Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
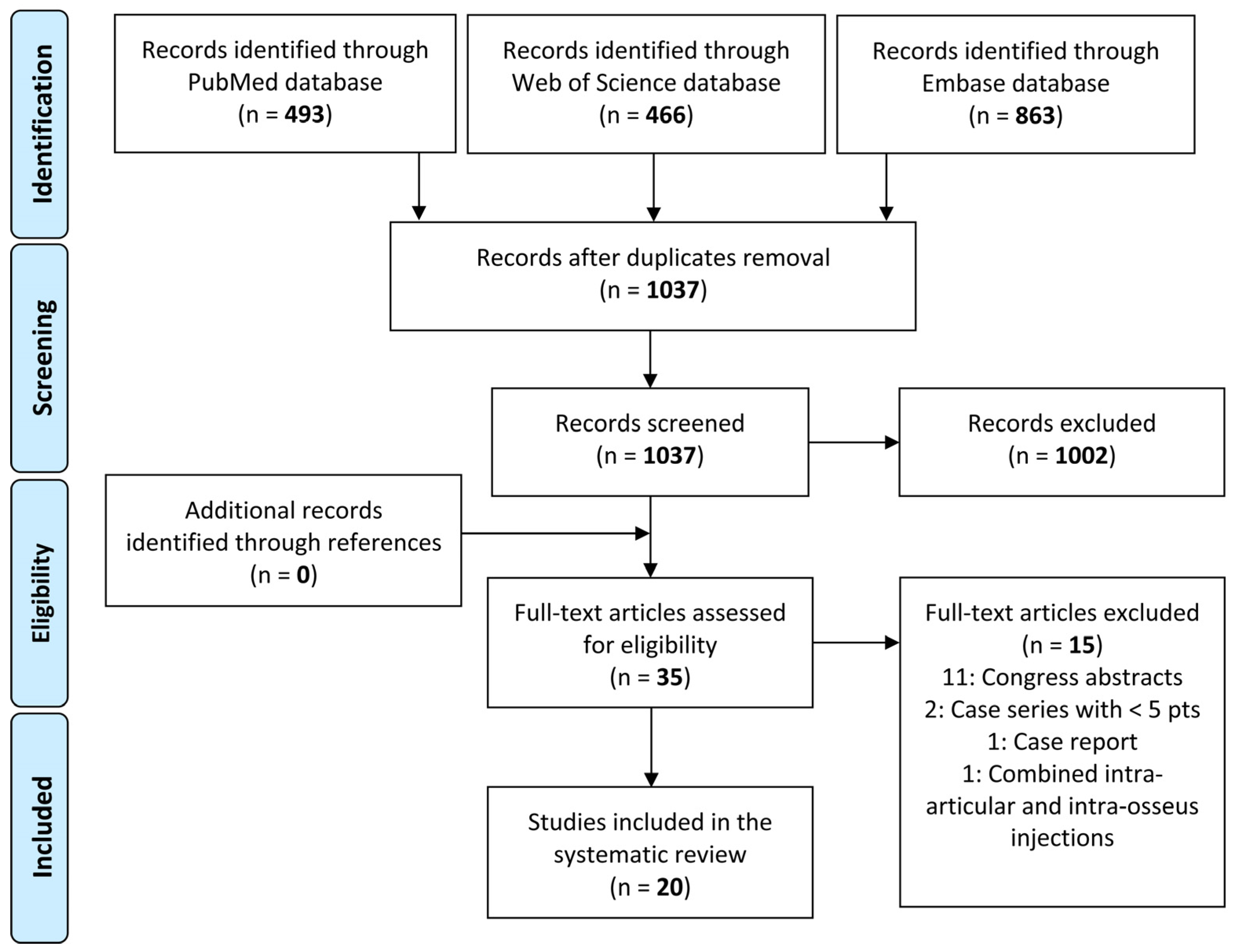
2.1. Search Strategy and Article Selection
2.2. Data Extraction, Outcome Measurement, and Quality Assessment
3. Results
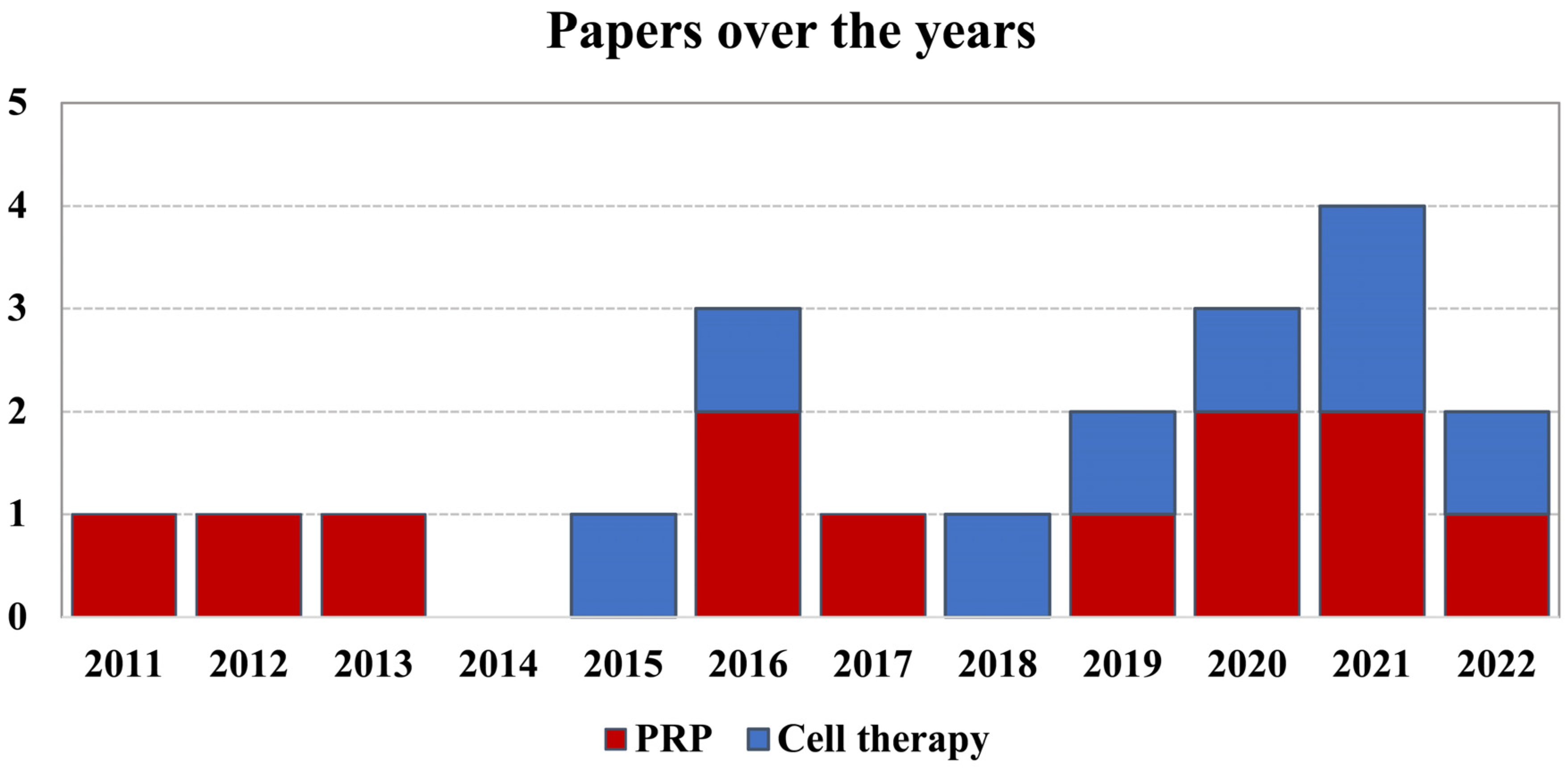
3.1. Article Selection and Characteristics
3.2. Orthobiologic Products
3.3. Safety
3.4. Clinical Efficacy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mobasheri, A.; Batt, M. An Update on the Pathophysiology of Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Zhang, Y. Epidemiology of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. The Epidemiology and Impact of Pain in Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- da Costa, B.R.; Reichenbach, S.; Keller, N.; Nartey, L.; Wandel, S.; Jüni, P.; Trelle, S. Effectiveness of Non-Steroidal Anti-Inflammatory Drugs for the Treatment of Pain in Knee and Hip Osteoarthritis: A Network Meta-Analysis. Lancet 2017, 390, e21–e33. [Google Scholar] [CrossRef]
- Leite, V.F.; Daud Amadera, J.E.; Buehler, A.M. Viscosupplementation for Hip Osteoarthritis: A Systematic Review and Meta-Analysis of the Efficacy on Pain and Disability, and the Occurrence of Adverse Events. Arch. Phys. Med. Rehabil. 2018, 99, 574–583. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Longo, U.G.; Madry, H.; Marchettini, P.; Marmotti, A.; Van Assche, D.; Zanon, G.; Peretti, G.M. Non-Surgical Treatments for the Management of Early Osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2016, 24, 1775–1785. [Google Scholar] [CrossRef]
- Pereira, L.C.; Kerr, J.; Jolles, B.M. Intra-Articular Steroid Injection for Osteoarthritis of the Hip Prior to Total Hip Arthroplasty: Is It Safe? A Systematic Review. Bone Jt. J. 2016, 98-B, 1027–1035. [Google Scholar] [CrossRef]
- Bayliss, L.E.; Culliford, D.; Monk, A.P.; Glyn-Jones, S.; Prieto-Alhambra, D.; Judge, A.; Cooper, C.; Carr, A.J.; Arden, N.K.; Beard, D.J.; et al. The Effect of Patient Age at Intervention on Risk of Implant Revision after Total Replacement of the Hip or Knee: A Population-Based Cohort Study. Lancet 2017, 389, 1424–1430. [Google Scholar] [CrossRef]
- Gronbeck, C.; Cote, M.P.; Lieberman, J.R.; Halawi, M.J. Risk Stratification in Primary Total Joint Arthroplasty: The Current State of Knowledge. Arthroplast. Today 2019, 5, 126–131. [Google Scholar] [CrossRef]
- Schreurs, B.W.; Hannink, G. Total Joint Arthroplasty in Younger Patients: Heading for Trouble? Lancet 2017, 389, 1374–1375. [Google Scholar] [CrossRef]
- Sullivan, S.W.; Aladesuru, O.M.; Ranawat, A.S.; Nwachukwu, B.U. The Use of Biologics to Improve Patient-Reported Outcomes in Hip Preservation. J. Hip Preserv. Surg. 2021, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, C.; Boffa, A.; Andriolo, L.; Silva, S.; Grigolo, B.; Zaffagnini, S.; Filardo, G. Bone Marrow Concentrate Injections for the Treatment of Osteoarthritis: Evidence from Preclinical Findings to the Clinical Application. Int. Orthop. 