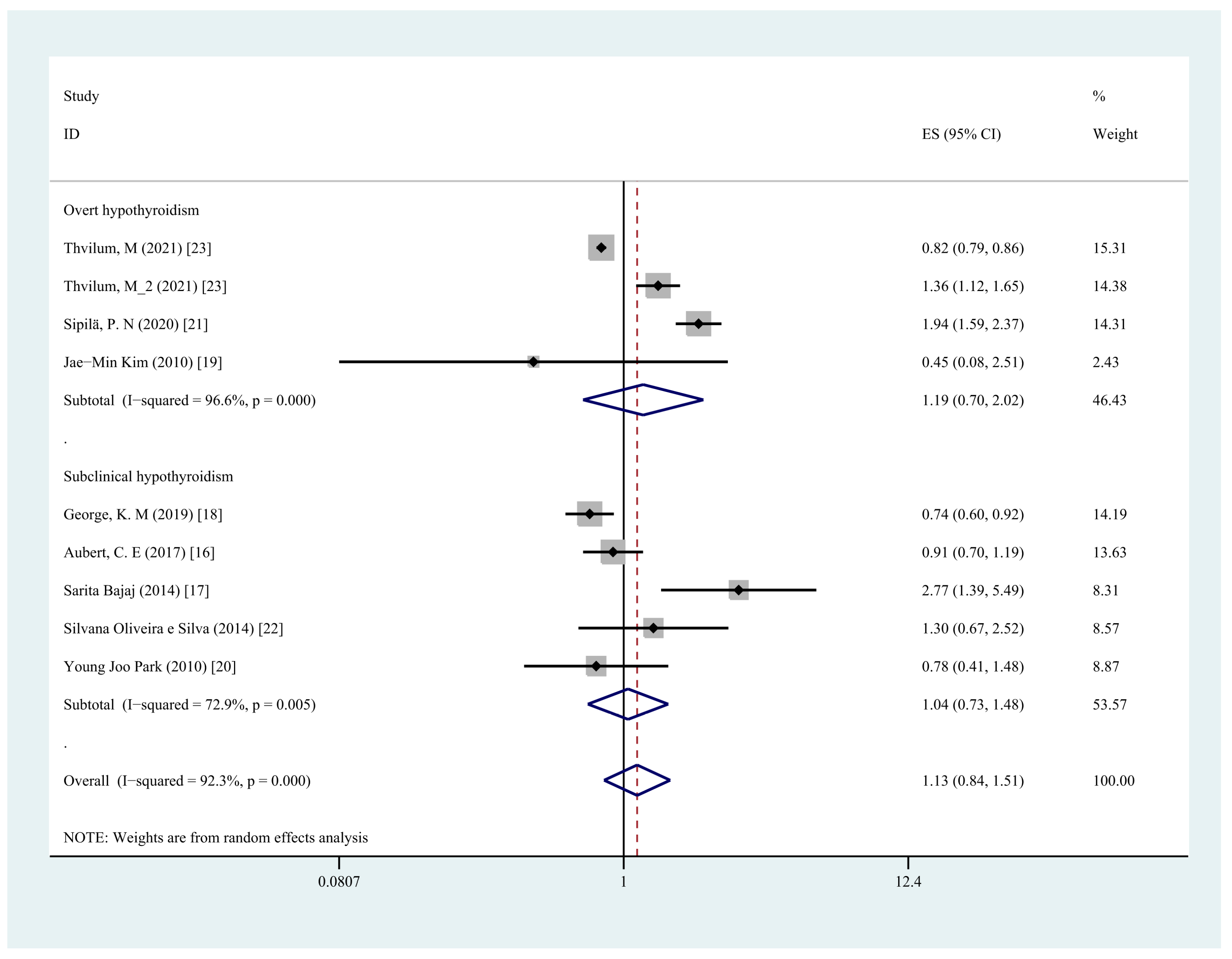
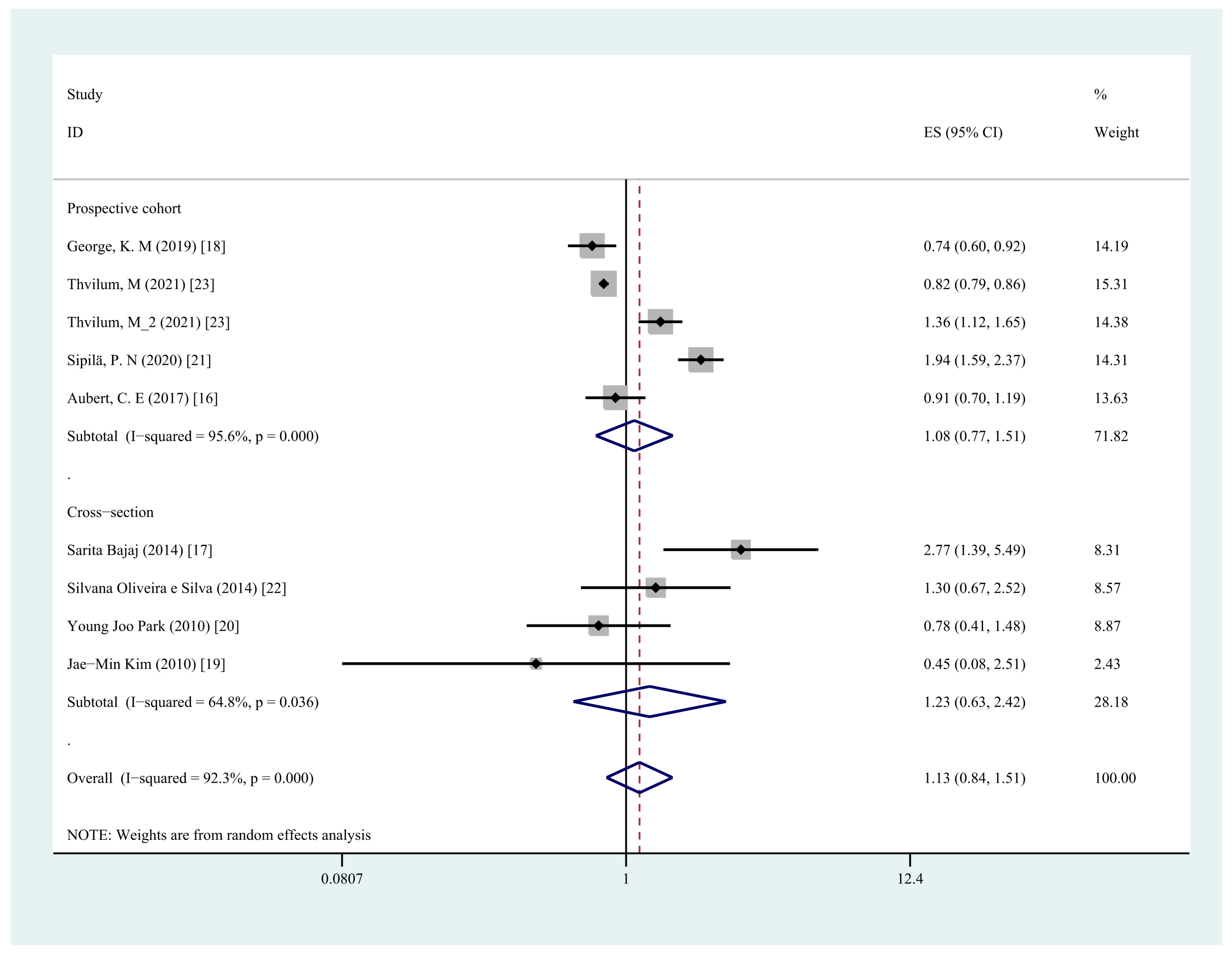
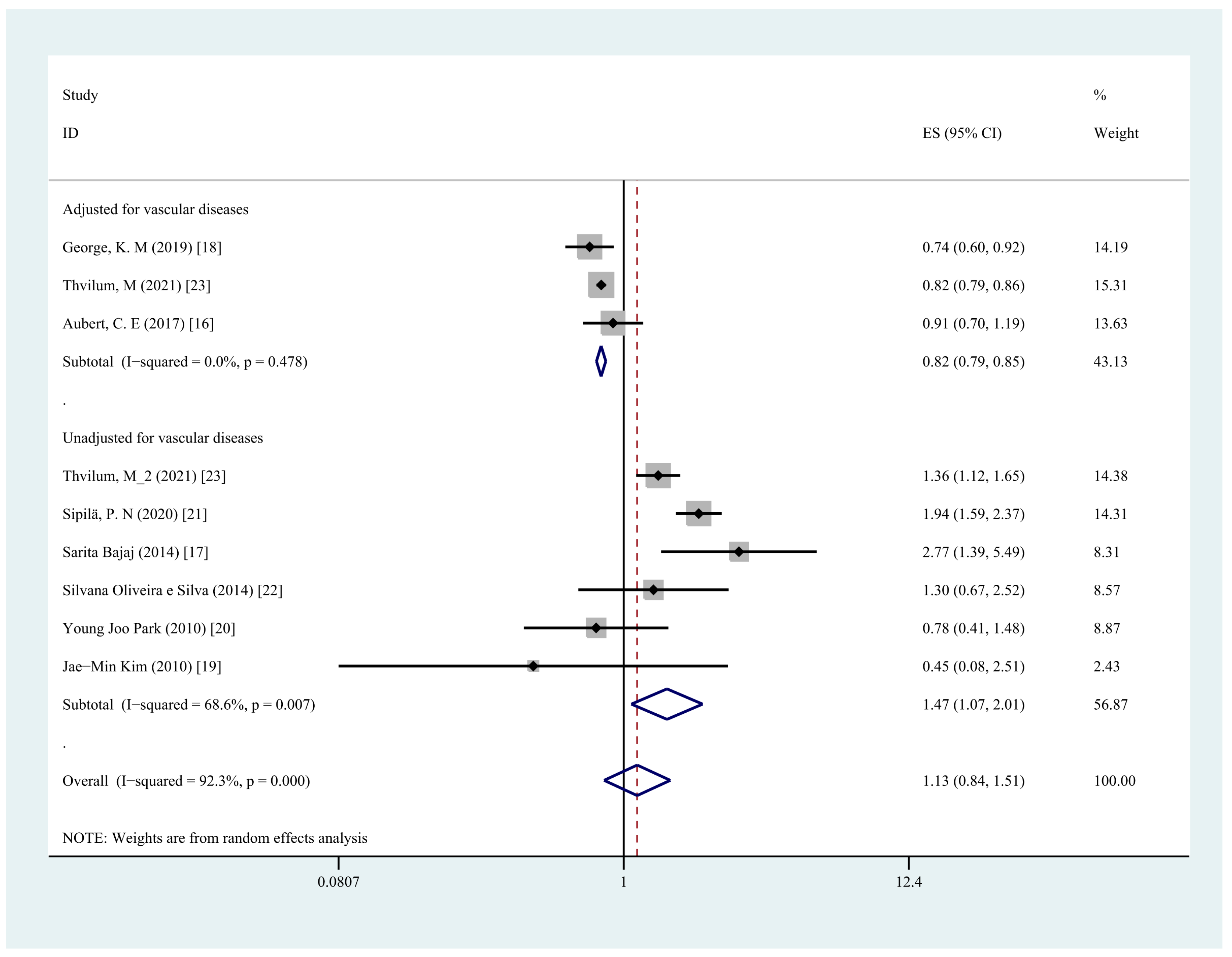
3.6. Subgroup Analysis
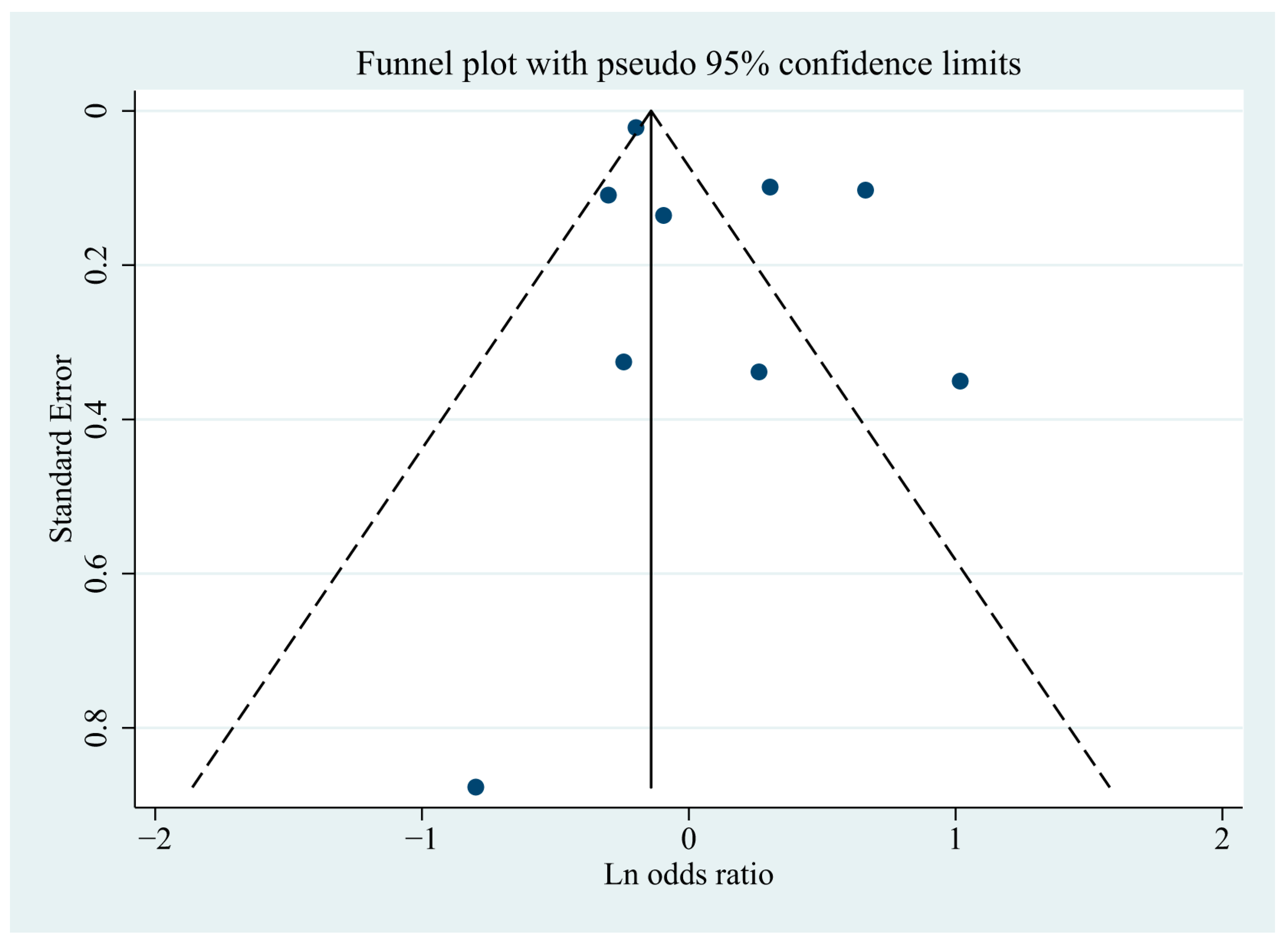
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burns, A.; Iliffe, S. Dementia. BMJ 2009, 338, b75. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Prina, M. World Alzheimer Report 2015. The Global Impact of Dementia. An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Petersen, R.C.; Roberts, R.O.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild Cognitive Impairment. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Waldemar, G.; Dubois, B.; Emre, M.; Georges, J.; McKeith, I.; Rossor, M.; Scheltens, P.; Tariska, P.; Winblad, B. Recommendations for the diagnosis and management of Alzheimer’s disease and other disorders associated with dementia: EFNS guideline. Eur. J. Neurol. 2007, 14, e1–e26. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, N.A.; van Heemst, D.; Almeida, O.P.; Åsvold, B.O.; Aubert, C.E.; Bin Bae, J.; Barnes, L.E.; Bauer, D.C.; Blauw, G.J.; Brayne, C.; et al. Association of Thyroid Dysfunction with Cognitive Function. JAMA Intern. Med. 2021, 181, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Akintola, A.A.; Jansen, S.W.; Bodegom, D.E.; Der Grond, J.E.; Westendorp, R.G.; De Craen, A.J.M.; Heemst, D.E. Subclinical hypothyroidism and cognitive function in people over 60 years: A systematic review and meta-analysis. Front. Aging Neurosci. 2015, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Joffe, R.T.; Pearce, E.N.; Hennessey, J.V.; Ryan, J.J.; Stern, R. Subclinical hypothyroidism, mood, and cognition in older adults: A review. Int. J. Geriatr. Psychiatry 2012, 28, 111–118. [Google Scholar] [CrossRef]
- Parsaik, A.K.; Singh, B.; Roberts, R.O.; Pankratz, S.; Edwards, K.K.; Geda, Y.E.; Gharib, H.; Boeve, B.F.; Knopman, D.S.; Petersen, R.C. Hypothyroidism and Risk of Mild Cognitive Impairment in Elderly Persons. JAMA Neurol. 2014, 71, 201–207. [Google Scholar] [CrossRef]
- Pasqualetti, G.; Pagano, G.; Rengo, G.; Ferrara, N.; Monzani, F. Subclinical Hypothyroidism and Cognitive Impairment: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2015, 100, 4240–4248. [Google Scholar] [CrossRef]
- Rieben, C.; Segna, D.; da Costa, B.R.; Collet, T.-H.; Chaker, L.; Aubert, C.E.; Baumgartner, C.; Almeida, O.; Hogervorst, E.; Trompet, S.; et al. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: A Meta-Analysis of Prospective Cohort Studies. J. Clin. Endocrinol. Metab. 2016, 101, 4945–4954. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, Y.; Wang, F.; Xu, D.; Cui, W. Higher FT4 or TSH below the normal range are associated with increased risk of dementia: A meta-analysis of 11 studies. Sci. Rep. 2016, 6, 31975. