Effects of Non-Immersive Virtual Reality and Video Games on Walking Speed in Parkinson Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
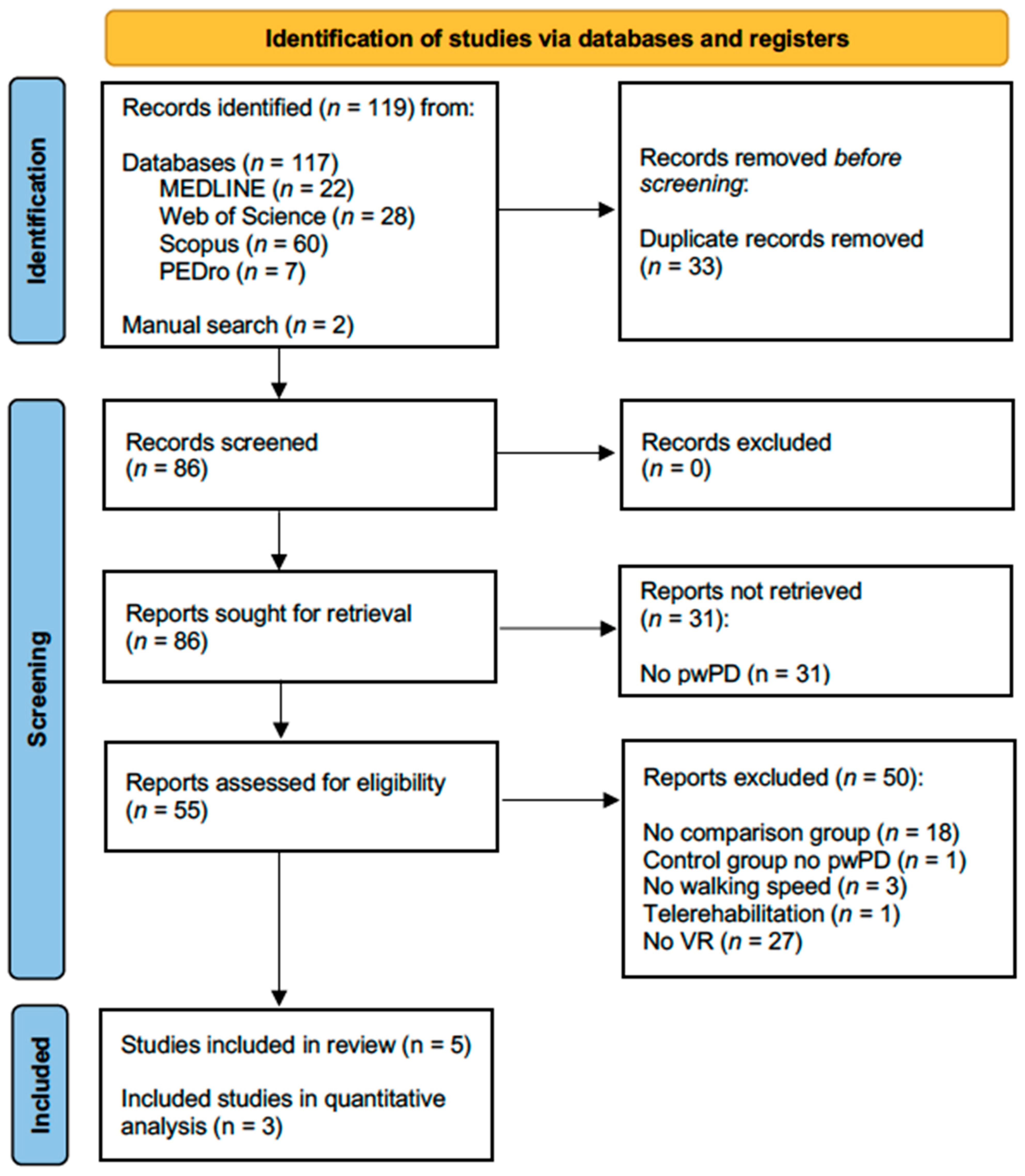
2.3. Selection Process and Data Extraction
2.4. Qualitative and Quantitative Assessment of Treatment Effects
3. Results
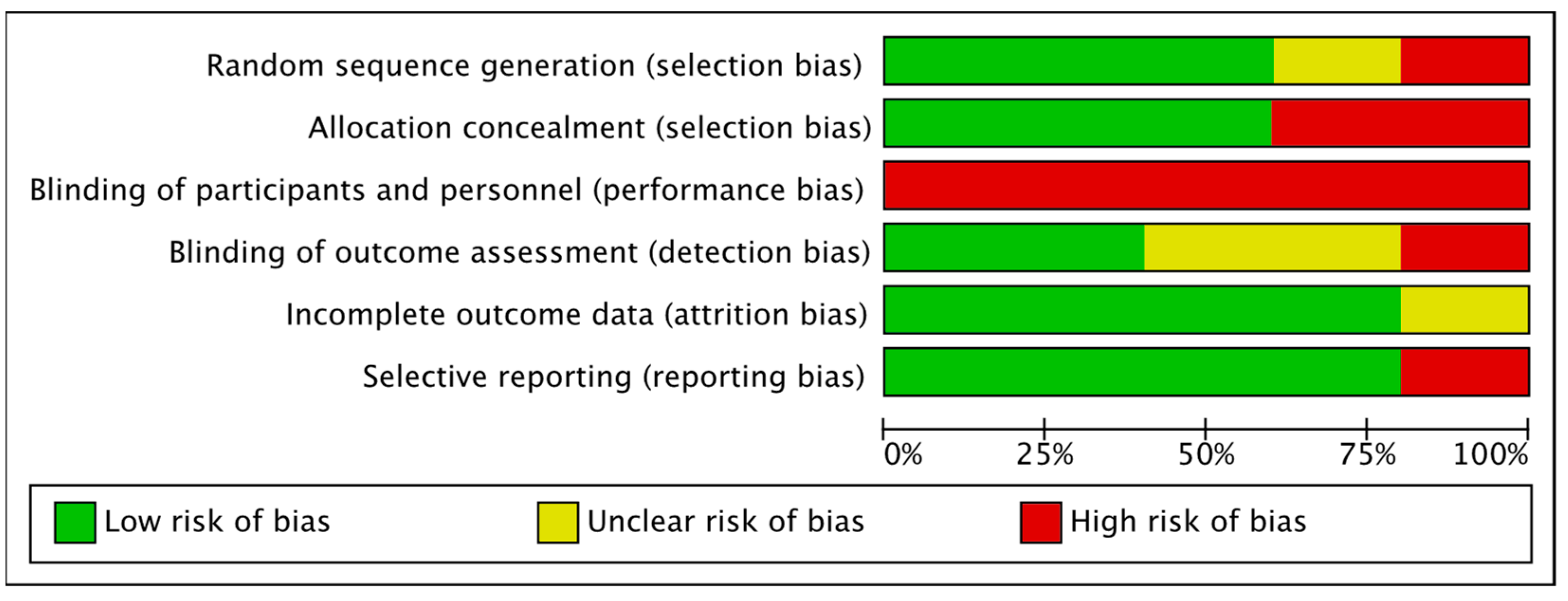
3.1. Assessment of Methodological Quality of the Studies
3.2. Synthesis of Results
3.3. Risk of Bias
3.4. Participant Characteristics
3.5. Intervention Characteristics
3.5.1. Commercial Systems Adapted for Therapeutic Use in Patients
- Nintendo Wii Balance Board (WBB)
- D’Alencar et al. [36] carried out an intervention based on seven Wii Fit virtual games with the Wii Balance Board (WBB) platform that required active movements from the participants during 35-minute sessions. The results were compared with a control group that received HR sessions of similar duration.
- Liao et al. [37] adapted the use of the Wii Fit Plus and the Wii Fit Balance Board to perform a 45-minute intervention protocol divided into three exercise modes (10 minutes of yoga, 20 minutes of balance, and 15 minutes of training strength), along with an additional 15 minutes of treadmill training. This group was compared with two groups. One of them received treadmill and CPT treatment for the same duration as the VR group, and the remaining group (called the control group) only received fall prevention talks.
- Kinect Xbox 360 (KX)
- De Melo et al. [38] chose the game “Your shape Fitness Evolved 2012”. In this video game, the patient had to perform during 20-minute sessions by simulating walking and running movements and using knee lifts without moving from his position. They also compared the effect of this intervention on two other groups. The first received CPT sessions and the second received treadmill training sessions.