2021, 45, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Dimarino, A.M.; Caplan, A.I.; Bonfield, T.L. Mesenchymal Stem Cells in Tissue Repair. Front. Immunol. 2013, 4, 201. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Tuan, R.S. Mesenchymal Stem Cells in Arthritic Diseases. Arthritis Res. Ther. 2008, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Belk, J.W.; Kraeutler, M.J.; Houck, D.A.; Goodrich, J.A.; Dragoo, J.L.; McCarty, E.C. Platelet-Rich Plasma Versus Hyaluronic Acid for Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Sports Med. 2021, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Previtali, D.; Napoli, F.; Candrian, C.; Zaffagnini, S.; Grassi, A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage 2021, 13, 364S–375S. [Google Scholar] [CrossRef]
- Harris, J.D.; Quatman, C.E.; Manring, M.M.; Siston, R.A.; Flanigan, D.C. How to Write a Systematic Review. Am. J. Sports Med. 2014, 42, 2761–2768. [Google Scholar] [CrossRef]
- Page, M.J.; Shamseer, L.; Tricco, A.C. Registration of Systematic Reviews in PROSPERO: 30,000 Records and Counting. Syst. Rev. 2018, 7, 32. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
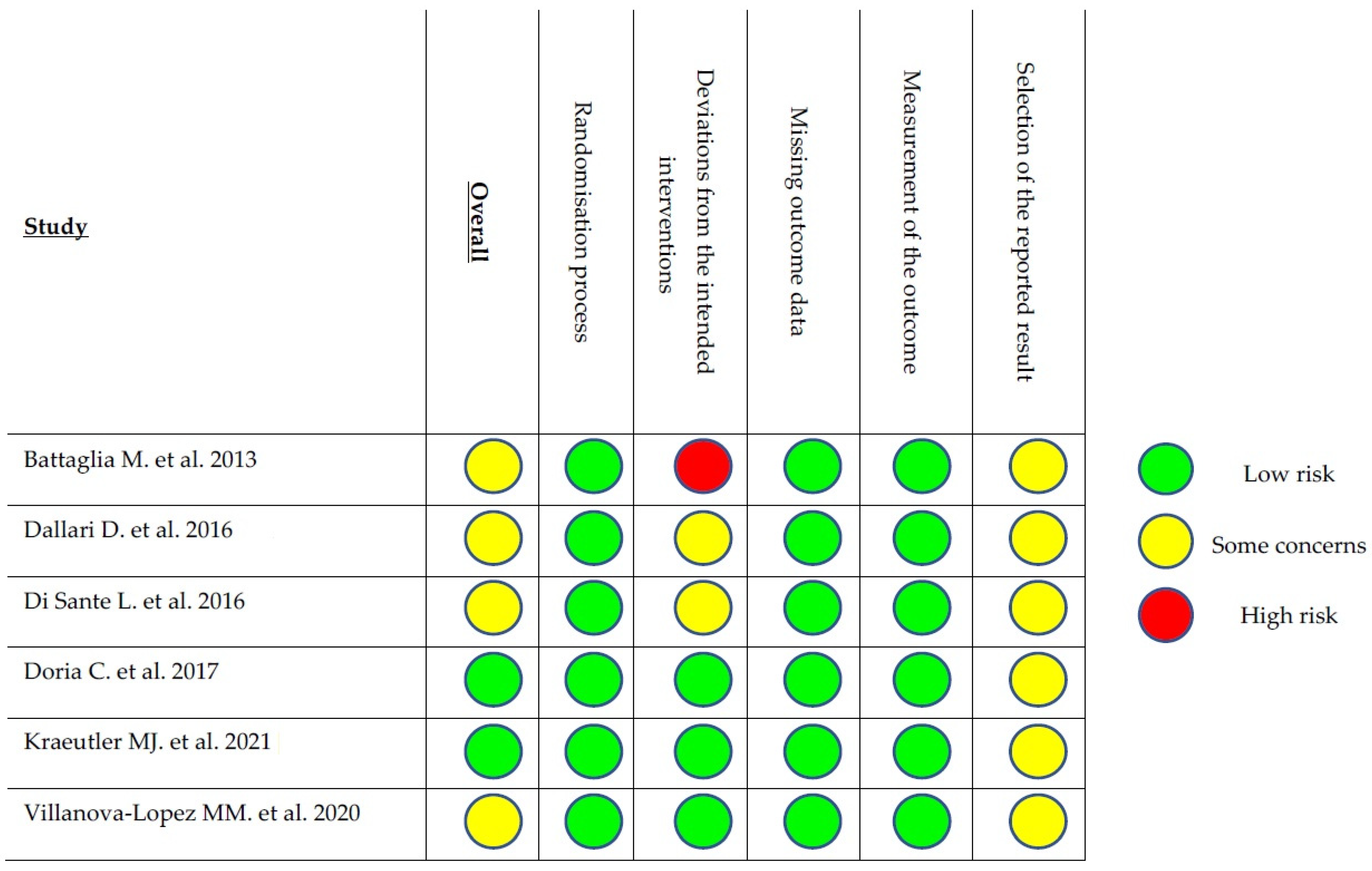
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Guaraldi, F.; Vannini, F.; Buscio, T.; Buda, R.; Galletti, S.; Giannini, S. Platelet-Rich Plasma (PRP) Intra-Articular Ultrasound-Guided Injections as a Possible Treatment for Hip Osteoarthritis: A Pilot Study. Clin. Exp. Rheumatol. 2011, 29, 754. [Google Scholar] [PubMed]
- Sánchez, M.; Guadilla, J.; Fiz, N.; Andia, I. Ultrasound-Guided Platelet-Rich Plasma Injections for the Treatment of Osteoarthritis of the Hip. Rheumatology 2012, 51, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.R.; Haffey, P.; Valimahomed, A.; Gellhorn, A.C. The Effectiveness of Autologous Platelet-Rich Plasma for Osteoarthritis of the Hip: A Retrospective Analysis. Pain Med. 2019, 20, 1611–1618. [Google Scholar] [CrossRef]
- Ortiz-Declet, V.; Iacobelli, D.A.; Battaglia, M.R.; Go, C.C.; Maldonado, D.R.; Lall, A.C.; Domb, B.G. The Effect of Platelet-Rich Plasma in Patients with Early Hip Osteoarthritis: A Pilot Study. J. Hip Preserv. Surg. 2020, 7, 496–502. [Google Scholar] [CrossRef]
- Battaglia, M.; Guaraldi, F.; Vannini, F.; Rossi, G.; Timoncini, A.; Buda, R.; Giannini, S. Efficacy of Ultrasound-Guided Intra-Articular Injections of Platelet-Rich Plasma versus Hyaluronic Acid for Hip Osteoarthritis. Orthopedics 2013, 36, e1501–e1508. [Google Scholar] [CrossRef]
- Dallari, D.; Stagni, C.; Rani, N.; Sabbioni, G.; Pelotti, P.; Torricelli, P.; Tschon, M.; Giavaresi, G. Ultrasound-Guided Injection of Platelet-Rich Plasma and Hyaluronic Acid, Separately and in Combination, for Hip Osteoarthritis: A Randomized Controlled Study. Am. J. Sports Med. 2016, 44, 664–671. [Google Scholar] [CrossRef]
- Di Sante, L.; Villani, C.; Santilli, V.; Valeo, M.; Bologna, E.; Imparato, L.; Paoloni, M.; Iagnocco, A. Intra-Articular Hyaluronic Acid vs. Platelet-Rich Plasma in the Treatment of Hip Osteoarthritis. Med. Ultrason. 2016, 18, 463–468. [Google Scholar] [CrossRef]