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ding, L. Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis [Internet]. In Figshare; 2022. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, S.; Geertzen, J.H.; Dijkstra, P.U. Association between cardiovascular diseases and mobility in persons with lower limb amputation: A systematic review. Disabil. Rehabil. 2017, 40, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Aubert, C.E.; Bauer, D.C.; da Costa, B.R.; Feller, M.; Rieben, C.; Simonsick, E.M.; Yaffe, K.; Rodondi, N. The Health ABC Study The association between subclinical thyroid dysfunction and dementia: The Health, Aging and Body Composition (Health ABC) Study. Clin. Endocrinol. 2017, 87, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Sachan, S.; Misra, V.; Varma, A.; Saxena, P. Cognitive function in subclinical hypothyroidism in elderly. Indian J. Endocrinol. Metab. 2014, 18, 811–814. [Google Scholar] [CrossRef]
- George, K.; Lutsey, P.L.; Selvin, E.; Palta, P.; Windham, B.G.; Folsom, A.R. Association Between Thyroid Dysfunction and Incident Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. J. Endocrinol. Metab. 2019, 9, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Stewart, R.; Kim, S.-Y.; Bae, K.-Y.; Yang, S.-J.; Kim, S.-W.; Shin, I.-S.; Yoon, J.-S. Thyroid Stimulating Hormone, Cognitive Impairment and Depression in an Older Korean Population. Psychiatry Investig. 2010, 7, 264–269. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, E.J.; Lee, Y.J.; Choi, S.H.; Park, J.H.; Lee, S.B.; Lim, S.; Lee, W.W.; Jang, H.C.; Cho, B.Y.; et al. Subclinical hypothyroidism (SCH) is not associated with metabolic derangement, cognitive impairment, depression or poor quality of life (QoL) in elderly subjects. Arch. Gerontol. Geriatr. 2010, 50, e68–e73. [Google Scholar] [CrossRef]
- Sipilä, P.N.; Lindbohm, J.V.; Singh-Manoux, A.; Shipley, M.J.; Kiiskinen, T.; Havulinna, A.S.; Vahtera, J.; Nyberg, S.T.; Pentti, J.; Kivimäki, M. Long-term risk of dementia following hospitalization due to physical diseases: A multicohort study. Alzheimer’s Dement. 2020, 16, 1686–1695. [Google Scholar] [CrossRef]
- E Silva, S.O.; Chan, I.T.; Lobo Santos, M.A.; Cohen, M.; de La Roque P Araujo, M.; da Silva Almeida, J.; Simões, A.; Givigi, H.R.B.; Vaisman, M.; Paixão, C.M., Jr.; et al. Impact of thyroid status and age on comprehensive geriatric assessment. Endocrine 2014, 47, 255–265. [Google Scholar] [CrossRef]