- Ferraz et al. [39] used a 30-minute intervention with the game "Kinect Adventures" for the experimental group. The patient was asked by the avatar that appeared on the screen to perform full-body movements to achieve goals with the avatar that appeared on the screen. They compared the intervention with two additional groups, both of which performed 30-minute sessions of aerobic exercise (via cycle ergometer or functional exercises). In addition to their specific intervention, all groups performed stretching, warm-ups, and breathing exercises for 20 minutes.
3.5.2. Combination of Treadmill + VR (Images on a Screen of an Avatar of Themselves)
3.6. Effects of Intervention
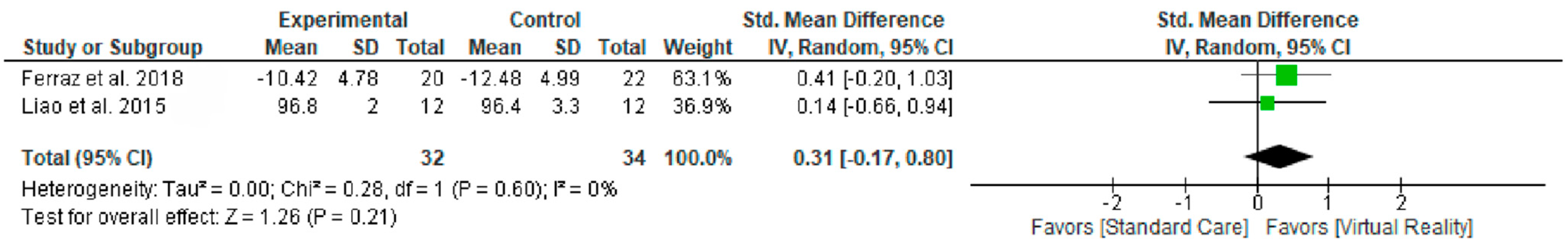
3.6.1. Comparation 1. Gait Speed
3.6.2. Comparation 2. Balance
4. Discussion
4.1. Walking Speed
4.2. Balance and Quality of Life
4.3. Limitations
4.4. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- de Lau, L.M.L.; Breteler, M.M.B. Epidemiology of Parkinson’s disease. Lancet. Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Tramonti, C.; Di Martino, S.; Unti, E.; Frosini, D.; Bonuccelli, U.; Rossi, B.; Ceravolo, R.; Chisari, C. Gait dynamics in Pisa syndrome and Camptocormia: The role of stride length and hip kinematics. Gait Posture 2017, 57, 130–135. [Google Scholar] [CrossRef] [PubMed]
- de Roiz, R.M.; Cacho, E.W.A.; Pazinatto, M.M.; Reis, J.G.; Cliquet, A.; Barasnevicius-Quagliato, E.M.A. Gait analysis comparing Parkinson’s disease with healthy elderly subjects. Arq. Neuropsiquiatr. 2010, 68, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Wang, R.-L.; Liou, D.-J.; Shaw, J.-S. Gait Disorders in Parkinson’s Disease: Assessment and Management. Int. J. Gerontol. 2013, 7, 189–193. [Google Scholar] [CrossRef]
- Tan, D.; Danoudis, M.; McGinley, J.; Morris, M.E. Relationships between motor aspects of gait impairments and activity limitations in people with Parkinson’s disease: A systematic review. Park. Relat. Disord. 2012, 18, 117–124. [Google Scholar] [CrossRef]
- Creaby, M.W.; Cole, M.H. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Park. Relat. Disord. 2018, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G.K.; Worringham, C.J.; Cole, M.H.; Lacherez, P.F.; Wood, J.M.; Silburn, P.A. Predictors of future falls in Parkinson disease. Neurology 2010, 75, 116–124. [Google Scholar] [CrossRef]
- Rubinstein, T.C.; Giladi, N.; Hausdorff, J.M. The power of cueing to circumvent dopamine deficits: A review of physical therapy treatment of gait disturbances in Parkinson’s disease. Mov. Disord. 2002, 17, 1148–1160. [Google Scholar] [CrossRef]
- Kwakkel, G.; de Goede, C.J.T.; van Wegen, E.E.H. Impact of physical therapy for Parkinson’s disease: A critical review of the literature. Park. Relat. Disord. 2007, 13 (Suppl. S3), S478–S487. [Google Scholar] [CrossRef]
- Hidalgo-Agudo, R.D.; Lucena-Anton, D.; Luque-Moreno, C.; Heredia-Rizo, A.M.; Moral-Munoz, J.A. Additional Physical Interventions to Conventional Physical Therapy in Parkinson’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2020, 9, 1038. [Google Scholar] [CrossRef]
- Abbruzzese, G.; Marchese, R.; Avanzino, L.; Pelosin, E. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Park. Relat. Disord. 2016, 22 (Suppl. S1), S60–S64. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; de Vries, N.M.; Ebersbach, G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov. Disord. 2015, 30, 1504–1520. [Google Scholar] [CrossRef] [PubMed]
