Post-Intensive Care Syndrome in Non-COVID-19 ICU Survivors during the COVID-19 Pandemic in South Korea: A Multicenter Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Measures
2.3.1. Mental Health
2.3.2. Cognitive Impairment
2.3.3. Physical Impairment
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
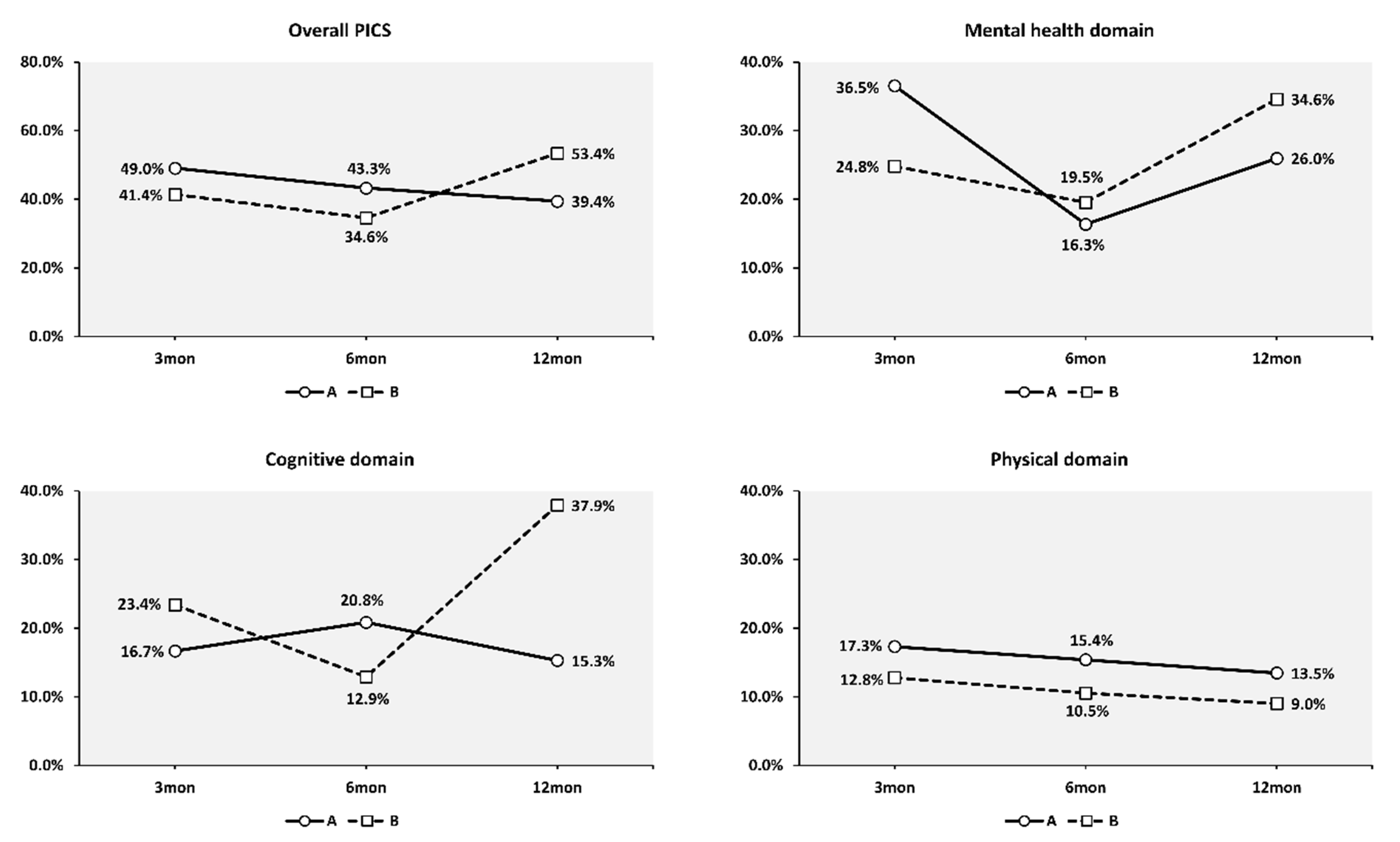
3.2. Prevalence of PICS
3.3. Effect of Calendar Period on the Prevalence of PICS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rengel, K.F.; Hayhurst, C.J.; Pandharipande, P.P.; Hughes, C.G. Long-term Cognitive and Functional Impairments After Critical Illness. Anesth. Analg. 2019, 128, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jeong, Y.J.; Hong, J. The effect of postintensive care syndrome on the quality of life of intensive care unit survivors: A secondary analysis. Aust. Crit. Care 2021, 34, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Iwashyna, T.J. Trajectories of recovery and dysfunction after acute illness, with implications for clinical trial design. Am. J. Respir. Crit. Care Med. 2012, 186, 302–304. [Google Scholar] [CrossRef]
- Marra, A.; Pandharipande, P.P.; Girard, T.D.; Patel, M.B.; Hughes, C.G.; Jackson, J.C.; Thompson, J.L.; Chandrasekhar, R.; Ely, E.W.; Brummel, N.E. Co-Occurrence of Post-Intensive Care Syndrome Problems Among 406 Survivors of Critical Illness. Crit. Care Med. 2018, 46, 1393–1401. [Google Scholar] [CrossRef]
- Haddad, D.N.; Mart, M.F.; Wang, L.; Lindsell, C.J.; Raman, R.; Nordness, M.F.; Sharp, K.W.; Pandharipande, P.P.; Girard, T.D.; Ely, E.W.; et al. Socioeconomic Factors and Intensive Care Unit-Related Cognitive Impairment. Ann. Surg. 2020, 272, 596–602. [Google Scholar] [CrossRef]
- Gandotra, S.; Lovato, J.; Case, D.; Bakhru, R.N.; Gibbs, K.; Berry, M.; Files, D.C.; Morris, P.E. Physical Function Trajectories in Survivors of Acute Respiratory Failure. Ann. Am. Thorac. Soc. 2019, 16, 471–477. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Gensichen, J.S.; Cakiroglu, A.S.; Cashmore, R.; Edbrooke, L.; Heintze, C.; Neumann, K.; Wollersheim, T.; Denehy, L.; Schmidt, K.F.R. Implications for post critical illness trial design: Sub-phenotyping trajectories of functional recovery among sepsis survivors. Crit. Care 2020, 24, 577. