Impact of Dietary Fats on Cardiovascular Disease with a Specific Focus on Omega-3 Fatty Acids
Abstract
1. Introduction
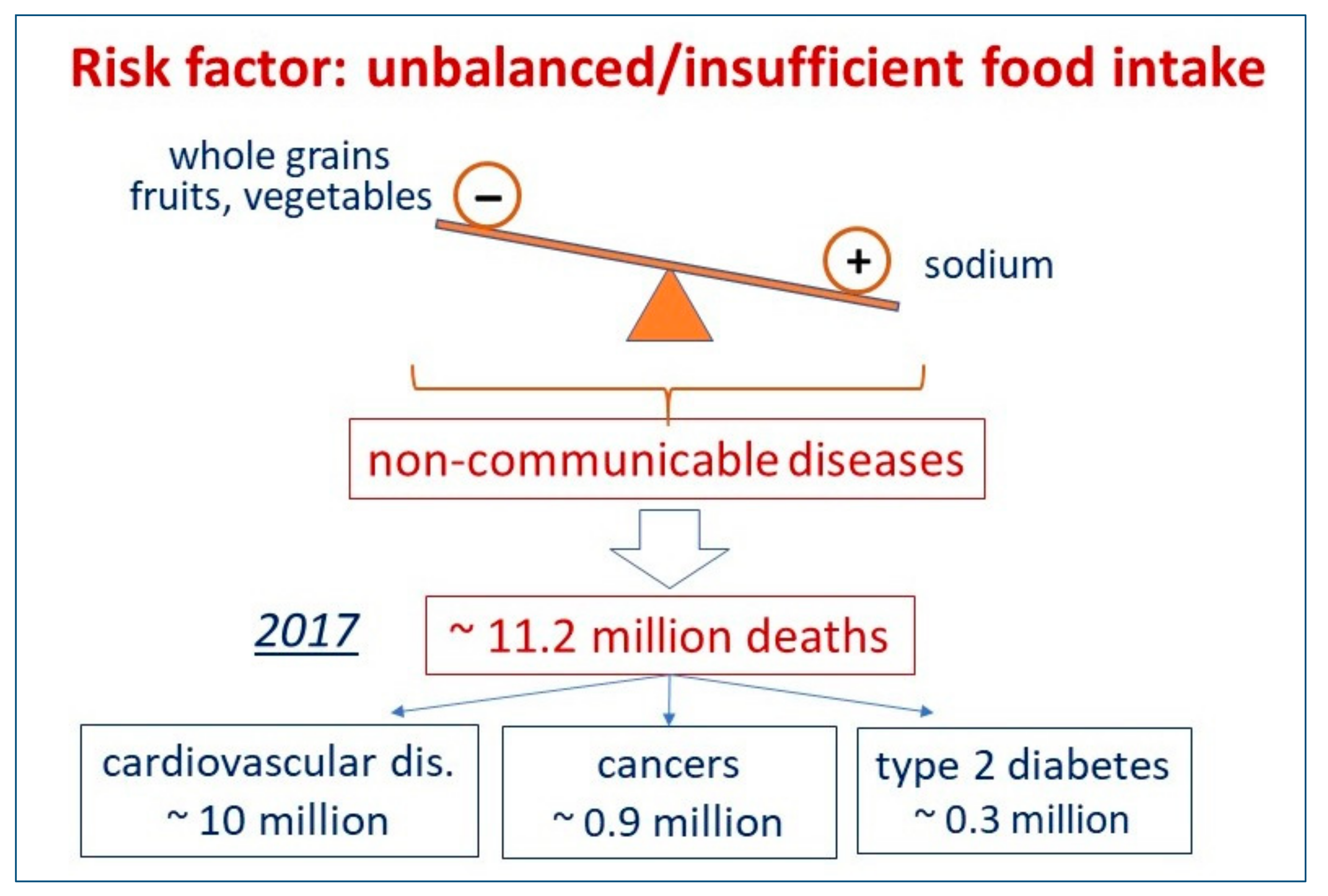
2. Which Foods, or Food Shortages, Can Impact Mortality in Different AREAS of the World? What Kind of Non-Communicable Disease Can Be Impacted by Different Dietary Habits?