- Saposnik, G.; Levin, M. Virtual reality in stroke rehabilitation: A meta-analysis and implications for clinicians. Stroke 2011, 42, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Luque-Moreno, C. A Decade of Progress Using Virtual Reality for Poststroke Lower Extremity Rehabilitation: Systematic Review of the Intervention Methods. BioMed Res. 2015, 2015, 342529. [Google Scholar] [CrossRef] [PubMed]
- Cano Porras, D.; Siemonsma, P.; Inzelberg, R.; Zeilig, G.; Plotnik, M. Advantages of virtual reality in the rehabilitation of balance and gait: Systematic review. Neurology 2018, 90, 1017–1025. [Google Scholar] [CrossRef]
- Sarma, S.V.; Cheng, M.L.; Eden, U.; Williams, Z.; Brown, E.N.; Eskandar, E. The effects of cues on neurons in the basal ganglia in Parkinson’s disease. Front. Integr. Neurosci. 2012, 6, 40. [Google Scholar] [CrossRef]
- Gómez-González, J.; Martín-Casas, P.; Cano-de-la-Cuerda, R. Efectos de los estímulos auditivos en la fase de iniciación de la marcha y de giro en pacientes con enfermedad de Parkinson. Neurología 2019, 34, 396–407. [Google Scholar] [CrossRef]
- Keus, S.H.J.; Bloem, B.R.; Hendriks, E.J.M.; Bredero-Cohen, A.B.; Munneke, M. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov. Disord. 2007, 22, 451–460. [Google Scholar] [CrossRef]
- Peñasco-Martín, B.; de los Reyes-Guzmán, A.; Gil-Agudo, Á.; Bernal-Sahún, A.; Pérez-Aguilar, B.; de la Peña-González, A.I. [Application of virtual reality in the motor aspects of neurorehabilitation]. Rev. Neurol. 2010, 51, 481–488. [Google Scholar]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef]
- Weiss, P.L.; Kizony, R.; Feintuch, U.; Katz, N. Virtual reality in neurorehabilitation. In Textbook of Neural Repair and Rehabilitation; Selzer, M., Clarke, S., Cohen, L., Duncan, P., Gage, F., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 182–197. [Google Scholar]
- Kiper, P.; Szczudlik, A.; Mirek, E.; Nowobilski, R.; Opara, J.; Agostini, M.; Tonin, P.; Turolla, A. The application of virtual reality in neuro-rehabilitation: Motor re-learning supported by innovative technologies. Rehabil. Med. 2013, 17, 29–36. [Google Scholar] [CrossRef]
- Caserman, P.; Garcia-Agundez, A.; Gámez Zerban, A.; Göbel, S. Cybersickness in current-generation virtual reality head-mounted displays: Systematic review and outlook. Virtual Real. 2021, 25, 1153–1170. [Google Scholar] [CrossRef]
- Howard, M.C.; Van Zandt, E.C. A meta-analysis of the virtual reality problem: Unequal effects of virtual reality sickness across individual differences. Virtual Real. 2021, 25, 1221–1246. [Google Scholar] [CrossRef]
- Fusco, A.; Tieri, G. Challenges and Perspectives for Clinical Applications of Immersive and Non-Immersive Virtual Reality. J. Clin. Med. 2022, 11, 4540. [Google Scholar] [CrossRef]
- Wang, B.; Shen, M.; Wang, Y.-X.; He, Z.-W.; Chi, S.-Q.; Yang, Z.-H. Effect of virtual reality on balance and gait ability in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2019, 33, 1130–1138. [Google Scholar] [CrossRef]
- Santos, P.; Scaldaferri, G.; Santos, L.; Ribeiro, N.; Neto, M.; Melo, A. Effects of the Nintendo Wii training on balance rehabilitation and quality of life of patients with Parkinson’s disease: A systematic review and meta-analysis. NeuroRehabilitation 2019, 44, 569–577. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; He, L.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Dockx, K.; Bekkers, E.M.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- da Santos, C.M.C.; de Pimenta, C.A.M.; Nobre, M.R.C. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enfermagem 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme (CASP). 11 Questions to Help You Make Sense of a Trial; CASP Checklists: Oxford, UK, 2013; pp. 1–7. [Google Scholar]