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Martillo, M.A.; Dangayach, N.S.; Tabacof, L.; Spielman, L.A.; Dams-O’Connor, K.; Chan, C.C.; Kohli-Seth, R.; Cortes, M.; Escalon, M.X. Postintensive Care Syndrome in Survivors of Critical Illness Related to Coronavirus Disease 2019: Cohort Study From a New York City Critical Care Recovery Clinic. Crit. Care Med. 2021, 49, 1427–1438. [Google Scholar] [CrossRef]
- Kotfis, K.; Williams Roberson, S.; Wilson, J.E.; Dabrowski, W.; Pun, B.T.; Ely, E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care 2020, 24, 176. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Nam, B.R.; Kwon, H.I.; Kwon, J.H. Psychometric Qualities of the Korean Version of the Posttraumatic Diagnosis scale (PDS-K). Korean J. Clin. Psychol. 2010, 29, 147–167. [Google Scholar]
- Foa, E.B.; Cashman, L.; Jaycox, L.; Perry, K. The validation of a self-report measure of posttraumatic stress disorder: The Posttraumatic Diagnostic Scale. Psychol. Assess. 1997, 9, 445–451. [Google Scholar] [CrossRef]
- McCarthy, S. Post-traumatic Stress Diagnostic Scale (PDS). Occup. Med. 2008, 58, 379. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Zietemann, V.; Kopczak, A.; Müller, C.; Wollenweber, F.A.; Dichgans, M. Validation of the Telephone Interview of Cognitive Status and Telephone Montreal Cognitive Assessment Against Detailed Cognitive Testing and Clinical Diagnosis of Mild Cognitive Impairment After Stroke. Stroke 2017, 48, 2952–2957. [Google Scholar] [CrossRef]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef]
- Carson, N.; Leach, L.; Murphy, K.J. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int. J. Geriatr. Psychiatry 2018, 33, 379–388. [Google Scholar] [CrossRef]
- Won, C.W.; Rho, Y.G.; Kim, S.Y.; Cho, B.R.; Lee, Y.S. The Validity and Reliability of Korean Activities of Daily Living(K-ADL) Scale. J. Korean Geriatr. Soc. 2002, 6, 98–106. [Google Scholar]
- Statistics Korea. Statistics Korea COVID-19. 2022. Available online: https://kosis.kr/covid_eng/covid_index.do (accessed on 7 November 2022).