- Thvilum, M.; Brandt, F.; Lillevang-Johansen, M.; Folkestad, L.; Brix, T.H.; Hegedüs, L. Increased risk of dementia in hypothyroidism: A Danish nationwide register-based study. Clin. Endocrinol. 2021, 94, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Randin, J.P.; Schwed, P.; Wertheimer, J.; Lemarchand-Béraud, T. Prevalence of thyroid disorders in psychogeriatric inpatients. A possible relationship of hypothyroidism with neurotic depression but not with dementia. J. Am. Geriatr. Soc. 1987, 35, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Percy, M.E.; Dalton, A.J.; Markovic, V.D.; McLachlan, D.R.C.; Gera, E.; Hummel, J.T.; Rusk, A.C.M.; Somerville, M.J.; Andrews, D.F.; Walfish, P.G. Autoimmune thyroiditis associated with mild “subclinical” hypothyroidism in adults with down syndrome: A comparison of patients with and without manifestations of Alzheimer disease. Am. J. Med Genet. 1990, 36, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Collacott, R.A.; Garrick, P.; Mitchell, C. Effect of thyroid stimulating hormone on adaptive behaviour in Down’s syndrome. J. Intellect. Disabil. Res. 1991, 35, 512–520. [Google Scholar] [CrossRef]
- Osterweil, D.; Syndulko, K.; Cohen, S.N.; Hershman, J.M.; Cummings, J.L.; Tourtellotte, W.W.; Solomon, D.H.; Pettier-Jennings, P.D. Cognitive Function in Non-Demented Older Adults with Hypothyroidism. J. Am. Geriatr. Soc. 1992, 40, 325–335. [Google Scholar] [CrossRef]
- Volpato, S.; Guralnik, J.M.; Fried, L.P.; Remaley, A.T.; Cappola, A.R.; Launer, L.J. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology 2002, 58, 1055–1061. [Google Scholar] [CrossRef]
- Münte, T.F.; Lill, C.; Ötting, G.; Brabant, G. Cognitive changes in short-term hypothyroidism assessed with event-related brain potentials. Psychoneuroendocrinology 2004, 29, 1109–1118. [Google Scholar] [CrossRef]
- Roberts, L.M.; Pattison, H.; Roalfe, A.; Franklyn, J.; Wilson, S.; Hobbs, F.R.; Parle, J.V. Is Subclinical Thyroid Dysfunction in the Elderly Associated with Depression or Cognitive Dysfunction? Ann. Intern. Med. 2006, 145, 573–581. [Google Scholar] [CrossRef]
- Suhanov, A.V.; Pilipenko, P.I.; Korczyn, A.D.; Hofman, A.; Voevoda, M.I.; Shishkin, S.V.; Simonova, G.I.; Nikitin, Y.P.; Feigin, V.L. Risk factors for Alzheimer’s disease in Russia: A case–control study. Eur. J. Neurol. 2006, 13, 990–995. [Google Scholar] [CrossRef]
- Tan, Z.S. Thyroid Function and the Risk of Alzheimer DiseaseThe Framingham Study. Arch. Intern. Med. 2008, 168, 1514–1520. [Google Scholar] [CrossRef]
- Ceresini, G.; Lauretani, F.; Maggio, M.; Ceda, G.P.; Morganti, S.; Usberti, E.; Chezzi, C.; Valcavi, R.; Bandinelli, S.; Guralnik, J.M.; et al. Thyroid Function Abnormalities and Cognitive Impairment in Elderly People: Results of the Invecchiare in Chianti Study. J. Am. Geriatr. Soc. 2009, 57, 89–93. [Google Scholar] [CrossRef]