- Manterola, C.; Asenjo-lobos, C.; Otzen, T. Jerarquización de la evidencia. Niveles de evidencia y grados de recomendación de uso actual. Rev. Chil. Infectol 2014, 31, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef] [PubMed]
- D’Alencar, M.S.; Sa, K.N.; Beatriz Carneiro Pinto, E.; Baptista, A.F.; Da Silva Pereira, I.; Ribeiro, J.A.M.; De Jesus Souza, R.B.; Evangelista Dos Santos, G.; Gonealves, B.O.; Santos, A.L.B.; et al. Correlation between disease severity and gait speed in elderly with Parkinson’s disease submitted to virtual reality exposure therapy. In Proceedings of the 2015 International Conference on Virtual Rehabilitation (ICVR), Valencia, Spain, 9–12 June 2015; pp. 123–124. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Yang, Y.-R.; Wu, Y.-R.; Wang, R.-Y. Virtual Reality-Based Wii Fit Training in Improving Muscle Strength, Sensory Integration Ability, and Walking Abilities in Patients with Parkinson’s Disease: A Randomized Control Trial. Int. J. Gerontol. 2015, 9, 190–195. [Google Scholar] [CrossRef]
- de Melo, G.E.L.; Kleiner, A.F.R.; Lopes, J.B.P.; Dumont, A.J.L.; Lazzari, R.D.; Galli, M.; Oliveira, C.S. Effect of virtual reality training on walking distance and physical fitness in individuals with Parkinson’s disease. NeuroRehabilitation 2018, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, D.D.; Trippo, K.V.; Duarte, G.P.; Neto, M.G.; Bernardes Santos, K.O.; Filho, J.O. The Effects of Functional Training, Bicycle Exercise, and Exergaming on Walking Capacity of Elderly Patients with Parkinson Disease: A Pilot Randomized Controlled Single-blinded Trial. Arch. Phys. Med. Rehabil. 2018, 99, 826–833. [Google Scholar] [CrossRef]
- Fundarò, C.; Maestri, R.; Ferriero, G.; Chimento, P.; Taveggia, G.; Casale, R. Self-selected speed gait training in Parkinson’s disease: Robot-assisted gait training with virtual reality versus gait training on the ground. Eur. J. Phys. Rehabil. Med. 2019, 55, 456–462. [Google Scholar] [CrossRef]
- Palacios-Navarro, G.; García-Magariño, I.; Ramos-Lorente, P. A Kinect-Based System for Lower Limb Rehabilitation in Parkinson’s Disease Patients: A Pilot Study. J. Med. Syst. 2015, 39, 103. [Google Scholar] [CrossRef]
- Holden, M.K. Virtual Environments for Motor Rehabilitation: Review. CyberPsychol. Behav. 2005, 8, 187–211. [Google Scholar] [CrossRef]
- Ehgoetz Martens, K.A.; Ellard, C.G.; Almeida, Q.J. Does Anxiety Cause Freezing of Gait in Parkinson’s Disease? PLoS ONE 2014, 9, e106561. [Google Scholar] [CrossRef]
- Freitas, L.; de Araújo Val, S.; Magalhães, F.; Marinho, V.; Ayres, C.; Teixeira, S.; Bastos, V.H. Virtual reality exposure therapy for neuro-psychomotor recovery in adults: A systematic review. Disabil. Rehabil. Assist. Technol. 2019, 16, 646–6521. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Darakjian, N.; Finley, J.M. Walking in fully immersive virtual environments: An evaluation of potential adverse effects in older adults and individuals with Parkinson’s disease. J. Neuroeng. Rehabil. 2017, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, M.J.; Wagner, W.P. Virtual, mixed, and augmented reality: A systematic review for immersive systems research. Virtual Real. 2021, 25, 773–799. [Google Scholar] [CrossRef]