- Doria, C.; Mosele, G.R.; Caggiari, G.; Puddu, L.; Ciurlia, E. Treatment of Early Hip Osteoarthritis: Ultrasound-Guided Platelet Rich Plasma versus Hyaluronic Acid Injections in a Randomized Clinical Trial. Joints 2017, 5, 152–155. [Google Scholar] [CrossRef]
- Villanova-López, M.M.; Núñez-Núñez, M.; Fernández-Prieto, D.; González-López, C.; García-Donaire, J.; Pérez-Pérez, A.; Sandoval Fernández Del Castillo, S.; Murillo-Izquierdo, M.; Camean-Fernández, M.; Gutiérrez-Pizarraya, A.; et al. Randomized, Double-Blind, Controlled Trial, Phase III, to Evaluate the Use of Platelet-Rich Plasma versus Hyaluronic Acid in Hip Coxarthrosis. Rev. Esp. Cir. Ortop. Traumatol. 2020, 64, 134–142. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Houck, D.A.; Garabekyan, T.; Miller, S.L.; Dragoo, J.L.; Mei-Dan, O. Comparing Intra-Articular Injections of Leukocyte-Poor Platelet-Rich Plasma Versus Low-Molecular Weight Hyaluronic Acid for the Treatment of Symptomatic Osteoarthritis of the Hip: A Double-Blind, Randomized Pilot Study. Orthop. J. Sport. Med. 2021, 9, 2325967120969210. [Google Scholar] [CrossRef]
- Palco, M.; Rizzo, P.; Basile, G.C.; Alito, A.; Bruschetta, D.; Accorinti, M.; Restuccia, R.; Leonetti, D. Short- and Midterm Comparison of Platelet-Rich Plasma with Hyaluronic Acid versus Leucocyte and Platelet-Rich Plasma on Pain and Function to Treat Hip Osteoarthritis. A Retrospective Study. Gels 2021, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, A.; Pennello, E.; Stagni, C.; Del Piccolo, N.; Boffa, A.; Cenacchi, A.; Buzzi, M.; Filardo, G.; Dallari, D. Umbilical Cord PRP vs. Autologous PRP for the Treatment of Hip Osteoarthritis. J. Clin. Med. 2022, 11, 4505. [Google Scholar] [CrossRef] [PubMed]
- Emadedin, M.; Ghorbani Liastani, M.; Fazeli, R.; Mohseni, F.; Moghadasali, R.; Mardpour, S.; Hosseini, S.E.; Niknejadi, M.; Moeininia, F.; Aghahossein Fanni, A.; et al. Long-Term Follow-up of Intra-Articular Injection of Autologous Mesenchymal Stem Cells in Patients with Knee, Ankle, or Hip Osteoarthritis. Arch. Iran. Med. 2015, 18, 336–344. [Google Scholar] [PubMed]
- Mardones, R.; Jofré, C.M.; Tobar, L.; Minguell, J.J. Mesenchymal Stem Cell Therapy in the Treatment of Hip Osteoarthritis. J. Hip Preserv. Surg. 2017, 4, 159–163. [Google Scholar] [CrossRef]
- Rodriguez-Fontan, F.; Piuzzi, N.S.; Kraeutler, M.J.; Pascual-Garrido, C. Early Clinical Outcomes of Intra-Articular Injections of Bone Marrow Aspirate Concentrate for the Treatment of Early Osteoarthritis of the Hip and Knee: A Cohort Study. PM R 2018, 10, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oca, C.; Breda, S.; Elena, N.; Valentini, R.; Samaila, E.M.; Magnan, B. Mesenchymal Stem Cells Injection in Hip Osteoarthritis: Preliminary Results. Acta Biomed. 2019, 90, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Whitney, K.E.; Briggs, K.K.; Chamness, C.; Bolia, I.K.; Huard, J.; Philippon, M.J.; Evans, T.A. Bone Marrow Concentrate Injection Treatment Improves Short-Term Outcomes in Symptomatic Hip Osteoarthritis Patients: A Pilot Study. Orthop. J. Sport. Med. 2020, 8, 2325967120966162. [Google Scholar] [CrossRef]
