Mid- and Long-Term Surgical Outcomes Due to Infective Endocarditis in Elderly Patients: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Patient Management
2.3. Surgical Management
2.4. Statistical Analysis
3. Results
3.1. Patients’ Baseline Characteristics and Clinical Presentation
3.2. Operative Data and Secondary Endpoints
3.3. Postoperative Data and Primary Endpoints
4. Discussion
4.1. Patients’ Baseline Characteristics and Clinical Presentation
4.2. Operative Data and Secondary Endpoints
4.3. Postoperative Data and Primary Endpoints
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ragnarsson, S.; Salto-Alejandre, S.; Ström, A.; Olaison, L.; Rasmussen, M. Surgery Is Underused in Elderly Patients with Left-Sided Infective Endocarditis: A Nationwide Registry Study. J. Am. Heart Assoc. 2021, 10, e020221. [Google Scholar] [CrossRef]
- Hoen, B.; Alla, F.; Selton-Suty, C.; Béguinot, I.; Bouvet, A.; Briançon, S.; Casalta, J.P.; Danchin, N.; Delahaye, F.; Etienne, J.; et al. Changing profile of infective endocarditis: Results of a 1-year survey in France. JAMA 2002, 288, 75–81. [Google Scholar] [CrossRef]
- Dayer, M.J.; Jones, S.; Prendergast, B.; Baddour, L.M.; Lockhart, P.B.; Thornhill, M.H. Incidence of infective endocarditis in England, 2000–2013: A secular trend, interrupted time-series analysis. Lancet 2015, 385, 1219–1228. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Bradley, S.; Selton-Suty, C.; Tripodi, M.F.; Barsic, B.; Bouza, E.; Cabell, C.H.; Ramos, A.I.; Fowler, V., Jr.; Hoen, B.; et al. Current features of infective endocarditis in elderly patients: Results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch. Intern. Med. 2008, 168, 2095–2103. [Google Scholar] [CrossRef]
- Selton-Suty, C.; Célard, M.; Le Moing, V.; Doco-Lecompte, T.; Chirouze, C.; Iung, B.; Strady, C.; Revest, M.; Vandenesch, F.; Bouvet, A.; et al. Preeminence of Staphylococcus aureus in infective endocarditis: A 1-year population-based survey. Clin. Infect. Dis. 2012, 54, 1230–1239. [Google Scholar] [CrossRef]
- Salem, M.; Friedrich, C.; Saad, M.; Frank, D.; Salem, M.; Puehler, T.; Schoettler, J.; Schoeneich, F.; Cremer, J.; Haneya, A. Active Infective Native and Prosthetic Valve Endocarditis: Short- and Long-Term Outcomes of Patients after Surgical Treatment. J. Clin. Med. 2021, 10, 1868. [Google Scholar] [CrossRef]
- Saran, N.; Locker, C.; Said, S.M.; Daly, R.C.; Maltais, S.; Stulak, J.M.; Greason, K.L.; Pochettino, A.; Schaff, H.V.; Dearani, J.A.; et al. Current trends in bilateral internal thoracic artery use for coronary revascularization: Extending benefit to high-risk patients. J. Thorac. Cardiovasc. Surg. 2018, 155, 2331–2343. [Google Scholar] [CrossRef]