- Cikajlo, I.; Peterlin Potisk, K. Advantages of using 3D virtual reality based training in persons with Parkinson’s disease: A parallel study. J. Neuroeng. Rehabil. 2019, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Foerde, K.; Braun, E.K.; Higgins, E.T.; Shohamy, D. Motivational modes and learning in Parkinson’s disease. Soc. Cogn. Affect. Neurosci. 2015, 10, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Friis, R.; Kugler, J.; Twork, S.; Storch, A.; Pohl, M. Treadmill training for patients with Parkinson’s disease. In Cochrane Database of Systematic Reviews; Mehrholz, J., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Luque-Moreno, C.; López-García, J.C.; Díaz-Argandoña, E. Analysis of sustained attention in patients with Parkinson’s disease being treated with dopamine precursors. Rev. Neurol. 2012, 55, 257–262. [Google Scholar] [PubMed]
- Gandolfi, M.; Geroin, C.; Dimitrova, E.; Boldrini, P.; Waldner, A.; Bonadiman, S.; Picelli, A.; Regazzo, S.; Stirbu, E.; Primon, D.; et al. Virtual Reality Telerehabilitation for Postural Instability in Parkinson’s Disease: A Multicenter, Single-Blind, Randomized, Controlled Trial. BioMed Res. Int. 2017, 2017, 7962826. [Google Scholar] [CrossRef]
- Gallagher, R.; Damodaran, H.; Werner, W.G.; Powell, W.; Deutsch, J.E. Auditory and visual cueing modulate cycling speed of older adults and persons with Parkinson’s disease in a Virtual Cycling (V-Cycle) system. J. Neuroeng. Rehabil. 2016, 13, 77. [Google Scholar] [CrossRef]
- Kobayashi, E.; Himuro, N.; Takahashi, M. Clinical utility of the 6-min walk test for patients with moderate Parkinson’s disease. Int. J. Rehabil. Res. 2017, 40, 66–70. [Google Scholar] [CrossRef]

| Database | Search Terms | Records |
|---|---|---|
| Web of Science | TOPIC: (“parkinson disease” OR “parkinson´s disease”) AND (“virtual reality” OR feedback OR “video games” OR “Kinect” OR “Wii”) AND (“gait speed” OR “gait velocity” OR “walking speed”) NOT (“Telerehabilitation" OR home*) | 28 |
| Scopus | TITLE-ABS-KEY (“parkinson disease” OR “parkinson´s disease") AND (“virtual reality” OR feedback OR “video games” OR “Kinect” OR “Wii”) AND (“gait speed” OR “gait velocity” OR “walking speed”) AND NOT (“Telerehabilitation” OR home*) | 60 |
| PEDro | “parkinson disease” “virtual reality” “video games” | 7 |
| Medline | (“parkinson disease” OR “parkinson´s disease”) AND (“virtual reality” OR feedback OR “video games” OR “Kinect” OR “Wii”) AND (“gait speed” OR “gait velocity” OR “walking speed”) NOT (“Telerehabilitation” OR home*) | 22 |
| Total | 117 |
| Study | D’Alencar et al., 2015 [36] | Liao et al., 2015 [37] | De Melo et al., 2018 [38] | Ferraz et al., 2018 [39] | Fundarò et al., 2019 [40] | |
|---|---|---|---|---|---|---|
| Is the basic study design valid for a randomized controlled trial? | Did the study address a clearly focused research question? | YES | YES | YES | YES | YES |
| Was the assignment of participants to interventions randomized? | UNK | YES | YES | YES | NO | |
| Were all participants who entered the study accounted for at its conclusion? | YES | YES | YES | YES | YES | |
| Was the study methodologically sound? | Were the participants and investigators ”blind”? | NO | NO | NO | NO | NO |
| Were the study groups similar at the start of the randomized controlled trial? | NO | YES | YES | YES | YES | |
| Did each study group receive the same level of care? | YES | YES | YES | YES | YES | |
| What are the results? | Were the effects of the intervention reported comprehensively? | YES | YES | YES | YES | YES |
| Was the precision of the estimate of the intervention or treatment effect reported? | YES | YES | YES | YES | YES | |