- Burnham, R.; Smith, A.; Hart, D. The Safety and Effectiveness of Bone Marrow Concentrate Injection for Knee and Hip Osteoarthritis: A Canadian Cohort. Regen. Med. 2021, 16, 619–628. [Google Scholar] [CrossRef]
- Meadows, M.C.; Elisman, K.; Nho, S.J.; Mowry, K.; Safran, M.R. A Single Injection of Amniotic Suspension Allograft Is Safe and Effective for Treatment of Mild to Moderate Hip Osteoarthritis: A Prospective Study. Arthroscopy 2021, 38, 325–331. [Google Scholar] [CrossRef]
- Heidari, N.; Slevin, M.; Zeinolabediny, Y.; Meloni, D.; Olgiati, S.; Wilson, A.; Noorani, A.; Azamfirei, L. Comparison of the Effect of MFAT and MFAT + PRP on Treatment of Hip Osteoarthritis: An Observational, Intention-to-Treat Study at One Year. J. Clin. Med. 2022, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Delanois, R.E.; Sax, O.C.; Chen, Z.; Cohen, J.M.; Callahan, D.M.; Mont, M.A. Biologic Therapies for the Treatment of Knee Osteoarthritis: An Updated Systematic Review. J. Arthroplast. 2022, in press. [CrossRef] [PubMed]
- Lim, A.; Zhu, J.B.; Khanduja, V. The Use of Intra-Articular Platelet-Rich Plasma as a Therapeutic Intervention for Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: An Expert Opinion and Proposal for a Novel Classification and Coding System. Expert. Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef]
- Boffa, A.; Salerno, M.; Merli, G.; De Girolamo, L.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G. Platelet-Rich Plasma Injections Induce Disease-Modifying Effects in the Treatment of Osteoarthritis in Animal Models. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 4100–4121. [Google Scholar] [CrossRef]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The Effect of Platelet-Rich Plasma Formulations and Blood Products on Human Synoviocytes: Implications for Intra-Articular Injury and Therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef]
- Di Martino, A.; Boffa, A.; Andriolo, L.; Romandini, I.; Altamura, S.A.; Cenacchi, A.; Roverini, V.; Zaffagnini, S.; Filardo, G. Leukocyte-Rich versus Leukocyte-Poor Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Double-Blind Randomized Trial. Am. J. Sports Med. 2022, 50, 609–617. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem Cells: Past, Present, and Future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic Mechanisms in Articular Cartilage and Role of Inflammation in Osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef]
- Filardo, G.; Perdisa, F.; Roffi, A.; Marcacci, M.; Kon, E. Stem Cells in Articular Cartilage Regeneration. J. Orthop. Surg. Res. 2016, 11, 42. [Google Scholar] [CrossRef]