- López, J.; Revilla, A.; Vilacosta, I.; Sevilla, T.; Villacorta, E.; Sarriá, C.; Pozo, E.; Rollán, M.J.; Gómez, I.; Mota, P.; et al. Age-dependent profile of left-sided infective endocarditis: A 3-center experience. Circulation 2010, 121, 892–897. [Google Scholar] [CrossRef]
- Armiñanzas, C.; Fariñas-Alvarez, C.; Zarauza, J.; Muñoz, P.; Ramallo, V.G.; Sellés, M.M.; Meda, J.M.; Pericás, J.M.; Goenaga, M.Á.; Burgos, G.O.; et al. Role of age and comorbidities in mortality of patients with infective endocarditis. Eur. J. Intern. Med. 2019, 64, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Menchi-Elanzi, M.; Ramos-Rincón, J.M.; Merino-Lucas, E.; Reus-Bañuls, S.; Torrús-Tendero, D.; Clíment-Paya, V.; Boix, V.; Portilla-Sogorb, J. Infective endocarditis in elderly and very elderly patients. Aging Clin. Exp. Res. 2020, 32, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, E.; Azeka, E.; Ignêz, Z.M.; Feltrim, Z.; Auler Júnior, J.O. Factors associated with failure of weaning from long-term mechanical ventilation after cardiac surgery. Int. Heart J. 2005, 46, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.M.; Yang, Z.; Yang, G.L.; Huang, Y.; Peng, J.R.; Wu, M.J. Lung injury after cardiopulmonary bypass: Alternative treatment prospects. World J. Clin. Cases 2022, 10, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Huffmyer, J.L.; Groves, D.S. Pulmonary complications of cardiopulmonary bypass. Best Pract. Res. Clin. Anaesthesiol. 2015, 29, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.L.; Alberts, A.R.; Hooft, L.; Mattace-Raso, F.; Mosk, C.A.; van der Laan, L. Prevention of postoperative delirium in elderly patients planned for elective surgery: Systematic review and meta-analysis. Clin. Interv. Aging 2019, 14, 1095–1117. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, G.B.; Hussain, S.T. Current AATS guidelines on surgical treatment of infective endocarditis. Ann. Cardiothorac. Surg. 2019, 8, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Nissen, H.; Nielsen, P.F.; Frederiksen, M.; Helleberg, C.; Nielsen, J.S. Native valve infective endocarditis in the general population: A 10-year survey of the clinical picture during the 1980s. Eur. Heart J. 1992, 13, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Huckaby, L.V.; Hong, Y.; Sultan, I.; Aranda-Michel, E.; Thoma, F.; Wang, Y.; Navid, F.; Gleason, T.G. Surgical treatment of infective endocarditis: Results in 831 patients from a single center. J. Card. Sur. 2020, 35, 2725–2733. [Google Scholar] [CrossRef]
| (a) | |||
| Predictors | Odds Ratio | 95% CI | p-Value |
| Female gender | 2.115 | 1.119–4.001 | 0.021 |
| Age ≥ 70 years | 1.925 | 1.046–3.543 | 0.035 |
| Dialysis (acute and chronic) | 2.853 | 1.295–6.283 | 0.009 |
