Postoperative Delirium after Reconstructive Surgery in the Head and Neck Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics of the Patient Cohort
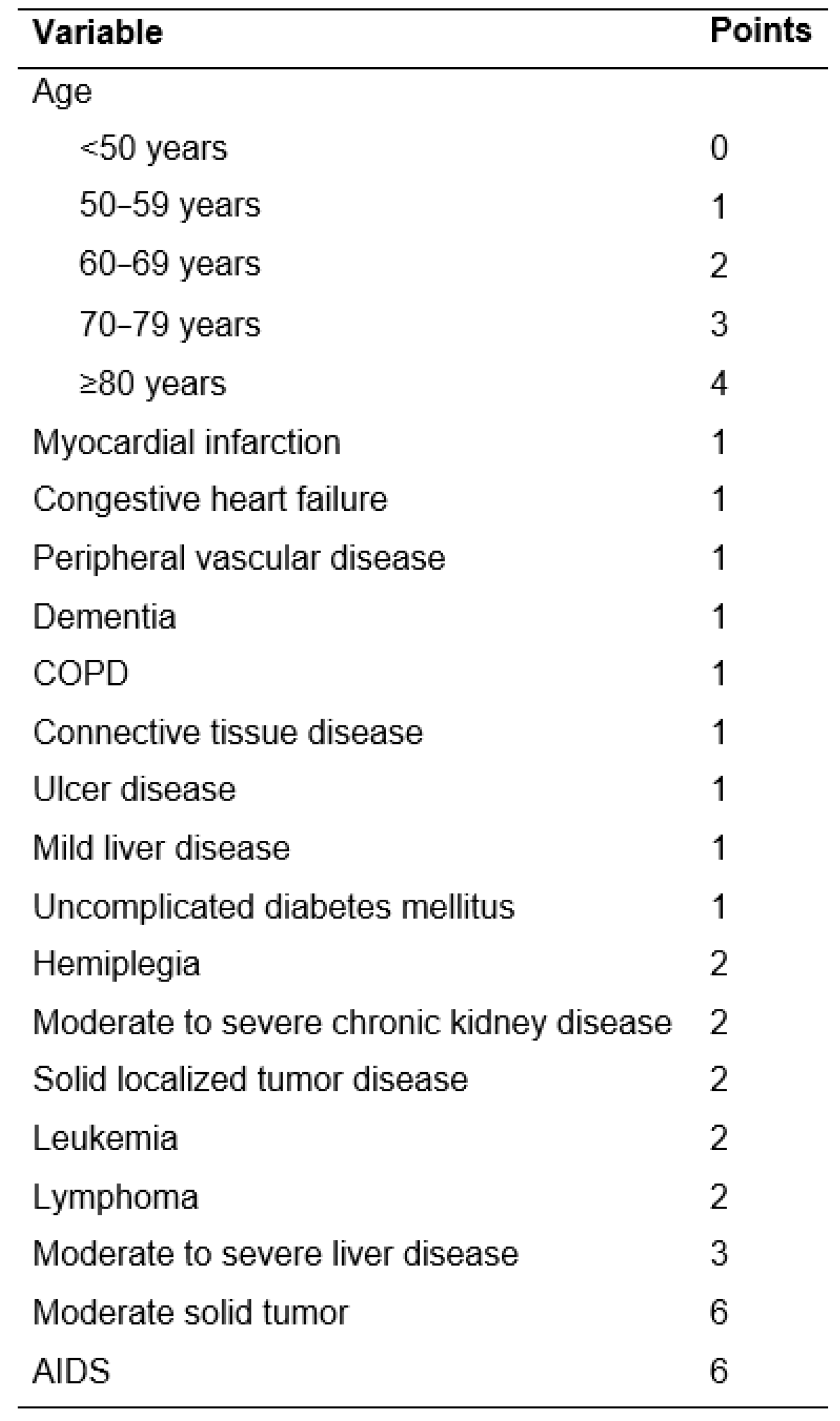
3.2. Association of Clinicopathologic Characteristics with Diagnosis of POD
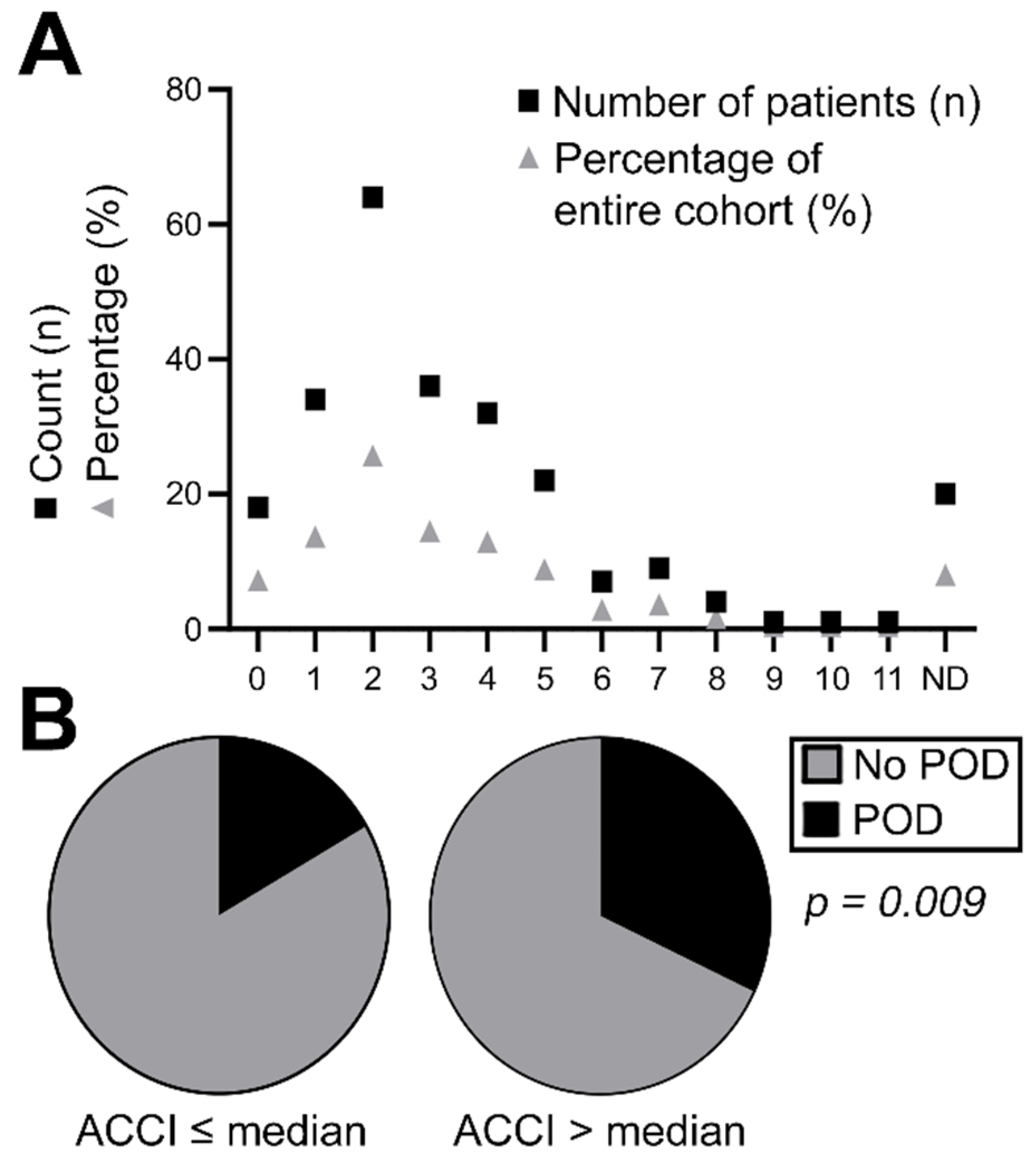
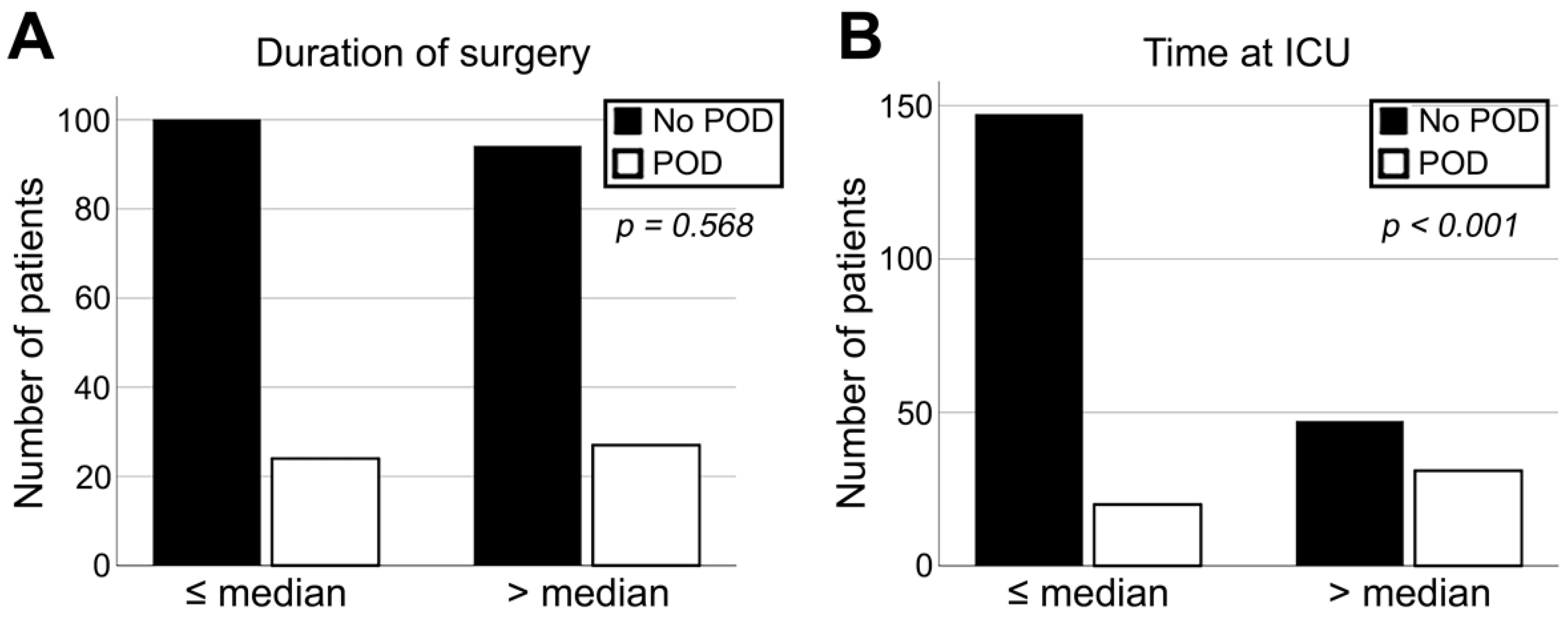
3.3. Correlation of Flap-Related Parameters with Diagnosis of POD
3.4. Binary Logistic Regression Analysis of POD Diagnosis and Clinicopathological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Inouye, S.K.; van Dyck, C.H.; Alessi, C.A.; Balkin, S.; Siegal, A.P.; Horwitz, R.I. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990, 113, 941–948. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013; p. 596. [Google Scholar]
- Meagher, D. Motor subtypes of delirium: Past, present and future. Int. Rev. Psychiatry 2009, 21, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Meagher, D.J.; O’Hanlon, D.; O’Mahony, E.; Casey, P.R.; Trzepacz, P.T. Relationship between symptoms and motoric subtype of delirium. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.N.; Raeburn, C.D.; Tran, Z.V.; Brenner, L.A.; Moss, M. Motor subtypes of postoperative delirium in older adults. Arch. Surg. 2011, 146, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, G.; Liu, S.; Zhou, S.; Lian, Y.; Zhang, C.; Yang, W. Risk factors for postoperative delirium in patients undergoing major head and neck cancer surgery: A meta-analysis. Jpn. J. Clin. Oncol. 2017, 47, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Densky, J.; Eskander, A.; Kang, S.; Chan, J.; Tweel, B.; Sitapara, J.; Ozer, E.; Agrawal, A.; Carrau, R.; Rocco, J.; et al. Risk Factors Associated With Postoperative Delirium in Patients Undergoing Head and Neck Free Flap Reconstruction. JAMA Otolaryngol. Head. Neck Surg. 2019, 145, 216–221. [Google Scholar] [CrossRef]
- Rengel, K.F.; Pandharipande, P.P.; Hughes, C.G. Postoperative delirium. Presse Med. 2018, 47, e53–e64. [Google Scholar] [CrossRef]