- Roffi, A.; Nakamura, N.; Sanchez, M.; Cucchiarini, M.; Filardo, G. Injectable Systems for Intra-Articular Delivery of Mesenchymal Stromal Cells for Cartilage Treatment: A Systematic Review of Preclinical and Clinical Evidence. Int. J. Mol. Sci. 2018, 19, 3322. [Google Scholar] [CrossRef] [PubMed]
- Perucca Orfei, C.; Boffa, A.; Sourugeon, Y.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G.; de Girolamo, L. Cell-Based Therapies Have Disease-Modifying Effects on Osteoarthritis in Animal Models. A Systematic Review by the ESSKA Orthobiologic Initiative. Part 1: Adipose Tissue-Derived Cell-Based Injectable Therapies. Knee Surg. Sports Traumatol. Arthrosc. 2022. [Google Scholar] [CrossRef] [PubMed]
- Previtali, D.; Merli, G.; Di Laura Frattura, G.; Candrian, C.; Zaffagnini, S.; Filardo, G. The Long-Lasting Effects of “Placebo Injections” in Knee Osteoarthritis: A Meta-Analysis. Cartilage 2021, 13, 185S–196S. [Google Scholar] [CrossRef] [PubMed]
- Civinini, R.; Nistri, L.; Martini, C.; Redl, B.; Ristori, G.; Innocenti, M. Growth Factors in the Treatment of Early Osteoarthritis. Clin. Cases Miner. Bone Metab. 2013, 10, 26–29. [Google Scholar] [CrossRef]
| Studies Using Kellgren–Lawrence Scale | Grade I | Grade II | Grade III | Grade IV |
| Battaglia M. et al., 2011 [22] | − | 4 | 8 | 8 |
| Singh JR. et al., 2019 [24] | 7 | 11 | 9 | 9 |
| Battaglia M. et al., 2013 [26] | − | 39 | 44 | 17 |
| Di Sante L. et al., 2016 [28] | − | 12 | 31 | − |
| Doria C. et al., 2017 [29] | + | + | + | − |
| Villanova-López MM. et al., 2020 [30] | 27 | 37 | 10 | |
| Kraeutler MJ. et al., 2021 [31] | − | 9 | 14 | − |
| Palco M. et al., 2021 [32] | − | 24 | 28 | − |
| Emadedin M. et al., 2015 [34] | − | − | + | + |
| Burnham R. et al., 2021 [39] | + | + | + | + |
| Heidari N. et al., 2022 [41] | 25 | 28 | 33 | 61 |
| Studies Using Tönnis Scale | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
| Sánchez M. et al., 2012 [23] | − | − | 12 | 28 |
| Ortiz-Declet V. et al., 2020 [25] | 3 | 6 | 2 | − |
| Mazzotta A. et al., 2022 [33] | − | 11 | 42 | 43 |
| Mardones R. et al., 2017 [35] | − | 2 | 9 | 2 |
| Rodriguez-Fontan F. et al., 2018 [36] | − | + | + | − |
| Dall’Oca C. et al., 2019 [37] | + | + | + | − |
| Whitney KE. et al., 2020 [38] | − | − | 6 | 12 |
| Meadows MC. et al., 2021 [40] | − | 3 | 6 | − |
| Author Year | Study Design | Injective Product | Product Manufacturing and Characteristics | Injection Schedule and Amount | Patients (Sex) Age Mean + SD | Final F-up | mCMS | Results |
|---|---|---|---|---|---|---|---|---|
| Battaglia M. 2011 [22] | Prospective Case Series | PRP | NR | 3 injections 2 weeks intervals 5 mL US guidance | 20 (13 M/7 F) 52 ± 13 | 12 m | 41 | PRP injections are safe and effective in reducing pain and improving articular function and quality of life in patients affected by hip OA. |
| Sánchez M. 2012 [23] | Prospective Case Series | PRP | LP-PRP (PRGF) Activation: Ca chloride (10%) | 3 injections 1–2 weeks intervals 8 mL US guidance | 40 (27 M/12 F) 56 ± 11.9 | 6 m | 49 | PRP injections improved pain and function in a limited number of patients with severe hip OA. |
