Intracranial-Pressure-Monitoring-Assisted Management Associated with Favorable Outcomes in Moderate Traumatic Brain Injury Patients with a GCS of 9–11
Abstract
1. Introduction
2. Patients and Methods
2.1. Design of the Study and Participants
2.2. Clinical Management
2.3. Data Collection
2.4. Outcome
2.5. Ethics Statement
2.6. Statistical Analysis
3. Results
3.1. Patients’ Demographic and Clinical Characteristics
3.2. Clinical Outcomes
3.3. The Relationship between ICP Characteristics and Neurological Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Abdelmalik, P.A.; Draghic, N.; Ling, G.S.F. Management of moderate and severe traumatic brain injury. Transfusion 2019, 59, 1529–1538. [Google Scholar] [CrossRef]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Stocchetti, N.; Maas, A.I. Traumatic intracranial hypertension. N. Engl. J. Med. 2014, 370, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Andriessen, T.M.; Horn, J.; Franschman, G.; van der Naalt, J.; Haitsma, I.; Jacobs, B.; Steyerberg, E.W.; Vos, P.E. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: A prospective multicenter study. J. Neurotrauma 2011, 28, 2019–2031. [Google Scholar] [CrossRef]
- Watanitanon, A.; Lyons, V.H.; Lele, A.V.; Krishnamoorthy, V.; Chaikittisilpa, N.; Chandee, T.; Vavilala, M.S. Clinical Epidemiology of Adults With Moderate Traumatic Brain Injury. Crit. Care Med. 2018, 46, 781–787. [Google Scholar] [CrossRef]
- Aiolfi, A.; Benjamin, E.; Khor, D.; Inaba, K.; Lam, L.; Demetriades, D. Brain Trauma Foundation Guidelines for Intracranial Pressure Monitoring: Compliance and Effect on Outcome. World J. Surg. 2017, 41, 1543–1549. [Google Scholar] [CrossRef]
- Compagnone, C.; d’Avella, D.; Servadei, F.; Angileri, F.F.; Brambilla, G.; Conti, C.; Cristofori, L.; Delfini, R.; Denaro, L.; Ducati, A.; et al. Patients with moderate head injury: A prospective multicenter study of 315 patients. Neurosurgery 2009, 64, 690–696; discussion 696–697. [Google Scholar] [CrossRef]
- Huijben, J.A.; Wiegers, E.J.A.; Lingsma, H.F.; Citerio, G.; Maas, A.I.R.; Menon, D.K.; Ercole, A.; Nelson, D.; van der Jagt, M.; Steyerberg, E.W.; et al. Changing care pathways and between-center practice variations in intensive care for traumatic brain injury across Europe: A CENTER-TBI analysis. Intensive Care Med. 2020, 46, 995–1004. [Google Scholar] [CrossRef]