- Leslie, D.L.; Inouye, S.K. The importance of delirium: Economic and societal costs. J. Am. Geriatr. Soc. 2011, 59 (Suppl. S2), S241–S243. [Google Scholar] [CrossRef]
- Pandharipande, P.; Cotton, B.A.; Shintani, A.; Thompson, J.; Costabile, S.; Truman Pun, B.; Dittus, R.; Ely, E.W. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007, 33, 1726–1731. [Google Scholar] [CrossRef]
- Pandharipande, P.; Cotton, B.A.; Shintani, A.; Thompson, J.; Pun, B.T.; Morris, J.A., Jr.; Dittus, R.; Ely, E.W. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J. Trauma 2008, 65, 34–41. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, J.; Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth 2020, 125, 492–504. [Google Scholar] [CrossRef]
- Oh, S.T.; Park, J.Y. Postoperative delirium. Korean J. Anesthesiol. 2019, 72, 4–12. [Google Scholar] [CrossRef]
- Montes, D.M. Postoperative delirium in head and neck cancer patients: A survey of oncologic oral and maxillofacial surgeon practices. J. Oral. Maxillofac. Surg. 2014, 72, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Foreman, M.D.; Mion, L.C.; Katz, K.H.; Cooney, L.M., Jr. Nurses’ recognition of delirium and its symptoms: Comparison of nurse and researcher ratings. Arch. Intern. Med. 2001, 161, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Weed, H.G.; He, X.; Agrawal, A.; Ozer, E.; Schuller, D.E. Alcohol-related predictors of delirium after major head and neck cancer surgery. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 266–271. [Google Scholar] [CrossRef]
- Booka, E.; Kamijo, T.; Matsumoto, T.; Takeuchi, M.; Kitani, T.; Nagaoka, M.; Imai, A.; Iida, Y.; Shimada, A.; Takebayashi, K.; et al. Incidence and risk factors for postoperative delirium after major head and neck cancer surgery. J. Craniomaxillofac. Surg. 2016, 44, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, Y.S.; Kim, Y.H.; Cho, K.J.; Jung, Y.H.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Early Ambulation to Prevent Delirium After Long-Time Head and Neck Cancer Surgery. Front. Surg. 2022, 9, 880092. [Google Scholar] [CrossRef]