- Correia, N.; Mullally, S.; Cooke, G.; Tun, T.K.; Phelan, N.; Feeney, J.; FitzGibbon, M.; Boran, G.; O’Mara, S.; Gibney, J. Evidence for a Specific Defect in Hippocampal Memory in Overt and Subclinical Hypothyroidism. J. Clin. Endocrinol. Metab. 2009, 94, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Resta, F.; Triggiani, V.; Barile, G.; Benigno, M.; Suppressa, P.; Giagulli, V.A.; Guastamacchia, E.; Sabba, C. Subclinical hypothyroidism and cognitive dysfunction in the elderly. Endocrine, Metab. Immune Disord. Drug Targets 2012, 12, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ishizawa, K.; Ishikawa, M.; Yamanaka, G.; Yamanaka, T.; Murakami, S.; Hiraiwa, T.; Okumiya, K.; Ishine, M.; Matsubayashi, K.; et al. Cognitive function with subclinical hypothyroidism in elderly people without dementia: One year follow up. Geriatr. Gerontol. Int. 2012, 12, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, L.W.; De Craen, A.J.M.; Trompet, S.; Gussekloo, J.; Stott, D.J.; Rodondi, N.; Welsh, P.; Jukema, J.W.; Westendorp, R.G.J.; Mooijaart, S.P. Subclinical Thyroid Dysfunction and Cognitive Decline in Old Age. PLoS ONE 2013, 8, e59199. [Google Scholar] [CrossRef][Green Version]
- Formiga, F.; Ferrer, A.; Padros, G.; Contra, A.; Corbella, X.; Pujol, R. Thyroid status and functional and cognitive status at baseline and survival after 3 years of follow-up: The OCTABAIX study. Eur. J. Endocrinol. 2014, 170, 69–75. [Google Scholar] [CrossRef]
- Paladugu, S.; Hanmayyagari, B.R.; Kudugunti, N.; Reddy, R.; Sahay, R.; Ramesh, J. Improvement in subclinical cognitive dysfunction with thyroxine therapy in hypothyroidism: A study from tertiary care center. Indian J. Endocrinol. Metab. 2015, 19, 829–833. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.-C.; Guo, Q.-H.; Cheng, W.; Chen, Y.-W. Is thyroid status associated with cognitive impairment in elderly patients in China? BMC Endocr. Disord. 2016, 16, 11. [Google Scholar] [CrossRef]
- Juárez-Cedillo, T.; Basurto-Acevedo, L.; Vega-García, S.; Martha, A.S.-R.; Retana-Ugalde, R.; Roberto, C.G.-M.; Escobedo-De-La-Peña, J. Prevalence of thyroid dysfunction and its impact on cognition in older mexican adults: (SADEM study). J. Endocrinol. Investig. 2017, 40, 945–952. [Google Scholar] [CrossRef]
- Szlejf, C.; Suemoto, C.K.; Santos, I.S.; Lotufo, P.A.; Diniz, M.D.F.H.S.; Barreto, S.M.; Benseñor, I.M. Thyrotropin level and cognitive performance: Baseline results from the ELSA-Brasil Study. Psychoneuroendocrinology 2018, 87, 152–158. [Google Scholar] [CrossRef]
- Wändell, P.; Carlsson, A.C.; Sundquist, J.; Sundquist, K. Effect of Levothyroxine Treatment on Incident Dementia in Adults with Atrial Fibrillation and Hypothyroidism. Clin. Drug Investig. 2019, 39, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.; Kumaraswamy, D.R.; Dharmalingam, M.; Saini, J.; Yadav, R. Neuropsychological Impairments in Young Patients with Subclinical Hypothyroidism: A Case Control Study. Ann. Neurosci. 2020, 27, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Mercaldo, N.D.; Wang, C.M.; Hersch, M.S.; Hersch, G.G.; Rosas, H.D. Association between Hypothyroidism Onset and Alzheimer Disease Onset in Adults with Down Syndrome. Brain Sci. 2021, 11, 1223. [Google Scholar] [CrossRef] [PubMed]