- Boede, M.; Gensichen, J.S.; Jackson, J.C.; Eißler, F.; Lehmann, T.; Schulz, S.; Petersen, J.J.; Wolf, F.P.; Dreischulte, T.; Schmidt, K.F.R. Trajectories of depression in sepsis survivors: An observational cohort study. Crit. Care 2021, 25, 161. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, J.; Jeong, Y.J. Risk factors for post-intensive care syndrome: A systematic review and meta-analysis. Aust. Crit. Care 2020, 33, 287–294. [Google Scholar] [CrossRef]
- Jouan, Y.; Grammatico-Guillon, L.; Teixera, N.; Hassen-Khodja, C.; Gaborit, C.; Salmon-Gandonnière, C.; Guillon, A.; Ehrmann, S. Healthcare trajectories before and after critical illness: Population-based insight on diverse patients clusters. Ann. Intensive Care 2019, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- van Veenendaal, N.; van der Meulen, I.C.; Onrust, M.; Paans, W.; Dieperink, W.; van der Voort, P.H.J. Six-month outcomes in COVID-19 ICU patients and their family members: A prospective cohort study. Healthcare 2021, 9, 865. [Google Scholar] [CrossRef]
- Shah, S.M.A.; Mohammad, D.; Qureshi, M.F.H.; Abbas, M.Z.; Aleem, S. Prevalence, Psychological Responses and Associated Correlates of Depression, Anxiety and Stress in a Global Population, During the Coronavirus Disease (COVID-19) Pandemic. Community Ment. Health J. 2021, 57, 101–110. [Google Scholar] [CrossRef]
- Bridgland, V.M.E.; Moeck, E.K.; Green, D.M.; Swain, T.L.; Nayda, D.M.; Matson, L.A.; Hutchison, N.P.; Takarangi, M.K.T. Why the COVID-19 pandemic is a traumatic stressor. PLoS ONE 2021, 16, e0240146. [Google Scholar] [CrossRef] [PubMed]
- Tsapanou, A.; Papatriantafyllou, J.D.; Yiannopoulou, K.; Sali, D.; Kalligerou, F.; Ntanasi, E.; Zoi, P.; Margioti, E.; Kamtsadeli, V.; Hatzopoulou, M.; et al. The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int. J. Geriatr. Psychiatry 2021, 36, 583–587. [Google Scholar] [CrossRef]
- Cai, Z.; Zheng, S.; Huang, Y.; Zhang, X.; Qiu, Z.; Huang, A.; Wu, K. Emotional and Cognitive Responses and Behavioral Coping of Chinese Medical Workers and General Population during the Pandemic of COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 6198. [Google Scholar] [CrossRef]