| NYHA 4 | 3.065 | 1.553–6.049 | 0.001 |
| Previous cardiac surgery | 2.232 | 1.126–4.423 | 0.021 |
| Cardiogenic shock | 4.167 | 1.256–13.829 | 0.020 |
| Neurological deficits (TIA or stroke) | 3.053 | 1.521–6.130 | 0.002 |
| Abscess | 2.252 | 1.199–4.228 | 0.012 |
| (b) | |||
| Predictors | Odds Ratio | 95% CI | p-Value |
| Age < 70 years | |||
| Female gender | 3.280 | 1.355–7.937 | 0.008 |
| Body mass index | 1.088 | 1.025–1.156 | 0.006 |
| Pulmonary hypertension (sPAP > 25 mm Hg) | 3.901 | 1.518–10.023 | 0.005 |
| Dialysis (acute and chronic) | 3.008 | 1.026–8.820 | 0.045 |
| Previous cardiac surgery | 4.032 | 1.734–9.374 | 0.001 |
| Cardiogenic shock | 20.763 | 5.107–84.417 | <0.001 |
| Culture negative endocarditis | 3.369 | 1.422–7.985 | 0.006 |
| Age ≥ 70 years | |||
| Arterial hypertension | 0.196 | 0.058–0.663 | 0.009 |
| NYHA 4 | 5.609 | 1.791–17.570 | 0.003 |
| Insulin dependent diabetes mellitus | 3.817 | 1.017–14.321 | 0.047 |
| Dialysis (acute and chronic) | 4.646 | 1.144–18.859 | 0.032 |
| Cerebral embolization | 5.724 | 1.512–21.669 | 0.010 |
| Aortic valve endocarditis | 0.229 | 0.060–0.869 | 0.030 |
| Abscess | 4.329 | 1.565–11.975 | 0.050 |
| All Patients (n = 413) | C-Group (n = 276, 67%) | E-Group (n = 137, 33%) | p-Value | |
|---|---|---|---|---|
| Age, years | 61.1 ± 14.9 64 (52; 73) | 53.7 ± 12.7 57 (47; 64) | 75.9 ± 3.8 76 (73; 79) | <0.001 |
| Female gender | 105 (25.4%) | 65 (23.6%) | 40 (29.2%) | 0.215 |
| Body mass index [kg/m2] | 25.9 (23.0; 29.4) | 25.5 (22.6; 29.3) | 26.2 (23.4; 29.9) | 0.151 |
| EuroSCORE II | 12.1 (5.2; 27.3) | 8.3 (3.6; 19.7) | 24.5 (12.0; 45.9) | <0.001 |
| COPD | 50 (12.1%) | 30 (10.9%) | 20 (14.6%) | 0.274 |
| Arterial hypertension | 240 (58.1%) | 134 (48.6%) | 106 (77.4%) | <0.001 |
| Pulmonary hypertension | 86 (20.9%) | 49 (17.8%) | 37 (27.2%) | 0.026 |
| LVEF (%), | 55 (49;55) | 55 (50;55) | 55 (47;55) | 0.381 |
| LVEF < 30% | 41 (10.5%) | 23 (8.9%) | 18 (13.6%) | 0.147 |
| Heart rhythm | ||||
| Atrial fibrillation | 81 (19.6%) | 40 (14.5%) | 41 (29.9%) | <0.001 |
| Pacemaker patient | 40 (9.7%) | 23 (8.3%) | 17 (12.4%) | 0.187 |
| Peripheral vascular disease | 36 (8.7%) | 22 (8.0%) | 14 (10.2%) | 0.446 |
| Drug abuse | 23 (5.6%) | 23 (8.3%) | 0 (0.0%) | 0.001 |
| Type 2 diabetes mellitus | 83 (20.1%) | 34 (12.3%) | 49 (35.8%) | <0.001 |
| IDDM | 45 (10.9%) | 20 (7.2%) | 25 (18.2%) | 0.001 |
| Hyperlipoproteinemia | 116 (28.1%) | 56 (20.3%) | 60 (43.8%) | <0.001 |
| Smoking | 103 (27.8%) | 86 (34.3%) | 17 (14.2%) | <0.001 |
| Immunosuppressive therapy | 11 (2.7%) | 10 (3.6%) | 1 (0.7%) | 0.110 |
| Acute renal insufficiency | 53 (12.8%) | 29 (10.5%) | 24 (17.5%) | 0.045 |