- Kolk, A.; Schwarzer, C.; Wolff, K.D.; Grill, F.; Weingart, J. Factors Associated With Postoperative Delirium in Patients Undergoing Complex Head and Neck Flap Surgery. J. Oral Maxillofac. Surg. 2022, 80, 372–379.e375. [Google Scholar] [CrossRef]
- Yamagata, K.; Onizawa, K.; Yusa, H.; Wakatsuki, T.; Yanagawa, T.; Yoshida, H. Risk factors for postoperative delirium in patients undergoing head and neck cancer surgery. Int. J. Oral Maxillofac. Surg. 2005, 34, 33–36. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Anaesthesiologie und Intensivmedizin e.V., Deutsche Interdisziplinaere Vereinigung für Intensiv- und Notfallmedizin e.V. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. 2020. Available online: https://www.awmf.org/uploads/tx_szleitlinien/001-012l_S3_Analgesie-Sedierung-Delirmanagement-in-der-Intensivmedizin-DAS_2021-08.pdf (accessed on 15 September 2022).
- Cavallazzi, R.; Saad, M.; Marik, P.E. Delirium in the ICU: An overview. Ann. Intensive Care 2012, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Jakob, S.M.; Ruokonen, E.; Grounds, R.M.; Sarapohja, T.; Garratt, C.; Pocock, S.J.; Bratty, J.R.; Takala, J. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012, 307, 1151–1160. [Google Scholar] [CrossRef]
- Austin, S.R.; Wong, Y.N.; Uzzo, R.G.; Beck, J.R.; Egleston, B.L. Why Summary Comorbidity Measures Such As the Charlson Comorbidity Index and Elixhauser Score Work. Med. Care 2015, 53, e65–e72. [Google Scholar] [CrossRef] [PubMed]
- de Groot, V.; Beckerman, H.; Lankhorst, G.J.; Bouter, L.M. How to measure comorbidity. a critical review of available methods. J. Clin. Epidemiol. 2003, 56, 221–229. [Google Scholar] [CrossRef]
- Singh, B.; Bhaya, M.; Stern, J.; Roland, J.T.; Zimbler, M.; Rosenfeld, R.M.; Har-El, G.; Lucente, F.E. Validation of the Charlson comorbidity index in patients with head and neck cancer: A multi-institutional study. Laryngoscope 1997, 107, 1469–1475. [Google Scholar] [CrossRef]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar]