- Alali, A.S.; Fowler, R.A.; Mainprize, T.G.; Scales, D.C.; Kiss, A.; de Mestral, C.; Ray, J.G.; Nathens, A.B. Intracranial pressure monitoring in severe traumatic brain injury: Results from the American College of Surgeons Trauma Quality Improvement Program. J. Neurotrauma 2013, 30, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observational cohort study. Lancet Neurol. 2021, 20, 548–558. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Z.; Yan, Z.; Ge, S.; Zhang, Y.; Yang, H.; Zhao, L.; Liu, L.; Zhang, X.; Cai, Y.; et al. Predicting Neurological Deterioration after Moderate Traumatic Brain Injury: Development and Validation of a Prediction Model Based on Data Collected on Admission. Neurotrauma 2022, 39, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.F.; Marshall, S.B.; Klauber, M.R.; van Berkum Clark, M.; Eisenberg, H.M.; Jane, J.A.; Luerssen, T.G.; Marmarou, A.; Foulkes, M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991, 75, s14–s20. [Google Scholar] [CrossRef]
- Frontera, J.A.; Claassen, J.; Schmidt, J.M.; Wartenberg, K.E.; Temes, R.; Connolly, E.S.; Macdonald, R.L.; Mayer, S.A. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: The modified fisher scale. Neurosurgery 2006, 59, 21–27; discussion 21–27. [Google Scholar] [PubMed]
- Zuercher, P.; Groen, J.L.; Aries, M.J.; Steyerberg, E.W.; Maas, A.I.; Ercole, A.; Menon, D.K. Reliability and Validity of the Therapy Intensity Level Scale: Analysis of Clinimetric Properties of a Novel Approach to Assess Management of Intracranial Pressure in Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1768–1774. [Google Scholar] [CrossRef]
- Weir, J.; Steyerberg, E.W.; Butcher, I.; Lu, J.; Lingsma, H.F.; McHugh, G.S.; Roozenbeek, B.; Maas, A.I.; Murray, G.D. Does the extended Glasgow Outcome Scale add value to the conventional Glasgow Outcome Scale? J. Neurotrauma 2012, 29, 53–58. [Google Scholar] [CrossRef]
- Bouzat, P.; Francony, G.; Declety, P.; Genty, C.; Kaddour, A.; Bessou, P.; Brun, J.; Jacquot, C.; Chabardes, S.; Bosson, J.-L.; et al. Transcranial Doppler to screen on admission patients with mild to moderate traumatic brain injury. Neurosurgery 2011, 68, 1603–1609; discussion 1609–1610. [Google Scholar] [CrossRef]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1189–1209. [Google Scholar]
- Godoy, D.A.; Rubiano, A.; Rabinstein, A.A.; Bullock, R.; Sahuquillo, J. Moderate Traumatic Brain Injury: The Grey Zone of Neurotrauma. Neurocrit. Care 2016, 25, 306–319. [Google Scholar] [CrossRef]