3. Is It True That the Consumption of Saturated Fat Has a Negative Impact on Cardiovascular Mortality?
4. We Are Confronted with More Doubts Than Certainties: How Reliable Is Nutritional Epidemiology Research?
5. From Uncertainties to Facts: What Are the Mechanisms Underlying the Metabolic Effects of Dietary Fats?
6. From Observational Studies to Randomized Controlled Trials: What Is the Role of Omega 3 Fatty Acids in the Prevention of Cardiovascular Diseases?
6.1. Primary Prevention
6.2. Secondary Prevention
6.3. Heart Failure
6.4. The Issue of Atrial Fibrillation
7. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.; Mente, A.; Maroleanu, A.; Cozma, I.A.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Harcombe, Z.; Baker, J.S.; Davies, B. Evidence from prospective cohort studies does not support current dietary fat guidelines: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1743–1749. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.; Broste, S.K.; Frantz, R.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: Analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. The Challenge of Reforming Nutritional Epidemiologic Research. JAMA 2018, 320, 969–970. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.-M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462–1473. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Ioannidis, J.P.A. Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Adv. Nutr. 2018, 9, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P. Implausible results in human nutrition research. BMJ 2013, 347, f6698. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Jorgenson, J.; Ioannidis, J.P.; Cifu, A. Observational studies often make clinical practice recommendations: An empirical evaluation of authors’ attitudes. J. Clin. Epidemiol. 2013, 66, 361–366. [Google Scholar] [CrossRef]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Zhu, Y.; Zhang, X. Omega-3 Polyunsaturated Fatty Acids: Versatile Roles in Blood Pressure Regulation. Antioxid Redox Signal. 2021, 34, 800–810. [Google Scholar] [CrossRef]
- Sottero, B.; Leonarduzzi, G.; Testa, G.; Gargiulo, S.; Poli, G.; Biasi, F. Lipid Oxidation Derived Aldehydes and Oxysterols Between Health and Disease. Eur. J. Lipid Sci. Technol. 2019, 121, 1700047. [Google Scholar] [CrossRef]
- Gargiulo, S.; Testa, G.; Gamba, P.; Staurenghi, E.; Poli, G.; Leonarduzzi, G. Oxysterols and 4-hydroxy-2-nonenal contribute to atherosclerotic plaque destabilization. Free Radic. Biol. Med. 2017, 111, 140–150. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; Van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef]
- Albert, C.; Hennekens, C.H.; O’Donnell, C.J.; Ajani, U.A.; Carey, V.J.; Willett, W.C.; Ruskin, J.N.; Manson, J.E. Fish consumption and risk of sudden cardiac death. JAMA 1998, 279, 23–28. [Google Scholar] [CrossRef]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. VITAL Research Group. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al.; ASCEND Study Collaborative Group Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT Investigators. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Gissi-HF Investigators; et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Nodari, S.; Triggiani, M.; Campia, U.; Manerba, A.; Milesi, G.; Cesana, B.M.; Gheorghiade, M.; Cas, L.D. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, B.; Huang, J. The Role of Omega-3 Polyunsaturated Fatty Acids in Heart Failure: A Meta-Analysis of Randomised Controlled Trials. Nutrients 2016, 9, 18. [Google Scholar] [CrossRef]
- Abdelhamid, A.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.; et al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD012345. [Google Scholar] [CrossRef]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M.; Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef]