- Stawicki, S.P.; Kalra, S.; Jones, C.; Justiniano, C.F.; Papadimos, T.J.; Galwankar, S.C.; Pappada, S.M.; Feeney, J.J.; Evans, D.C. Comorbidity polypharmacy score and its clinical utility: A pragmatic practitioner’s perspective. J. Emerg. Trauma Shock 2015, 8, 224–231. [Google Scholar] [CrossRef]
- van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef]
- Galyfos, G.C.; Geropapas, G.E.; Sianou, A.; Sigala, F.; Filis, K. Risk factors for postoperative delirium in patients undergoing vascular surgery. J. Vasc. Surg. 2017, 66, 937–946. [Google Scholar] [CrossRef]
- Nazemi, A.K.; Gowd, A.K.; Carmouche, J.J.; Kates, S.L.; Albert, T.J.; Behrend, C.J. Prevention and Management of Postoperative Delirium in Elderly Patients Following Elective Spinal Surgery. Clin. Spine Surg. 2017, 30, 112–119. [Google Scholar] [CrossRef]
| Clinical Diagnosis of Postoperative Delirium | ||||||||
|---|---|---|---|---|---|---|---|---|
| No | Yes | Total | χ2 | |||||
| N | % | N | % | N | % | ρ | ||
| Sex | Female | 61 | 34.7% | 16 | 32.7% | 77 | 34.2% | 0.756 |
| Male | 115 | 65.3% | 33 | 67.3% | 148 | 65.8% | ||
| ACCI | ≤Median | 125 | 71.0% | 25 | 51.0% | 150 | 66.7% | 0.009 |
| >Median | 51 | 29.0% | 24 | 49.0% | 75 | 33.3% | ||
| Positive history of nicotine and alcohol abuse | No | 103 | 58.5% | 23 | 47.0% | 126 | 56.0% | 0.093 |
| Yes | 73 | 41.5% | 26 | 53.0% | 99 | 44.0% | ||
| Diagnosis for surgery | OSCC | 147 | 83.5% | 43 | 87.8% | 190 | 84.4% | |
| ORN | 20 | 11.4% | 4 | 8.2% | 24 | 10.7% | ||
| MRONJ | 2 | 1.1% | 1 | 2.0% | 3 | 1.3% | ||
| Osteomyelitis (not ORN or MRONJ) | 6 | 3.4% | 1 | 2.0% | 7 | 3.1% | ||
| Other | 1 | 0.6% | 0 | 0.0% | 1 | 0.4% | ||
| Site of reconstruction | Mandible | 57 | 32.6% | 17 | 35.4% | 74 | 33.0% | |
| Upper alveolus and gingiva & Hard palate | 20 | 11.4% | 5 | 10.4% | 25 | 11.2% | ||
| Tongue & Floor of mouth | 64 | 36.6% | 20 | 41.7% | 84 | 37.5% | ||