- Mulat, B.; Ambelu, A.; Yitayih, S.; Gela, Y.Y.; Adera, A.; Yeshaw, Y.; Akalu, Y. Cognitive Impairment and Associated Factors Among Adult Hypothyroid Patients in Referral Hospitals, Amhara Region, Ethiopia: Multicenter Cross-Sectional Study. Neuropsychiatr. Dis. Treat. 2021, 17, 935–943. [Google Scholar] [CrossRef]
- Szlejf, C.; Suemoto, C.K.; Janovsky, C.C.P.S.; Bertola, L.; Barreto, S.M.; Lotufo, P.A.; Benseñor, I.M. Subtle Thyroid Dysfunction Is Not Associated with Cognitive Decline: Results from the ELSA-Brasil. J. Alzheimers Dis. 2021, 81, 1529–1540. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.S.; Kim, Y.H.; Kwon, M.J.; Kim, J.-H.; Min, C.Y.; Yoo, D.M.; Choi, H.G. The Association Between Thyroid Diseases and Alzheimer’s Disease in a National Health Screening Cohort in Korea. Front. Endocrinol. 2022, 13, 815063. [Google Scholar] [CrossRef]
- Biondi, B.; Cappola, A.R. Subclinical hypothyroidism in older individuals. Lancet Diabetes Endocrinol. 2022, 10, 129–141. [Google Scholar] [CrossRef]
- Hogervorst, E.; Huppert, F.; Matthews, F.; Brayne, C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology 2008, 33, 1013–1022. [Google Scholar] [CrossRef]
- Cheng, P.-K.; Chen, H.-C.; Kuo, P.-L.; Chang, J.-W.; Chang, W.-T.; Huang, P.-C. Associations between Oxidative/Nitrosative Stress and Thyroid Hormones in Pregnant Women–Tainan Birth Cohort Study (TBCS). Antioxidants 2022, 11, 334. [Google Scholar] [CrossRef]
- Parle, J.; Roberts, L.; Wilson, S.; Pattison, H.; Roalfe, A.; Haque, M.S.; Heath, C.; Sheppard, M.; Franklyn, J.; Hobbs, F.D.R. A Randomized Controlled Trial of the Effect of Thyroxine Replacement on Cognitive Function in Community-Living Elderly Subjects with Subclinical Hypothyroidism: The Birmingham Elderly Thyroid Study. J. Clin. Endocrinol. Metab. 2010, 95, 3623–3632. [Google Scholar] [CrossRef]
- Khaleghzadeh-Ahangar, H.; Talebi, A.; Mohseni-Moghaddam, P. Thyroid Disorders and Development of Cognitive Impairment: A Review Study. Neuroendocrinology 2022, 112, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Boucai, L.; Hollowell, J.G.; Surks, M.I. An Approach for Development of Age-, Gender-, and Ethnicity-Specific Thyrotropin Reference Limits. Thyroid 2011, 21, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Bladowska, J.; Budrewicz, S.; Sąsiadek, M.; Dziadkowiak, E.; Ejma, M. The evaluation of Hashimoto’s thyroiditis with event-related potentials and magnetic resonance spectroscopy and its relation to cognitive function. Sci. Rep. 2021, 11, 2480. [Google Scholar] [CrossRef]