| Chronic renal insufficiency | 116 (28.1%) | 60 (21.7%) | 56 (40.9%) | <0.001 |
| NYHA IV | 83 (20.2%) | 53 (19.3%) | 30 (22.1%) | 0.519 |
| Coronary heart disease | 178 (43.2%) | 94 (34.1%) | 84 (61.8%) | <0.001 |
| Previous PCI | 37 (9.0%) | 15 (5.4%) | 22 (16.1%) | <0.001 |
| Previous cardiac surgery | 171 (41.4%) | 94 (34.1%) | 77 (56.2%) | <0.001 |
| CABG | 9 (2.2%) | 4 (1.4%) | 5 (3.6%) | |
| Aortic valve replacement | 69 (16.7%) | 33 (12.0%) | 36 (26.3%) | |
| Mitral valve replacement/repair | 6 (1.5%) | 3 (1.1%) | 3 (2.2%) | |
| Combined valve surgery | 79 (19.1%) | 48 (17.4%) | 31 (22.6%) | |
| TAVI | 2 (0.5%) | 0 (0%) | 2 (1.5%) | |
| Others | 6 (1.5%) | 6 (2.2%) | 0 (0%) | |
| Clinical presentation | ||||
| Acute myocardial infarction (≤48 h) | 14 (3.4%) | 12 (4.4%) | 2 (1.5%) | 0.156 |
| Cardiogenic shock | 21 (5.1%) | 14 (5.1%) | 7 (5.1%) | 0.987 |
| CPR (≤48 h) | 9 (2.2%) | 5 (1.8%) | 4 (2.9%) | 0.487 |
| Preoperative state | ||||
| Emergency | 90 (21.8%) | 69 (25.0%) | 21 (15.3%) | 0.025 |
| Transfer from intensive care unit | 109 (26.5%) | 79 (28.7%) | 30 (21.9%) | 0.139 |
| Intubated at admission | 38 (9.2%) | 25 (9.1%) | 13 (9.5%) | 0.887 |
| Neurological deficits | 81 (19.6%) | 58 (21.0%) | 23 (16.8%) | 0.308 |
| Embolization | 114 (27.6%) | 83 (30.1%) | 31 (22.6%) | 0.111 |
| Fever | 270 (66.5%) | 192 (70.6%) | 78 (58.2%) | 0.013 |
| 1 = up to surgery | 63 (15.5%) | 51 (18.8%) | 12 (9.0%) | |
| 2 = until 72 h before surgery | 15 (3.7%) | 12 (4.4%) | 3 (2.2%) | |
| 3 = 4–7 days before surgery | 39 (9.6%) | 28 (10.3%) | 11 (8.2%) | |
| 4 = over 7 days | 153 (37.7%) | 101 (37.1%) | 52 (38.8%) | |
| Tumor/malignancy | 55 (13.3%) | 29 (10.5%) | 26 (19.0%) | 0.017 |
| Rheumatic disease | 23 (5.6%) | 17 (6.2%) | 6 (4.4%) | 0.458 |
| Previous endocarditis | 60 (14.5%) | 43 (15.6%) | 17 (12.4%) | 0.389 |
| Liver disease | 55 (13.3%) | 44 (16.0%) | 11 (8.0%) | 0.025 |
| Time from diagnosis to surgery | 0.003 | |||
| 1 ≤ 1 day | 65 (15.9%) | 53 (19.4%) | 12 (8.8%) | |
| 2 = 2–3 days | 46 (11.2%) | 35 (12.8%) | 11 (8.0%) | |
| 3 = 4–7 days | 56 (13.7%) | 40 (14.7%) | 16 (11.7%) | |
| 4 ≥7 days | 243 (59.3%) | 145 (53.1%) | 98 (71.5%) | |
| Time from antibiotic start to surgery | 0.003 | |||
| 1 =< 1 day | 59 (14.5%) | 48 (17.6%) | 11 (8.1%) | |
| 2 = 2–3 days | 38 (9.3%) | 28 (10.3%) | 10 (7.4%) | |
| 3 = 4–7 days | 47 (11.5%) | 37 (13.6%) | 10 (7.4%) | |
| 4 => 7 days | 264 (64.7%) | 160 (58.6%) | 104 (77.0%) | |
| Pathogens | ||||
| 1 = staph. aureus | 82 (20.0%) | 64 (23.4%) | 18 (13.1%) | |
| 2 = enterococcus | 61 (14.8%) | 28 (10.2%) | 33 (24.1%) | |
| 3 = streptok. viridans | 43 (10.5%) | 34 (12.4%) | 9 (6.6%) | |
| 4 = grampos. Streptococcus | 37 (9.0%) | 22 (8.0%) | 15 (10.9%) | |