- Iaccarino, C.; Lippa, L.; Munari, M.; Castioni, C.A.; Robba, C.; Caricato, A.; Pompucci, A.; Signoretti, S.; Zona, G.; Rasulo, F.A.; et al. Management of intracranial hypertension following traumatic brain injury: A best clinical practice adoption proposal for intracranial pressure monitoring and decompressive craniectomy. Joint statements by the Traumatic Brain Injury Section of the Italian Society of Neurosurgery (SINch) and the Neuroanesthesia and Neurocritical Care Study Group of the Italian Society of Anesthesia, Analgesia, Resuscitation and Intensive Care (SIAARTI). J. Neurosurg. Sci. 2021, 65, 219–238. [Google Scholar] [PubMed]
- Ahl, R.; Sarani, B.; Sjolin, G.; Mohseni, S. The Association of Intracranial Pressure Monitoring and Mortality: A Propensity Score-Matched Cohort of Isolated Severe Blunt Traumatic Brain Injury. J. Emerg. Trauma Shock 2019, 12, 18–22. [Google Scholar] [PubMed]
- Donnelly, J.; Czosnyka, M.; Adams, H.; Cardim, D.; Kolias, A.; Zeiler, F.; Lavinio, A.; Aries, M.; Robba, C.; Smielewski, P.; et al. Twenty-Five Years of Intracranial Pressure Monitoring After Severe Traumatic Brain Injury: A Retrospective, Single-Center Analysis. Neurosurgery 2019, 85, E75–E82. [Google Scholar] [CrossRef] [PubMed]
- Khormi, Y.H.; Senthilselvan, A.; O’Kelly, C.; Zygun, D. Adherence to brain trauma foundation guidelines for intracranial pressure monitoring in severe traumatic brain injury and the effect on outcome: A population-based study. Surg. Neurol. Int. 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Mouchtouris, N.; Turpin, J.; Chalouhi, N.; Al Saiegh, F.; Theofanis, T.; Das, S.; Shah, S.O.; Jallo, J. Statewide Trends in Intracranial Pressure Monitor Use in 36,915 Patients with Severe Traumatic Brain Injury in a Mature Trauma System over the Past 18 Years. World Neurosurg. 2019, 130, e166–e171. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.; Lewis, M.; Benjamin, E.; Aiolfi, A.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe traumatic brain injuries: A closer look at level 1 trauma centers in the United States. Injury 2017, 48, 1944–1950. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, X.; Sun, Y.; Yu, J.; Li, Z.; Du, Z.; Mao, Y.; Zhou, L.; Hu, J. Impact of intracranial pressure monitoring on mortality in patients with traumatic brain injury: A systematic review and meta-analysis. J. Neurosurg. 2015, 122, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Al Saiegh, F.; Philipp, L.; Mouchtouris, N.; Chalouhi, N.; Khanna, O.; Shah, S.O.; Jallo, J. Comparison of Outcomes of Severe Traumatic Brain Injury in 36,929 Patients Treated with or without Intracranial Pressure Monitoring in a Mature Trauma System. World Neurosurg. 2020, 136, e535–e541. [Google Scholar] [CrossRef]