| Face & Neck | 15 | 8.6% | 2 | 4.2% | 17 | 7.6% | ||
| Buccal mucosa | 19 | 10.6% | 4 | 8.3% | 23 | 10.2% | ||
| Previous head and neck surgery | No | 93 | 52.8% | 29 | 59.2% | 122 | 54.2% | 0.239 |
| Yes | 83 | 47.2% | 20 | 40.8% | 103 | 45.8% | ||
| Microvascular surgery | No | 21 | 12.0% | 3 | 6.1% | 24 | 10.7% | 0.185 |
| Yes | 154 | 88.0% | 46 | 93.9% | 200 | 89.3% | ||
| Flap type | Radial forearm flap | 75 | 42.6% | 28 | 57.1% | 103 | 45.8% | |
| Anterolateral thigh flap | 18 | 10.2% | 3 | 6.1% | 21 | 9.3% | ||
| Free upper arm flap | 1 | 0.6% | 0 | 0.0% | 1 | 0.4% | ||
| Latissimus dorsi flap | 7 | 4.0% | 2 | 4.1% | 9 | 4.0% | ||
| Free fibula flap | 47 | 26.7% | 11 | 22.4% | 58 | 25.8% | ||
| Deep circumflex iliac artery flap | 4 | 2.3% | 2 | 4.1% | 6 | 2.7% | ||
| Scapular flap | 1 | 0.6% | 0 | 0.0% | 1 | 0.4% | ||
| Pectoralis major myocutaneos flap | 19 | 10.8% | 3 | 6.1% | 22 | 9.8% | ||
| Submental island flap | 4 | 2.3% | 0 | 0.0% | 4 | 1.8% | ||
| Size of reconstruction | ≤Median | 74 | 49.7% | 21 | 53.8% | 95 | 50.5% | 0.642 |
| >Median | 75 | 50.3% | 18 | 46.2% | 93 | 49.5% | ||
| Flap success | No | 18 | 9.3% | 6 | 11.8% | 24 | 9.8% | 0.743 |
| Yes | 176 | 90.7% | 45 | 88.2% | 221 | 90.2% | ||
| Impaired wound healing | No | 145 | 74.7% | 32 | 62.7% | 177 | 72.2% | 0.089 |
| Yes | 49 | 23.3% | 19 | 37.3% | 68 | 27.8% | ||
| Tracheostomy | No | 96 | 49.5% | 27 | 52.9% | 123 | 50.2% | 0.660 |
| Yes | 98 | 50.5% | 24 | 47.1% | 122 | 49.8% | ||
| Time at ICU | ≤Median | 147 | 75.8% | 20 | 39.2% | 167 | 68.2% | 0.000 |
| >Median | 47 | 24.2% | 31 | 60.8% | 78 | 31.8% | ||
| Duration of surgery | ≤Median | 100 | 51.5% | 24 | 47.0% | 124 | 50.6% | 0.568 |
| >Median | 94 | 48.5% | 27 | 53.0% | 121 | 49.4% | ||
| NRS score after end of sedation | 0 | 141 | 80.6% | 29 | 67.4% | 170 | 78.0% | 0.052 |
| ≠0 | 34 | 19.4% | 14 | 32.6% | 48 | 22.0% | ||
| Factor | p Value | Odds Ratio | 95% Confidence Interval, Lowest Value | 95% Confidence Interval, Highest Value |