- Iodice, F.; Cassano, V.; Rossini, P.M. Direct and indirect neurological, cognitive, and behavioral effects of COVID-19 on the healthy elderly, mild-cognitive-impairment, and Alzheimer’s disease populations. Neurol. Sci. 2021, 42, 455–465. [Google Scholar] [CrossRef]
- Yanagi, N.; Kamiya, K.; Hamazaki, N.; Matsuzawa, R.; Nozaki, K.; Ichikawa, T.; Valley, T.S.; Nakamura, T.; Yamashita, M.; Maekawa, E.; et al. Post-intensive care syndrome as a predictor of mortality in patients with critical illness: A cohort study. PLoS ONE 2021, 16, e0244564. [Google Scholar] [CrossRef]
- Geense, W.W.; van den Boogaard, M.; van der Hoeven, J.G.; Vermeulen, H.; Hannink, G.; Zegers, M. Nonpharmacologic Interventions to Prevent or Mitigate Adverse Long-Term Outcomes Among ICU Survivors: A Systematic Review and Meta-Analysis. Crit. Care Med. 2019, 47, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Katayama, O.; Lee, S.; Bae, S.; Makino, K.; Chiba, I.; Harada, K.; Morikawa, M.; Tomida, K.; Shimada, H. Association between Non-Face-to-Face Interactions and Incident Disability in Older Adults. J. Nutr. Health Aging 2022, 26, 147–152. [Google Scholar] [CrossRef]
- Noguchi, T.; Nojima, I.; Inoue-Hirakawa, T.; Sugiura, H. Role of non-face-to-face social contacts in moderating the association between living alone and mental health among community-dwelling older adults: A cross-sectional study. Public Health 2021, 194, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jeon, K.; Chung, C.R.; Yang, J.H.; Cho, Y.H.; Cho, J.; Park, C.M.; Park, H.; Cho, J.; Guallar, E.; et al. A nationwide analysis of intensive care unit admissions, 2009–2014-The Korean ICU National Data (KIND) study. J. Crit. Care 2018, 44, 24–30. [Google Scholar] [CrossRef] [PubMed]
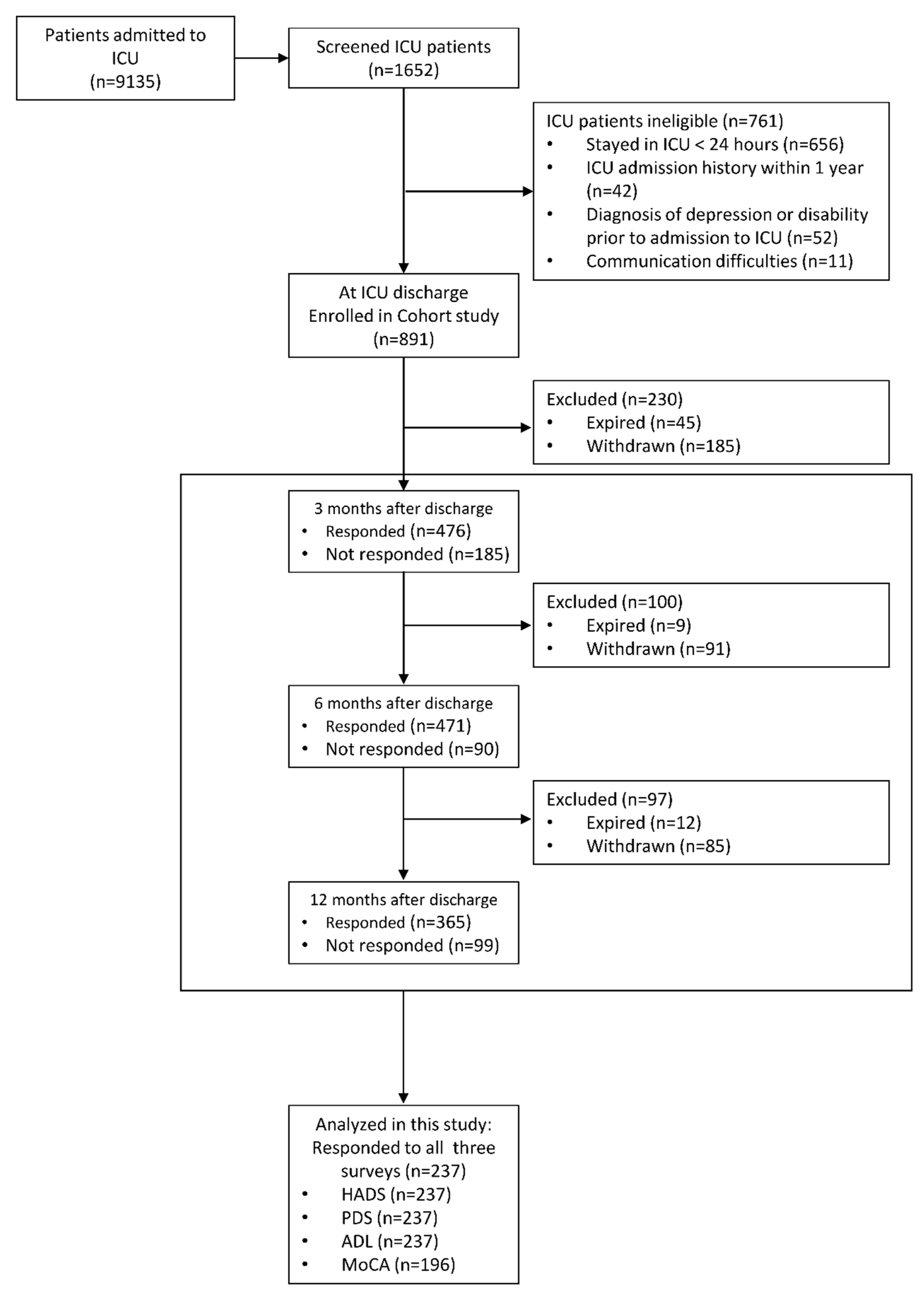

| Variables | Categories | Total (N = 237) | Group A (N = 104) | Group B (N = 133) | p |
|---|---|---|---|---|---|
| Age, years | 58.30 ± 13.30 | 57.55 ± 12.73 | 58.89 ± 13.75 | 0.443 | |
| Male sex | 143 (60.3) | 64 (61.5) | 79 (59.4) | 0.738 | |
| Employed prior to admission | 119 (50.2) | 49 (47.1) | 70 (52.6) | 0.441 | |
| FCI prior to admission | 0 | 100 (42.2) | 43 (41.3) | 57 (42.9) | 0.812 |
| 1 | 78 (32.9) | 33 (31.7) | 45 (33.8) | ||
| ≥2 | 59 (24.9) | 28 (26.9) | 31 (23.3) | ||