| Do the benefits of the experimental intervention outweigh the harms and costs? | YES | YES | YES | YES | YES | |
| Will the results help locally? | Can the results be applied to your local population/in your context? | YES | YES | YES | YES | YES |
| Would the experimental intervention provide greater value to the people in your care than any of the existing interventions? | YES | YES | YES | YES | YES | |
| Total | 8/11 | 10/11 | 10/11 | 10/11 | 9/11 | |
| Author (y) LE, GR | Study Design | Age Mean | Sample | Stage * (Mean) | Levels of Immersion-Intervention-FB | Sessions | Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|
| D’Alencar et al. (2015) B, 2b [36] | RCT | 70 | IG = 15 CPTG = 16 | IG = 2.2 CPTG = 2.3 |
| 10 ses 35 m/ses 3 d/wk | 10MWT | No statistically significant improvement in ws post-intervention. Correlation between Parkinson disease stage and ws. |
| Liao et al. (2015) A, 1b [37] | RCT | 66 | IG = 12 CPTG = 12 CG = 11 | GE = 2 CPTG = 2 CG = 1.9 |
| 12 ses 40 m/ses 2 d/wk | STP (GAITRite); FGA; Dynamometer; SOT | IG and CPTG significant improvements in stride length, speed, and FGA over CG (post one-month follow-up). No difference between IG and CPTG. SOT: Significant improvements in IG and CPTG over CG in visual (post, one-month). Improvements in vestibular in IG with respect to CG. |
| De Melo et al. (2018) A, 1b [38] | RCT | 62 | IG = 12 TG = 13 CPTG = 12 | IG = 1.4 TG =1.5 CPTG = 2.08 |
| 12 ses 20 m/ses 3 d/wk | UPDRS; 6MWT; IMU | Statistically significant increase in ws of IG and TG with respect to CPTG. |
| Ferraz et al. (2018) A, 1b [39] | RCT | 69 | IG = 20 CPTG = 22 CEG = 20 | IG = 2.5 CPTG = 2.5 CEG = 2.5 |
| 18 ses 30 m/ses 3 d/8wk | UPDRS; PDQ-39; 6MWT; 10MWT | Only IG achieved significant improvements in 10 MWT. IG also showed significant improvements in 6MWT and PDQ-39 as well as the other two groups. |
| Fundarò et al. (2019) B, 2b [40] | NRS | 68 | VRLG = 10 CPTG = 10 | VRLG = 2.5 CPTG = 2.5 |
| 20 ses 30 m/ses 5 d/wk | UPDRS; FIM; 10MWT; Speed in Lokomat; VR score | Only CPTG improved in 10MWT significantly without significant differences in VRLG. VRLG had significant improvements in the speed of the Lokomat treadmill and the VR score, although inversely correlated with the results of 10MWT. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Lozano, F.; Kiper, P.; Carmona-Pérez, C.; Rutkowski, S.; Pinero-Pinto, E.; Luque-Moreno, C. Effects of Non-Immersive Virtual Reality and Video Games on Walking Speed in Parkinson Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 6610. https://doi.org/10.3390/jcm11226610
Navarro-Lozano F, Kiper P, Carmona-Pérez C, Rutkowski S, Pinero-Pinto E, Luque-Moreno C. Effects of Non-Immersive Virtual Reality and Video Games on Walking Speed in Parkinson Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(22):6610. https://doi.org/10.3390/jcm11226610
Chicago/Turabian StyleNavarro-Lozano, Francisco, Pawel Kiper, Cristina Carmona-Pérez, Sebastian Rutkowski, Elena Pinero-Pinto, and Carlos Luque-Moreno. 2022. "Effects of Non-Immersive Virtual Reality and Video Games on Walking Speed in Parkinson Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 22: 6610. https://doi.org/10.3390/jcm11226610
APA StyleNavarro-Lozano, F., Kiper, P., Carmona-Pérez, C., Rutkowski, S., Pinero-Pinto, E., & Luque-Moreno, C. (2022). Effects of Non-Immersive Virtual Reality and Video Games on Walking Speed in Parkinson Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(22), 6610. https://doi.org/10.3390/jcm11226610