| 5 = HACEK group | 1 (0.2%) | 1 (0.4%) | 0 (0%) | |
| 6 = mycosis | 6 (1.5%) | 5 (1.8%) | 1 (0.7%) | |
| 7 = other | 39 (9.5%) | 27 (9.9%) | 12 (8.8%) | |
| 8 = non-pathogen | 113 (27.5%) | 75 (27.4%) | 38 (27.7%) | |
| 9 = Staphylococcus epidermidis | 28 (6.8%) | 17 (6.2%) | 11 (8.0%) | |
| 10 = 2 + 7 | 1 (0.2%) | 1 (0.4%) | 0 (0%) | |
| MRSA | 14 (3.4%) | 11 (4.0%) | 3 (2.2%) | 0.403 |
| Affected valves | 0.027 | |||
| 1 = AV | 128 (31.0%) | 95 (34.4%) | 33 (24.1%) | |
| 2 = MV | 92 (22.3%) | 61 (22.1%) | 31 (22.6%) | |
| 3 = TV | 7 (1.7%) | 6 (2.2%) | 1 (0.7%) | |
| 5 = AV + MV | 33 (8.0%) | 24 (8.7%) | 9 (6.6%) | |
| 6 = MV + TV | 2 (0.5%) | 2 (0.7%) | 0 (0%) | |
| 7 = only prosthetic valve endocarditis | 143 (34.6%) | 82 (29.7%) | 61 (44.5%) | |
| 8 = TAVI | 1 (0.2%) | 0 (0%) | 1 (0.7%) | |
| 9 = AV + TV | 5 (1.2%) | 5 (1.8%) | 0 (0%) | |
| 10 = AV + TV + MV | 2 (0.5%) | 1 (0.4%) | 1 (0.7%) | |
| Insufficiency (at least grade II, medium) and localization | 359 (87.3%) | 244 (89.1%) | 115 (83.9%) | 0.142 |
| 1 = AV | 108 (26.3%) | 89 (32.5%) | 19 (13.9%) | |
| 2 = MV | 78 (19.0%) | 55 (20.1%) | 23 (16.8%) | |
| 3 = TV | 8 (1.9%) | 6 (2.2%) | 2 (1.5%) | |
| 5 = AV + MV | 32 (7.8%) | 19 (6.9%) | 13 (9.5%) | |
| 6 = MV + TV | 3 (0.7%) | 3 (1.1%) | 0 (0%) | |
| 7 = Prostheses | 71 (17.3%) | 41 (15.0%) | 30 (21.9%) | |
| 8 = paravalv. leakage | 17 (4.1%) | 11 (4.0%) | 6 (4.4%) | |
| 9 = prosthetic endocarditis + parav. leak | 17 (4.1%) | 6 (2.2%) | 11 (8.0%) | |
| 10 = prosthetic endocarditis + flap | 13 (3.2%) | 10 (3.6%) | 3 (2.2%) | |
| 11 = parav. leak + valve | 2 (0.5%) | 0 (0.0%) | 2 (1.5%) | |
| 12 = more than 2 valves | 10 (2.4%) | 4 (1.5%) | 6 (4.4%) |
| All Patients (n = 413) | Patients Aged < 70 Years (n = 276, 67%) | Patients ≥ 70 Years (n = 137, 33%) | p-Value | |
|---|---|---|---|---|
| Length of surgery [min] | 273 (220;355) | 265 (210;338) | 305 (242;385) | 0.001 |
| Cardiopulmonary bypass time [min] | 166 (125;215) | 161 (120;208) | 176 (135;225) | 0.044 |
| Cross-clamp time [min] | 116 (86;156) | 111 (83;156) | 122 (92;157) | 0.089 |
| Circulatory arrest [min] | 0 (0–36) | 0 (0–31) | 0 (0–36) | 0.294 |
| Number of packed red blood cells, unit | 3 (0–27) | 2 (0–27) | 4 (0–17) | <0.001 |
| Number of fresh frozen plasma, unit | 0 (0–13) | 0 (0–12) | 0 (0–13) | 0.744 |
| Number of platelets, unit | 1 (0–6) | 1 (0–6) | 1 (0–4) | 0.045 |
| Abscess | 113 (27.8%) | 67 (24.7%) | 46 (33.8%) | 0.053 |
| Vegetation | 285 (70.4%) | 194 (72.1%) | 91 (66.9%) | 0.278 |
| 1 =< 5 mm | 49 (12.1%) | 39 (14.5%) | 10 (7.4%) | |
| 2 = 5–10 mm | 63 (15.6%) | 38 (14.1%) | 25 (18.4%) | |
| 3 = 11–20 mm | 134 (33.1%) | 89 (33.1%) | 45 (33.1%) | |