- Okazaki, T.; Kawakita, K.; Kuroda, Y. Hospital-level intracranial pressure monitoring utilization and functional outcome in severe traumatic brain injury: A post hoc analysis of prospective multicenter observational study. Scand. J. Trauma, Resusc. Emerg. Med. 2021, 29, 5. [Google Scholar] [CrossRef]
- Aiolfi, A.; Khor, D.; Cho, J.; Benjamin, E.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe blunt head trauma: Does the type of monitoring device matter? J. Neurosurg. 2018, 128, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.; Abi-Aad, K.; Bunch, K.M.; Beutler, T.; Otite, F.O.; Chin, L.S. Outcomes associated with brain tissue oxygen monitoring in patients with severe traumatic brain injury undergoing intracranial pressure monitoring. J. Neurosurg. 2021, 135, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, F.; Li, Y.; Wang, R.; Zhang, Z.; Qu, Y. Assessment of intracranial pressure monitoring in patients with moderate traumatic brain injury: A retrospective cohort study. Clin. Neurol. Neurosurg. 2020, 189, 105538. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.M.; Videtta, W. Situational Intracranial Pressure Management: An Argument Against a Fixed Treatment Threshold. Crit. Care Med. 2020, 48, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C.; Goldenberg, F.D. Intracranial Pressure in Traumatic Brain Injury: From Thresholds to Heuristics. Crit. Care Med. 2020, 48, 1210–1213. [Google Scholar] [CrossRef]
- Güiza, F.; Depreitere, B.; Piper, I.; Citerio, G.; Chambers, I.; Jones, P.A.; Lo, T.-Y.M.; Enblad, P.; Nillson, P.; Feyen, B.; et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015, 41, 1067–1076. [Google Scholar] [CrossRef]
- Launey, Y.; Fryer, T.D.; Hong, Y.T.; Steiner, L.A.; Nortje, J.; Veenith, T.V.; Hutchinson, P.J.; Ercole, A.; Gupta, A.K.; Aigbirhio, F.I.; et al. Spatial and Temporal Pattern of Ischemia and Abnormal Vascular Function Following Traumatic Brain Injury. JAMA Neurol. 2020, 77, 339–349. [Google Scholar] [CrossRef]

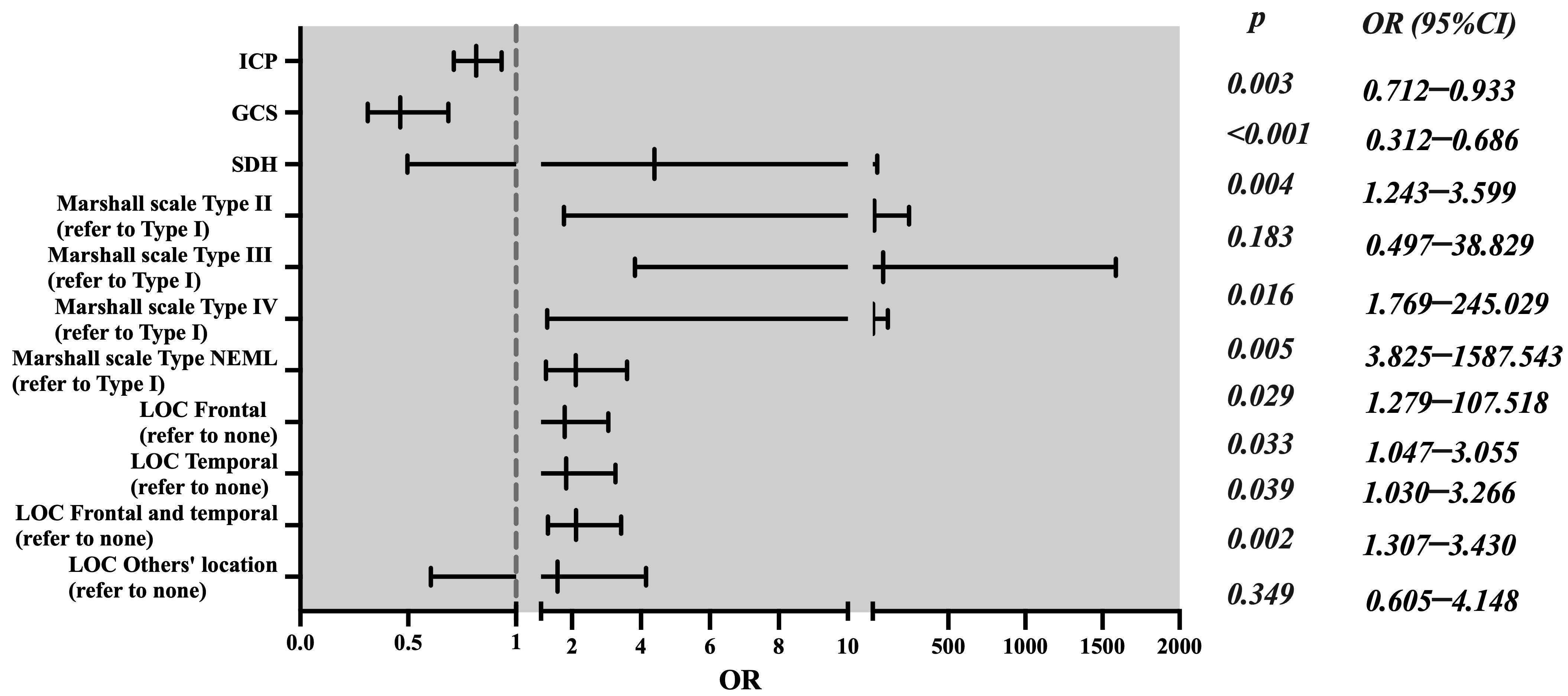
| Characteristics | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| ICP monitoring | Yes | 0.815 | 0.712–0.933 | 0.003 |
| No * | 1 | |||
| GCS score | 0.463 | 0.312–0.686 | <0.001 | |