| Battaglia M. 2013 [26] | RCT | PRP | LR-PRP Activation: Ca chloride (10%) Plts: Increased 600% vs. WB each unit contained 6 to 8 mln plts Leukocytes: 8.3 × 103/μL | 3 injections 2 weeks intervals 5 mL US guidance | 52 (20 M/30 F) 51 ± 12 | 12 m | 73 | IA injections of PRP are efficacious in terms of functional improvement and pain reduction but are not superior to HA in patients with symptomatic hip OA at 12-month F-up. |
| HA | HMW-HA (1500 kDa) (Hyalubrix 30 mg/2 mL) | 3 injections 2 weeks intervals 2 mL US guidance | 52 (17M/33F) 56 ± 12 | |||||
| Dallari D. 2016 [27] | RCT | PRP | LR-PRP Activation: Ca chloride (10%) | 3 injections 1-week interval 5 mL US guidance | 44 (20 M/24 F) NR | 12 m | 80 | IA PRP injections offer a significant clinical improvement in patients with hip OA without relevant side effects. The addition of PRP+HA did not lead to a significant improvement in pain symptoms. |
| HA | HMW-HA (1500 kDa) (Hyalubrix 30 mg/2 mL) | 3 injections 1-week interval 2 mL US guidance | 36 (26 M/10 F) NR | |||||
| PRP+HA | LR-PRP + HA | 3 injections 1-week interval 7 mL (5 mL PRP + 2 mL HA) US guidance | 31 (12 M/19 F) NR | |||||
| Di Sante L. 2016 [28] | RCT | PRP | LP-PRP Plts: 100–150% vs. WB | 3 injections 1-week interval 3 mL US guidance | 21 (11 M/10 F) 71.37 ± 6.03 | 4 m | 66 | IA PRP had an immediate effect on pain that was not maintained at longer term F-up when, on the contrary, the effects of IA HA were evident. |
| HA | Na-HA (30 mg/2 mL of HA with HMW 1000 to 2900 kDa) | 3 injections 1-week interval 2 mL US guidance | 22 (9 M/13 F) 73.62 ± 7.87 | |||||
| Doria C. 2017 [29] | RCT | PRP | NR | 3 injections 1-week interval 5 mL US guidance | 40 (NR) 67.3 ± 5.8 | 12 m | 68 | PRP did not offer significantly better results compared with HA in patients with moderate signs of OA. |
| HA | HA (Hyalubrix 15 mg/mL) | 3 injections 1-week interval NR US guidance | 40 (NR) 68 ± 4.6 | |||||
| Singh JR. 2019 [24] | Retrospective Case Series | PRP | LP-PRP No activation | Single injection 6 mL IA + 1 mL extracapsular US or fluoroscopy guidance | 36 (12 M/24 F) 66.0 ± 12.1 | 6 m | 51 | In patients with mild/moderate hip OA, PRP may provide pain relief and functional improvement for up to 6 months. |
| Ortiz-Declet V. 2020 [25] | Prospective Case Series | PRP | LP-PRP Plts: 2–3 times the level of WB | 3 injections 1-week interval 4–7 mL US guidance | 9 (4 M/5 F) 51.3 ± 9.4 | 12 m | 61 | Patients with early hip OA had significant improvements up to 12 months after PRP injections. |
| Villanova- López MM. 2020 [30] | RCT | PRP | LR-PRP Plts: 2.22 times the level of WB Leukocytes: 3.87 ± 2.11 × 103/μL | Single injection 6 mL US guidance | 38 (14 M/24 F) 61.2 ± 9.72 | 12 m | 70 | PRP is as effective and safe as those of HA for the treatment of hip OA in its initial stages. |
| HA | HA (Synvisc-One® 60 mg/6 mL) | Single injection 6 mL US guidance | 36 (19 M/17 F) 61.1 ± 12.3; | |||||