- Waliszewska-Prosół, M.; Ejma, M. Assessment of Visual and Brainstem Auditory Evoked Potentials in Patients with Hashimoto’s Thyroiditis. J. Immunol. Res. 2021, 2021, 3258942. [Google Scholar] [CrossRef] [PubMed]
- Haji, M.; Kimura, N.; Hanaoka, T.; Aso, Y.; Takemaru, M.; Hirano, T.; Matsubara, E. Evaluation of Regional Cerebral Blood Flow in Alzheimer’s Disease Patients with Subclinical Hypothyroidism. Dement. Geriatr. Cogn. Disord. 2015, 39, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, P.B.S.; Ferreira, A.F.F.; Britto, L.R.; Doussoulin, A.P.; Torrão, A.D.S. Association between thyroid function and Alzheimer’s disease: A systematic review. Metab. Brain Dis. 2021, 36, 1523–1543. [Google Scholar] [CrossRef] [PubMed]
- Brix, T.H.; Kyvik, K.O.; Hegedüs, L. A Population-Based Study of Chronic Autoimmune Hypothyroidism in Danish Twins 1. J. Clin. Endocrinol. Metab. 2000, 85, 536–539. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Gamaldo, A.A.; Teel, A.; Zonderman, A.B.; Wang, Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health 2014, 14, 643. [Google Scholar] [CrossRef]
- Benseñor, I.M.; A Lotufo, P.; Menezes, P.R.; Scazufca, M. Subclinical hyperthyroidism and dementia: The Sao Paulo Ageing & Health Study (SPAH). BMC Public Heal. 2010, 10, 298. [Google Scholar] [CrossRef]
- Agarwal, R.; Kushwaha, S.; Chhillar, N.; Kumar, A.; Dubey, D.; Tripathi, C.B. A cross-sectional study on thyroid status in North Indian elderly outpatients with dementia. Ann. Indian Acad. Neurol. 2013, 16, 333–337. [Google Scholar] [CrossRef]
- Zhang, M.; Gong, W.; Zhang, D.; Ji, M.; Chen, B.; Chen, B.; Li, X.; Zhou, Y.; Dong, C.; Wen, G.; et al. Ageing related thyroid deficiency increases brain-targeted transport of liver-derived ApoE4-laden exosomes leading to cognitive impairment. Cell Death Dis. 2022, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Booms, S.; Hill, E.; Kulhanek, L.; Vredeveld, J.; Gregg, B. Iodine Deficiency and Hypothyroidism from Voluntary Diet Restrictions in the US: Case Reports. Pediatrics 2016, 137, e20154003. [Google Scholar] [CrossRef] [PubMed]
- Nepal, A.K.; Suwal, R.; Gautam, S.; Shah, G.S.; Baral, N.; Andersson, M.; Zimmermann, M.B. Subclinical Hypothyroidism and Elevated Thyroglobulin in Infants with Chronic Excess Iodine Intake. Thyroid 2015, 25, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Famà, F.; Perdichizzi, L.G.; Antonelli, A.; Brenta, G.; Vermiglio, F.; Moleti, M. Fish and the Thyroid: A Janus Bifrons Relationship Caused by Pollutants and the Omega-3 Polyunsaturated Fatty Acids. Front. Endocrinol. 2022, 13, 891233. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, Y.; Ke, S.; Huang, J.; Gao, X.; Li, B.; Lin, X.; Liu, X.; Liu, X.; Ma, L.; et al. Lifestyle is associated with thyroid function in subclinical hypothyroidism: A cross-sectional study. BMC Endocr. Disord. 2021, 21, 112. [Google Scholar] [CrossRef]