| 4 => 20 mm | 39 (9.6%) | 28 (10.4%) | 11 (8.1%) | |
| AVR | 305 (74.2%) | 201 (73.1%) | 104 (76.5%) | 0.461 |
| 1 = AVR biological | 190 (46.2%) | 117 (42.5%) | 73 (53.7%) | |
| 2 = AVR mechanical | 27 (6.6%) | 25 (9.1%) | 2 (1.5%) | |
| 3 = AVr | 3 (0.7%) | 2 (0.7%) | 1 (0.7%) | |
| 4 = Aortic root replacement biological | 80 (19.5%) | 52 (18.9%) | 28 (20.6%) | |
| 5 = Aortic root replacement mechanical | 5 (1.2%) | 5 (1.8%) | 0 (0%) | |
| MVR | 155 (37.7%) | 102 (37.1%) | 53 (39.0%) | 0.711 |
| 1 = MVR biological | 111 (27.0%) | 73 (26.5%) | 38 (27.9%) | |
| 2 = MVR mechanical | 13 (3.2%) | 11 (4.0%) | 2 (1.5%) | |
| 3 = MVr | 31 (7.5%) | 18 (6.5%) | 13 (9.6%) | |
| TVR | 15 (3.6%) | 13 (4.7%) | 2 (1.5%) | 0.159 |
| 1 = TVR biological | 3 (0.7%) | 3 (1.1%) | 0 (0%) | |
| 3 = TVr | 12 (2.9%) | 10 (3.6%) | 2 (1.5%) | |
| With: | 193 (47.0%) | 125 (45.5%) | 68 (50.0%) | 0.385 |
| 1 = ACB | 49 (11.9%) | 32 (11.6%) | 17 (12.5%) | |
| 2 = VSD closure | 1 (0.2%) | 1 (0.4%) | 0 (0%) | |
| 3 = PM | 12 (2.9%) | 9 (3.3%) | 3 (2.2%) | |
| 4 = other | 101 (24.6%) | 67 (24.4%) | 34 (25.0%) | |
| 5 = ASD closure | 4 (1.0%) | 2 (0.7%) | 2 (1.5%) | |
| 6 = several | 26 (6.3%) | 14 (5.1%) | 12 (8.8%) |
| All Patients (n = 413) | Patients Aged < 70 Years (n = 276, 67%) | Patients ≥ 70 Years (n = 137, 33%) | p-Value | |
|---|---|---|---|---|
| 24 h drainage loss [mL] | 600 (300;1100) | 500 (300;950) | 775 (500;1350) | <0.001 |
| Re-thoracotomy due to bleeding/tamponade | 50 (12.4%) | 29 (10.8%) | 21 (15.6%) | 0.169 |
| 24 h number of packed red blood cells, unit | 2 (0–27) | 2 (0–27) | 2 (0–20) | 0.611 |
| 24 h number of fresh frozen plasma, unit | 0 (0–29) | 0 (0–29) | 3 (0–18) | 0.006 |
| 24 h number of platelets, unit | 0 (0–8) | 0 (0–8) | 0 (0–5) | 0.032 |
| 48 h number of packed red blood cells, unit | 2 (0–27) | 2 (0–27) | 2 (0–23) | 0.142 |
| 48 h number of fresh frozen plasma | 0 (0–35) | 0 (0–35) | 3 (0–24) | 0.006 |
| 48 h number of platelets, unit | 0 (0–9) | 0 (0–9) | 0 (0–8) | 0.004 |
| Ventilation time [h] | 16 (9;45) | 14 (8;37) | 23 (11;85) | <0.001 |
| Reintubation | 49 (12.3%) | 26 (9.7%) | 23 (17.4%) | 0.027 |
| Tracheotomy | 57 (14.5%) | 31 (11.8%) | 26 (20.2%) | 0.027 |
| Bronchopulmonary infection | 45 (11.1%) | 21 (7.7%) | 24 (17.9%) | 0.002 |
| New onset of hemodialysis | 61 (15.6%) | 28 (10.6%) | 33 (25.6%) | <0.001 |
| Hemodialysis, days | 5 (3;9) | 4 (3;8) | 5 (3;10) | 0.602 |
| ICU time [d] | 3 (1;7) | 2 (1;6) | 4 (2;9) | 0.001 |
| Re-admission to the ICU | 34 (8.5%) | 24 (9.0%) | 10 (7.6%) | 0.634 |
| Re-admission POD | 9.5 (5.0;16.3) | 10.0 (5.0;17.0) | 8.0 (4.0;14.0) | 0.513 |
| Postoperative days | 10 (7;16) | 10 (7;16) | 10 (6;16) | 0.578 |
| Postoperative delirium | 64 (16.1%) | 27 (10.2%) | 37 (28.2%) | <0.001 |