| Kraeutler MJ. 2021 [31] | RCT | PRP | LP-PRP Activation: Ca chloride. Plts: 2–3 times the level of WB No leukocytes | 3 injections 1-week interval 4–8 mL PRP No guidance | 19 (8 M/10 F) 53.3 ± 8.4 | 24 m | 79 | LP-PRP resulted in an improvement in WOMAC scores and hip internal rotation at 6 months and delayed the need for THA compared with treatment with LMW-HA. |
| HA | Na-HA (Supartz; 10 mg/2.5 mL) | 3 injections 1-week interval 2.5 mL PRP No guidance | 15 (10 M/3 F) 53.6 ± 7.6 | |||||
| Palco M. 2021 [32] | Retrospective Comparative Study | LR-PRP | LR-PRP Plts: 370,000/μL Leukocytes: 4 × 103/μL | 2 injections 2 weeks interval 5 mL US guidance | 26 (16 M/10 F) 50.62 ± 16.14 | 12 m | 57 | Both treatments are effective at reducing pain in the short to medium term. LR-PRP could be the treatment of choice due to a more marked effect over time. |
| PRP +HA | Cellular Matrix A-CP-HA centrifugation | 2 injections 2 weeks interval 5 mL (3 mL PRP + 2 mL HA) US guidance | 26 (12 M/14 F) 64.81 ± 10.81 | |||||
| Mazzotta A. 2022 [33] | Prospective Comparative Study | C-PRP | LR-PRP Activation: Ca-gluconate (10%) Plts increased by 4–5 times vs. the baseline mean plts concentration of 1000 × 109/L ± 20% | 3 injections 1-week interval 5 mL US guidance | 50 (26 M/20 F) 47 ± 11.9 | 12 m | 63 | C-PRP is a safe approach for the treatment of patients with hip OA, with a low rate of adverse events and failures, although it provided only a mild clinical improvement comparable with A-PRP. |
| A-PRP | LR-PRP Activation: Ca-gluconate (10%) Plts increased by 4–5 times vs. the baseline mean plts concentration of 1000 × 109/L ± 20% | 3 injections 1-week interval 5 mL US guidance | 50 (34 M/16 F) 49.5 ± 12.2 |
| Author Year | Study Design | Injective Product | Product Manufacturing and Characteristics | Injection Schedule and Amount | Patients (Sex) Age Mean + SD | Final F-up | mCMS | Results |
|---|---|---|---|---|---|---|---|---|
| Emadedin M. 2015 [34] | Prospective Case Series | BM-MSC | Autologous Harvest from both iliac crests Expanded Characterized for membrane markers Tested for possible microbial contamination | Single injection 10 mL Fluoroscopy guidance | 6 (NR) NR | 30 m | 51 | BM-MSC injection is safe and therapeutically beneficial in patients with hip OA. |
| Mardones R. 2017 [35] | Prospective Case Series | BM-MSC | Autologous Harvest form posterior iliac crest Expanded Characterized for membrane markers Tested for possible microbial contamination | 3 injections 1-week interval NR No guidance | 10 (5 M/5 F) 54.7 | 40 m | 54 | The IA injection of 3 consecutive weekly doses of expanded autologous BM-MSC proved to be a safe and clinically effective in patients with hip OA. |
| Rodriguez- Fontan F. 2018 [36] | Prospective Case Series | BMAC | Autologous Harvest from the anterior iliac crest Not expanded BioCUE Platelet Concentration System | Single injection 12 mL US or RX guidance | 13 (NR); 58 ± 12.7 (also knee) | 24 m | 54 | IA injections of BMAC were safe and demonstrated satisfactory results for the treatment of early hip OA. |