| Authors | Country | Study Type | Sample Size | Follow-Up Years | Age (Years) | Diagnosis of Hypothyroidism | Hormone Test Methods and Sample Collection Time | Diagnosis of Dementia/Cognitive Impairment | Hypothyroidism Type | Dementia Type | Confounders Adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|
| George et al., 2019 [18] | USA | Prospective cohort | 12,481 | 21.9 | 57 ± 5.7 | TSH and FT4 concentration | Elecsys 2010 analyzer, a sandwich immunoassay method for TSH and competition immunoassay method for FT4; Time: NA | Cognitive test performance, neuropsychological examinations, clinician review of suspected cases, telephone interviews, relevant hospital, and death-certificate codes | Overt hypothyroidism, subclinical hypothyroidism | MCI, dementia | Age, sex, race-center, APOE ε4, income and education, BMI, smoking status, hypertension, diabetes, drinking status, HDL-C and total cholesterol, prevalent CVD, and baseline thyroid medication use |
| Thvilum et al., 2021 [23] | Denmark | Prospective cohort | 557,825 | 6.2 | 55.8 (43.3–68.5) | ICD-10 codes: E03.2-E03.9; ATC code: H03A | NA | ICD-10 codes; ATC codes | Hypothyroidism | Dementia, Alzheimer’s disease, vascular dementia, and other forms of dementia | Charlson Comorbidity Index |
| Thvilum et al., 2021 [23] | Denmark | Prospective cohort | 233,844 | 7.2 | Hypothyroid Individuals: 56.4 (42.6–69.2); reference Individuals: 50.3 (36.4–64.2) | TSH concentration | NA | ICD-10 codes | Hypothyroidism | Dementia | NA |
| Sipilä et al., 2020 [21] | Finland | Prospective cohort | 283,414 | 19 | 33.5 (18.0–87.9) | ICD-10 codes: E03 | NA | ICD-10 codes | Hypothyroidism | Dementia | Age, sex, low education/socioeconomic status, hypertension, smoking, depression, physical inactivity, diabetes, marital status (a proxy for social isolation), obesity (available in all cohorts except STW), and APOE genotype (available in WHII) |
| Aubert et al., 2017 [16] | Switzerland | Prospective cohort | 2558 | over 10 years | 75.1 (2.8) | TSH and FT4 concentration | ACS; Chiron Diagnostics Corp, Emeryville, Calif, immunoassay for TSH and competitive immunoassay for FT4; Time: NA | Application of 3 MS score, diagnosis of hospitalization or prescription of dementia drug | Subclinical hypothyroidism | Dementia | Age, sex, race, education, and baseline 3 MS, and then further for cardiovascular risk factors |