| Reason for ICU admission | Postoperative care | 74 (31.2) | 36 (34.6) | 38 (28.6) | 0.477 |
| Neurological | 66 (27.8) | 26 (25.0) | 40 (30.1) | ||
| Cardiovascular | 44 (18.6) | 16 (15.4) | 28 (21.1) | ||
| Respiratory | 38 (16.0) | 20 (19.2) | 18 (13.5) | ||
| Trauma | 15 (6.3) | 6 (5.8) | 9 (6.8) | ||
| ICU type | Surgical | 104 (43.9) | 53 (51.0) | 51 (38.3) | 0.292 |
| Cardiovascular | 47 (19.8) | 18 (17.3) | 29 (21.8) | ||
| Neurological | 43 (18.1) | 14 (13.5) | 29 (21.8) | ||
| Medical | 18 (7.6) | 8 (7.7) | 10 (7.5) | ||
| Others | 25 (10.5) | 11 (10.6) | 14 (10.5) | ||
| ICU admission route | ED | 110 (46.4) | 50 (48.1) | 60 (45.1) | 0.650 |
| Others | 127 (53.6) | 54 (51.9) | 73 (54.9) | ||
| Mechanical ventilation use | 38 (16.0) | 17 (16.3) | 21 (15.8) | 0.908 | |
| Surgery | 134 (56.5) | 63 (60.6) | 71 (53.4) | 0.268 | |
| Delirium in ICU | 25 (10.5) | 12 (11.5) | 13 (9.8) | 0.661 | |
| Discharge place | Home | 209 (88.2) | 93 (89.4) | 116 (87.2) | 0.602 |
| LTC | 28 (11.8) | 11 (10.6) | 17 (12.8) | ||
| Severity | APACHE II | 10.70 ± 5.81 | 11.50 ± 6.55 | 9.97 ± 4.99 | 0.141 |
| SAPS | 31.92 ± 15.08 | 34.57 ± 11.91 | 30.64 ± 16.33 | 0.259 | |
| SOFA | 4.37 ± 2.61 | 4.34 ± 3.10 | 4.41 ± 1.64 | 0.908 | |
| ICU length of stay, days | 4.02 ± 4.28 | 3.52 ± 4.45 | 4.41 ± 4.11 | 0.113 | |
| CCI | 1.03 ± 1.19 | 0.95 ± 1.22 | 1.10 ± 1.17 | 0.350 |
| Variables | Time | Repeated Measures ANOVA Model | |||||
|---|---|---|---|---|---|---|---|
| Linear | Non-Linear (Quadratic) | ||||||
| 3 Months | 6 Months | 12 Months | F | p | F | p | |
| Anxiety | 3.22 ± 3.42 | 2.76 ± 3.51 | 3.35 ± 3.64 | 0.312 | 0.577 | 7.242 | 0.008 |
| Depression | 4.70 ± 4.19 | 3.27 ± 3.57 | 4.92 ± 4.73 | 0.646 | 0.422 | 53.997 | <0.001 |
| PTSD | 4.45 ± 5.58 | 3.98 ± 5.70 | 5.53 ± 5.83 | 8.764 | 0.003 | 13.238 | <0.001 |
| Cognition | 25.12 ± 4.15 | 25.77 ± 3.67 | 24.06 ± 4.64 | 11.913 | 0.001 | 24.644 | <0.001 |
| ADL | 7.61 ± 2.09 | 7.32 ± 1.12 | 7.39 ± 1.57 | 3.789 | 0.053 | 6.206 | 0.013 |
| Variable | Group | Time | Group | Time | Group × Time | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 Months | 6 Months | 12 Months | Linear | Non-Linear (Quadratic) | Linear | Non-Linear (Quadratic) | ||||