- Nilsen, D.W.; Albrektsen, G.; Landmark, K.; Moen, S.; Aarsland, T.; Woie, L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am. J. Clin. Nutr. 2001, 74, 50–56. [Google Scholar] [CrossRef]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of Omega-3 Fatty Acid Supplement Use with Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; MacFadyen, J.; Glynn, R.J.; Jiao, L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Tardif, J.C.; et al. Effects of Randomized Treatment with Icosapent Ethyl and a Mineral Oil Comparator on Interleukin-1β, Interleukin-6, C-Reactive Protein, Oxidized Low-Density Lipoprotein Cholesterol, Homocysteine, Lipoprotein(a), and Lipoprotein-Associated Phospholipase A2: A REDUCE-IT Biomarker Substudy. Circulation 2022, 146, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, B.; Chung, M.K.; Budoff, M.J.; Philip, S.; Jiao, L.; Doyle, R.T., Jr.; Copland, C.; Giaquinto, A.; Juliano, R.A.; Bhatt, D.L. Mineral oil: Safety and use as placebo in REDUCE-IT and other clinical studies. Eur. Heart J. Suppl. 2020, 22, J34–J48. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L. Omega-3 for Cardiovascular Diseases: Where Do We Stand After REDUCE-IT and STRENGTH? Circulation 2021, 144, 183–185. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Bryson, C.L.; Lemaitre, R.N.; Burke, G.L.; Siscovick, D.S. Fish intake and risk of incident heart failure. J. Am. Coll. Cardiol. 2005, 45, 2015–2021. [Google Scholar] [CrossRef]
- Kojuri, J.; Ostovan, M.; Rezaian, G.R.; Dialameh, P.A.; Zamiri, N.; Sharifkazemi, M.; Jannati, M. Effect of omega-3 on brain natriuretic peptide and echocardiographic findings in heart failure: Double-blind placebo-controlled randomized trial. J. Cardiovasc. Dis. Res. 2013, 4, 20–24. [Google Scholar] [CrossRef]
- Kohashi, K.; Nakagomi, A.; Saiki, Y.; Morisawa, T.; Kosugi, M.; Kusama, Y.; Atarashi, H.; Shimizu, W. Effects of eicosapentaenoic acid on the levels of inflammatory markers, cardiac function and long-term prognosis in chronic heart failure patients with dyslipidemia. J. Atheroscler. Thromb. 2014, 21, 712–729. [Google Scholar] [CrossRef]
- Nodari, S.; Metra, M.; Milesi, G.; Manerba, A.; Cesana, B.M.; Gheorghiade, M.; Cas, L.D. The role of n-3 PUFAs in preventing the arrhythmic risk in patients with idiopathic dilated cardiomyopathy. Cardiovasc. Drugs Ther. 2009, 23, 5–15. [Google Scholar] [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients After Myocardial Infarction: A Randomized, Controlled Trial. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef]
- Curfman, G. Omega-3 Fatty Acids and Atrial Fibrillation. JAMA 2021, 325, 1063. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggioni, A.P.; Poli, G.; Mannucci, P.M. Impact of Dietary Fats on Cardiovascular Disease with a Specific Focus on Omega-3 Fatty Acids. J. Clin. Med. 2022, 11, 6652. https://doi.org/10.3390/jcm11226652
Maggioni AP, Poli G, Mannucci PM. Impact of Dietary Fats on Cardiovascular Disease with a Specific Focus on Omega-3 Fatty Acids. Journal of Clinical Medicine. 2022; 11(22):6652. https://doi.org/10.3390/jcm11226652
Chicago/Turabian StyleMaggioni, Aldo Pietro, Giuseppe Poli, and Pier Mannuccio Mannucci. 2022. "Impact of Dietary Fats on Cardiovascular Disease with a Specific Focus on Omega-3 Fatty Acids" Journal of Clinical Medicine 11, no. 22: 6652. https://doi.org/10.3390/jcm11226652
APA StyleMaggioni, A. P., Poli, G., & Mannucci, P. M. (2022). Impact of Dietary Fats on Cardiovascular Disease with a Specific Focus on Omega-3 Fatty Acids. Journal of Clinical Medicine, 11(22), 6652. https://doi.org/10.3390/jcm11226652