| Neurologic damage | 27 (6.8%) | 14 (5.3%) | 13 (9.9%) | 0.085 |
| TIA | 9 (2.3%) | 5 (1.9%) | 4 (3.1%) | |
| Stroke | 18 (4.5%) | 9 (3.4%) | 9 (6.9%) | |
| CPR | 22 (5.5%) | 15 (5.6%) | 7 (5.3%) | 0.903 |
| Newly appeared atrial fibrillation | 17 (4.9%) | 7 (2.9%) | 10 (9.4%) | 0.010 |
| Pacemaker patient | 47 (11.6%) | 27 (10.0%) | 20 (14.9%) | 0.142 |
| Postoperative myocardial infarction | 5 (1.3%) | 3 (1.1%) | 2 (1.5%) | 0.666 |
| Sepsis | 54 (13.3%) | 31 (11.4%) | 23 (17.0%) | 0.114 |
| Sternal wound infection | 9 (2.5%) | 8 (3.2%) | 1 (0.9%) | 0.282 |
| 7-day mortality | 50 (12.1%) | 27 (9.8%) | 23 (16.8%) | 0.040 |
| 30-day mortality | 74 (17.9%) | 38 (13.8%) | 36 (26.3%) | 0.002 |
| Hospital mortality | 68 (16.6%) | 36 (13.1%) | 32 (23.5%) | 0.008 |
| Cardiac death | 10 (14.3%) | 5 (13.5%) | 5 (15.2%) | |
| Cerebral death | 1 (1.4%) | 1 (2.7%) | 0 (0%) | |
| Sepsis | 9 (12.9%) | 5 (13.5%) | 4 (12.1%) | |
| Multi-organ failure | 50 (71.4%) | 26 (70.3%) | 24 (72.7%) |
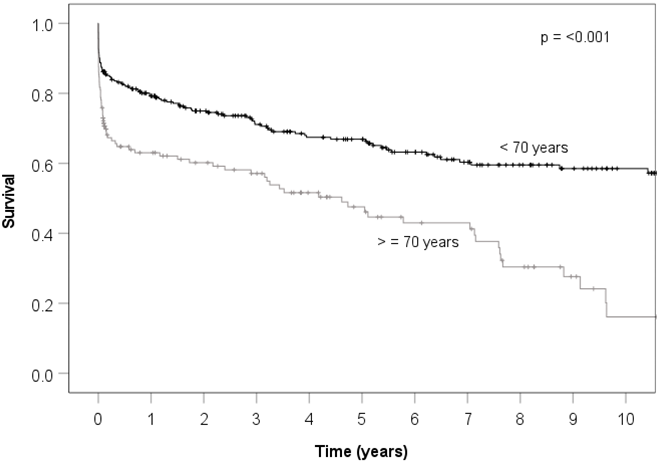 | |||||
| Time (years) | 1 | 3 | 5 | 10 | |
| Patients < 70 years | |||||
| N at risk | 263 | 158 | 121 | 50 | 6 |
| Survival | 0.79 | 0.71 | 0.67 | 0.58 | 0.53 |
| Patients ≥ 70 years | |||||
| N at risk | 128 | 59 | 39 | 8 | |
| Survival | 0.62 | 0.56 | 0.47 | 0.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jussli-Melchers, J.; Salem, M.A.; Schoettler, J.; Friedrich, C.; Huenges, K.; Elke, G.; Puehler, T.; Cremer, J.; Haneya, A. Mid- and Long-Term Surgical Outcomes Due to Infective Endocarditis in Elderly Patients: A Retrospective Cohort Study. J. Clin. Med. 2022, 11, 6693. https://doi.org/10.3390/jcm11226693
Jussli-Melchers J, Salem MA, Schoettler J, Friedrich C, Huenges K, Elke G, Puehler T, Cremer J, Haneya A. Mid- and Long-Term Surgical Outcomes Due to Infective Endocarditis in Elderly Patients: A Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(22):6693. https://doi.org/10.3390/jcm11226693
Chicago/Turabian StyleJussli-Melchers, Jill, Mohamed Ahmed Salem, Jan Schoettler, Christine Friedrich, Katharina Huenges, Gunnar Elke, Thomas Puehler, Jochen Cremer, and Assad Haneya. 2022. "Mid- and Long-Term Surgical Outcomes Due to Infective Endocarditis in Elderly Patients: A Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 22: 6693. https://doi.org/10.3390/jcm11226693
APA StyleJussli-Melchers, J., Salem, M. A., Schoettler, J., Friedrich, C., Huenges, K., Elke, G., Puehler, T., Cremer, J., & Haneya, A. (2022). Mid- and Long-Term Surgical Outcomes Due to Infective Endocarditis in Elderly Patients: A Retrospective Cohort Study. Journal of Clinical Medicine, 11(22), 6693. https://doi.org/10.3390/jcm11226693