| Anxiety | Group A | 3.68 ± 3.31 | 2.44 ± 3.13 | 3.25 ± 4.00 | F-value | 0.005 | 0.091 | 8.893 | 4.376 | 5.465 |
| Group B | 2.85 ± 3.48 | 3.02 ± 3.77 | 3.43 ± 3.35 | p-value | 0.942 | 0.763 | 0.003 | 0.038 | 0.020 | |
| Depression | Group A | 5.20 ± 3.85 | 3.28 ± 3.76 | 3.94 ± 4.50 | F-value | 0.371 | 0.058 | 51.364 | 23.585 | 1.085 |
| Group B | 4.30 ± 4.41 | 3.26 ± 3.43 | 5.69 ± 4.78 | p-value | 0.543 | 0.810 | 0.000 | 0.000 | 0.299 | |
| PTSD | Group A | 5.42 ± 5.97 | 4.03 ± 6.11 | 4.71 ± 5.73 | F-value | 0.036 | 6.272 | 13.076 | 20.338 | 0.011 |
| Group B | 3.68 ± 5.15 | 3.95 ± 5.37 | 6.17 ± 5.85 | p-value | 0.850 | 0.013 | 0.000 | 0.000 | 0.918 | |
| Cognition | Group A | 25.75 ± 3.43 | 25.74 ± 3.89 | 25.46 ± 4.06 | F-value | 4.340 | 8.072 | 16.019 | 3.687 | 11.930 |
| Group B | 24.75 ± 4.49 | 25.79 ± 3.55 | 23.24 ± 4.78 | p-value | 0.039 | 0.005 | 0.000 | 0.056 | 0.001 | |
| ADL | Group A | 7.82 ± 2.49 | 7.50 ± 1.51 | 7.62 ± 2.03 | F-value | 4.037 | 3.653 | 6.350 | 0.019 | 0.179 |
| Group B | 7.45 ± 1.69 | 7.18 ± 0.65 | 7.22 ± 1.06 | p-value | 0.046 | 0.057 | 0.012 | 0.891 | 0.673 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Hong, J.; Jeong, J.-H. Post-Intensive Care Syndrome in Non-COVID-19 ICU Survivors during the COVID-19 Pandemic in South Korea: A Multicenter Prospective Cohort Study. J. Clin. Med. 2022, 11, 6653. https://doi.org/10.3390/jcm11226653
Kang J, Hong J, Jeong J-H. Post-Intensive Care Syndrome in Non-COVID-19 ICU Survivors during the COVID-19 Pandemic in South Korea: A Multicenter Prospective Cohort Study. Journal of Clinical Medicine. 2022; 11(22):6653. https://doi.org/10.3390/jcm11226653
Chicago/Turabian StyleKang, Jiyeon, Jiwon Hong, and Jin-Heon Jeong. 2022. "Post-Intensive Care Syndrome in Non-COVID-19 ICU Survivors during the COVID-19 Pandemic in South Korea: A Multicenter Prospective Cohort Study" Journal of Clinical Medicine 11, no. 22: 6653. https://doi.org/10.3390/jcm11226653
APA StyleKang, J., Hong, J., & Jeong, J.-H. (2022). Post-Intensive Care Syndrome in Non-COVID-19 ICU Survivors during the COVID-19 Pandemic in South Korea: A Multicenter Prospective Cohort Study. Journal of Clinical Medicine, 11(22), 6653. https://doi.org/10.3390/jcm11226653