|---|---|---|---|---|
| Duration of surgery | 0.392 | 1.467 | 0.610 | 3.529 |
| ACCI | 0.022 | 2.579 | 1.144 | 5.816 |
| Sex | 0.812 | 0.898 | 0.369 | 2.183 |
| Time at ICU | <0.001 | 4.753 | 2.172 | 10.403 |
| Impaired wound healing | 0.390 | 1.489 | 0.601 | 3.690 |
| Positive history of nicotine and alcohol abuse | 0.068 | 2.248 | 0.941 | 5.368 |
| Microvascular surgery | 0.187 | 2.700 | 0.618 | 11.796 |
| Previous head and neck surgery | 0.846 | 0.913 | 0.366 | 2.280 |
| Flap success | 0.801 | 1.183 | 0.320 | 4.381 |
| Tracheostomy | 0.391 | 0.666 | 0.264 | 1.685 |
| Postoperative NRS score | 0.005 | 3.678 | 1.496 | 9.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxis, J.; Spoerl, S.; Broszio, A.; Eichberger, J.; Grau, E.; Schuderer, J.; Ludwig, N.; Gottsauner, M.; Spanier, G.; Bundscherer, A.; et al. Postoperative Delirium after Reconstructive Surgery in the Head and Neck Region. J. Clin. Med. 2022, 11, 6630. https://doi.org/10.3390/jcm11226630
Taxis J, Spoerl S, Broszio A, Eichberger J, Grau E, Schuderer J, Ludwig N, Gottsauner M, Spanier G, Bundscherer A, et al. Postoperative Delirium after Reconstructive Surgery in the Head and Neck Region. Journal of Clinical Medicine. 2022; 11(22):6630. https://doi.org/10.3390/jcm11226630
Chicago/Turabian StyleTaxis, Juergen, Steffen Spoerl, Andreas Broszio, Jonas Eichberger, Elisabeth Grau, Johannes Schuderer, Nils Ludwig, Maximilian Gottsauner, Gerrit Spanier, Annika Bundscherer, and et al. 2022. "Postoperative Delirium after Reconstructive Surgery in the Head and Neck Region" Journal of Clinical Medicine 11, no. 22: 6630. https://doi.org/10.3390/jcm11226630
APA StyleTaxis, J., Spoerl, S., Broszio, A., Eichberger, J., Grau, E., Schuderer, J., Ludwig, N., Gottsauner, M., Spanier, G., Bundscherer, A., Reichert, T. E., & Ettl, T. (2022). Postoperative Delirium after Reconstructive Surgery in the Head and Neck Region. Journal of Clinical Medicine, 11(22), 6630. https://doi.org/10.3390/jcm11226630