| Midline shift (mm) | 1.144 | 0.982–1.323 | 0.057 | |
| Marshall’s scale | Type II DI | 4.393 | 0.497–38.829 | 0.183 |
| Type III DI | 20.821 | 1.769–245.029 | 0.016 | |
| Type IV DI | 77.928 | 3.825–1587.543 | 0.005 | |
| NEML | 11.725 | 1.279–107.518 | 0.029 | |
| Type I DI * | 1 | |||
| SDH | Yes | 2.115 | 1.243–3.599 | 0.004 |
| No * | 1 | |||
| Location of contusion (LOC) | Frontal | 1.788 | 1.047–3.055 | 0.033 |
| Temporal | 1.834 | 1.03–3.266 | 0.039 | |
| Frontal and temporal | 2.118 | 1.307–3.43 | 0.002 | |
| Other location | 1.584 | 0.605–4.148 | 0.349 | |
| None * | 1 |
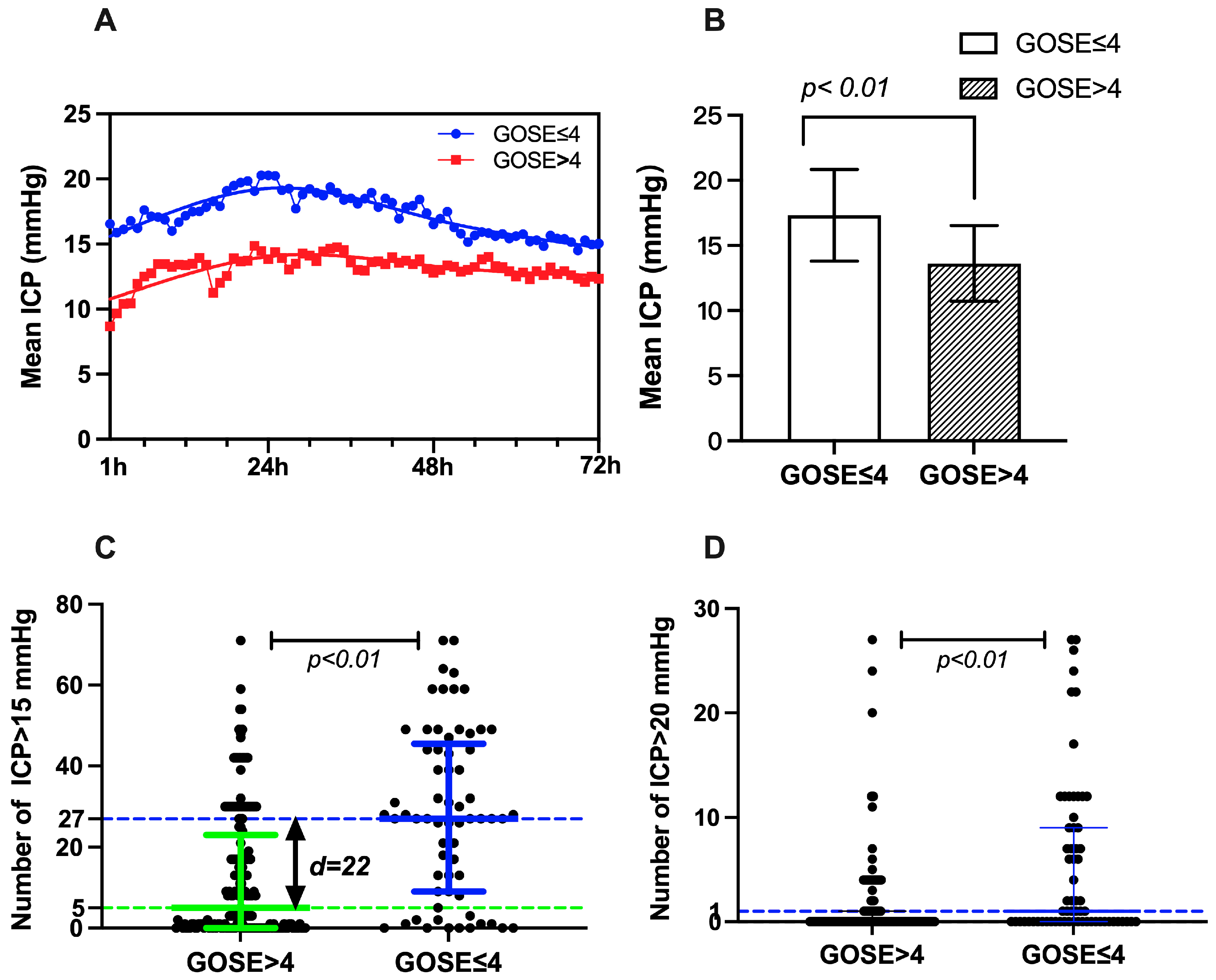
| GOS-E ≤ 4 | GOS-E > 4 | Difference and 95% CI | Z/T | p | |
|---|---|---|---|---|---|
| Mean ICP (mmHg) ± SE | 17.32 ± 3.52 | 13.62 ± 2.91 | 3.70 (1.82–4.58) | −6.047 | <0.001 |
| Median number of ICP > 15 mmHg (IQR) | 27 (9–45.5) | 5 (0–23) | 17 (10–23) | −5.406 | <0.001 |
| Median number of ICP > 20 mmHg (IQR) | 1 (0–9) | 0 (0–1) | 1 (0–1) | −4.635 | <0.001 |
| Proportion of ICP > 15 mmHg % (IQR) | 37.50 (12.50–63.19) | 6.94 (0–31.94) | 23.60 (13.90–31.90) | −5.406 | <0.001 |
| Proportion of ICP > 20 mmHg % | 1.39 (0–12.50) | 0 (0–1.39) | 1.40 (0–1.40) | −4.635 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Wu, H.; Li, Z.; Ge, S.; Zhao, L.; Zhang, X.; Qu, Y. Intracranial-Pressure-Monitoring-Assisted Management Associated with Favorable Outcomes in Moderate Traumatic Brain Injury Patients with a GCS of 9–11. J. Clin. Med. 2022, 11, 6661. https://doi.org/10.3390/jcm11226661
Chen M, Wu H, Li Z, Ge S, Zhao L, Zhang X, Qu Y. Intracranial-Pressure-Monitoring-Assisted Management Associated with Favorable Outcomes in Moderate Traumatic Brain Injury Patients with a GCS of 9–11. Journal of Clinical Medicine. 2022; 11(22):6661. https://doi.org/10.3390/jcm11226661
Chicago/Turabian StyleChen, Mingsheng, Haiyang Wu, Zhihong Li, Shunnan Ge, Lanfu Zhao, Xingye Zhang, and Yan Qu. 2022. "Intracranial-Pressure-Monitoring-Assisted Management Associated with Favorable Outcomes in Moderate Traumatic Brain Injury Patients with a GCS of 9–11" Journal of Clinical Medicine 11, no. 22: 6661. https://doi.org/10.3390/jcm11226661
APA StyleChen, M., Wu, H., Li, Z., Ge, S., Zhao, L., Zhang, X., & Qu, Y. (2022). Intracranial-Pressure-Monitoring-Assisted Management Associated with Favorable Outcomes in Moderate Traumatic Brain Injury Patients with a GCS of 9–11. Journal of Clinical Medicine, 11(22), 6661. https://doi.org/10.3390/jcm11226661