| Bajaj et al., 2014 [17] | India | Cross-section | 206 | - | Cases:75.74 ± 9.37; Controls:75.72 ± 9.40 | TSH, FT3 and FT4 concentration | Immulite-1000 TSH, chemiluminescent detection for TSH; Time: NA | MMSE test and The Clock Drawing Test | Subclinical hypothyroidism | Cognitive function | NA |
| SO, E.S., et al., 2014 [22] | Brazil | Cross-section | 284 | - | 80.7 ± 6.7 | TSH and FT4 concentration | Immulite 2000®, chemiluminescence for TSH and FT4; Time: at morning | MMSE test | Subclinical hypothyroidism | Cognitive deficit | NA |
| Park et al., 2010 [20] | Korea | Cross-section | 918 | - | Subclinical hypothyroidism: 76.5 ± 9.0; euthyroidism: 76.8 ± 9.0 | TSH and FT4 concentration | Immunoradiometric assays (TSH: CIS bio international, Gif-sur-Yvette, France; FT4: DiaSorin S.p.A, Saluggia, Italy); Time: NA | DSM-IV diagnostic criteria | Subclinical hypothyroidism | Dementia | Age, gender, and duration of education |
| Kim et al., 2010 [19] | Korea | Cross-section | 495 | - | 72.4 (5.6) | TSH concentration | Chemiluminescent immunoassay (Cobas: Roche Diagnostics, West Sussex, UK) for TSH; Time: at morning | CSID test | Hypothyroidism | Cognitive impairment | Age, gender, education, smoking, physical activity, systolic BP, diabetes mellitus, total cholesterol, albumin, levothyroxine treatment, and depression |
| Subgroups | Included Studies | OR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
| I2 (%) | p-Values | |||
| Hypothyroidism Type | ||||
| Overt hypothyroidism | 4 | 1.19 (0.70,2.02) | 96.6% | 0.53 |
| Subclinical hypothyroidism | 5 | 1.04 (0.73,1.48) | 72.9% | 0.83 |
| Research type | ||||
| Prospective cohort | 5 | 1.08 (0.77,1.51) | 95.6% | 0.67 |
| Cross-section | 4 | 1.23 (0.63,2.42) | 64.8% | 0.55 |
| Adjustment of comorbidities | ||||
| Adjusted for vascular diseases | 3 | 0.82 (0.79,0.85) | 0.0% | <0.001 |
| Unadjusted for vascular diseases | 6 | 1.47 (1.07,2.01) | 68.6% | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Wang, Y.; Li, S.; Guo, J.; Ding, L.; Liu, M. Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis. J. Clin. Med. 2022, 11, 6726. https://doi.org/10.3390/jcm11226726
Ye Y, Wang Y, Li S, Guo J, Ding L, Liu M. Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis. Journal of Clinical Medicine. 2022; 11(22):6726. https://doi.org/10.3390/jcm11226726
Chicago/Turabian StyleYe, Yuanyuan, Yiqing Wang, Shiwei Li, Jiyun Guo, Li Ding, and Ming Liu. 2022. "Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis" Journal of Clinical Medicine 11, no. 22: 6726. https://doi.org/10.3390/jcm11226726
APA StyleYe, Y., Wang, Y., Li, S., Guo, J., Ding, L., & Liu, M. (2022). Association of Hypothyroidism and the Risk of Cognitive Dysfunction: A Meta-Analysis. Journal of Clinical Medicine, 11(22), 6726. https://doi.org/10.3390/jcm11226726