| Dall’Oca C. 2019 [37] | Retrospective Case Series | MF-AT | Autologous Harvest from abdominal wall adipose tissue Not expanded Lipogems® system | Single injection 5–10 mL Fluoroscopy guidance Traction | 6 (5 M/1 F) 52 (37–60) | 6 m | 42 | MF-AT injection provided a significant clinical improvement in patients with early hip OA. |
| Whitney KE. 2020 [38] | Prospective Case Series | BMAC | Autologous Harvest from the posterior iliac crest Not expanded Hematology analysis | Single injection 6–12 mL US guidance | 21 (7 M/9 F) 57.6 ± 11 | 6 m | 57 | A single BMAC injection can significantly improve subjective pain and function scores up to 6 months in patients with symptomatic hip OA. |
| Burnham R. 2021 [39] | Prospective Case Series | BMAC | Autologous Harvest from the posterior iliac crest Not expanded | Single injection 8–10 mL US guidance | 30 (64 M/48 F) 64.1 ± 9.1 | 12 m | 60 | Hip OA treated with a single BMAC injection resulted in significant improvements in pain, disability, and quality of life with a low complication rate. |
| Meadows MC. 2021 [40] | Prospective Case Series | ASA | Homologous Not expanded | Single injection 4 mL US guidance | 10 (5 M/4 F) 54.2 ± 6.0 | 12 m | 53 | Promising results for relief of pain and improvement in patient-reported outcomes with IA ASA in patients affected by hip OA. |
| Heidari N. 2022 [41] | Prospective Comparative Study | MF-AT | Autologous Harvest from abdominal wall adipose tissue Lipogems® system | Single injection 6 mL US guidance | 57 (21 M/36 F) 60 | 12 m | 59 | Positive role for IA injection of MF-AT + PRP as a treatment for hip OA which may be important particularly in low BMI patients where the difficulty in obtaining sufficient MF-AT. |
| MF-AT + PRP | LP-PRP Activation: Ca-chloride Rich in plts | Single injection 6ml (4 mL MF-AT + 2 mL PRP) US guidance | 90 (53 M/37 F) 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaffagnini, M.; Boffa, A.; Andriolo, L.; Raggi, F.; Zaffagnini, S.; Filardo, G. Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review. J. Clin. Med. 2022, 11, 6663. https://doi.org/10.3390/jcm11226663
Zaffagnini M, Boffa A, Andriolo L, Raggi F, Zaffagnini S, Filardo G. Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review. Journal of Clinical Medicine. 2022; 11(22):6663. https://doi.org/10.3390/jcm11226663
Chicago/Turabian StyleZaffagnini, Marco, Angelo Boffa, Luca Andriolo, Federico Raggi, Stefano Zaffagnini, and Giuseppe Filardo. 2022. "Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review" Journal of Clinical Medicine 11, no. 22: 6663. https://doi.org/10.3390/jcm11226663
APA StyleZaffagnini, M., Boffa, A., Andriolo, L., Raggi, F., Zaffagnini, S., & Filardo, G. (2022). Orthobiologic Injections for the Treatment of Hip Osteoarthritis: A Systematic Review. Journal of Clinical Medicine, 11(22), 6663. https://doi.org/10.3390/jcm11226663