Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients, Design and Data Collection
2.2. Inclusion and Exclusion Criteria, Study Endpoints
2.3. Measurement of NT-Pro BNP and cTNI
2.4. Statistical Methods
2.4.1. Diagnostic Performance of NT-Pro BNP and cTNI
2.4.2. Prognostic Performance of NT-Pro BNP and cTNI
3. Results
3.1. Study Population
3.2. Association of cTNI and NT-Pro BNP with Clinical and Laboratory Data
3.3. Diagnostic Performance of cTNI and NT-Pro BNP
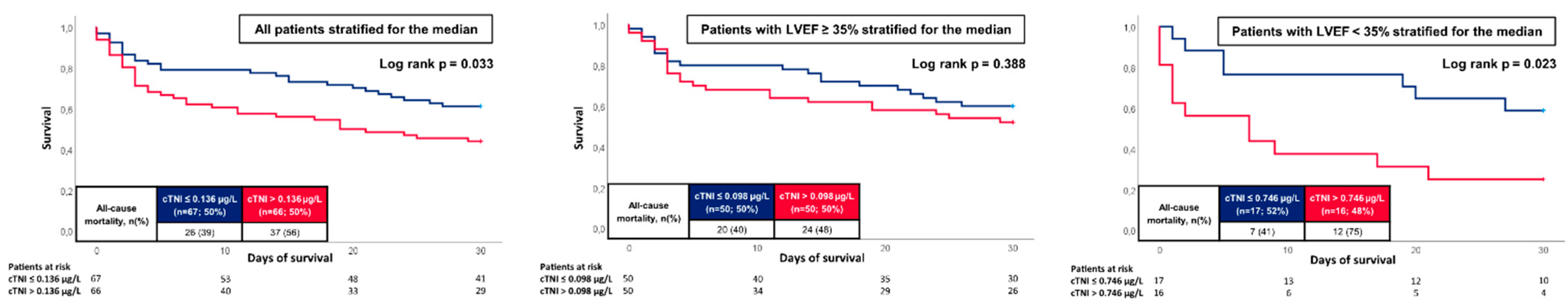
3.4. Prognostic Performance of cTNI and NT-Pro BNP
3.5. Multivariable Cox Regression Models
3.6. NT-Pro BNP Adjusted for eGFR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Charpentier, J.; Luyt, C.E.; Fulla, Y.; Vinsonneau, C.; Cariou, A.; Grabar, S.; Dhainaut, J.F.; Mira, J.P.; Chiche, J.D. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit. Care Med. 2004, 32, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Price, S.; Anning, P.B.; Mitchell, J.A.; Evans, T.W. Myocardial dysfunction in sepsis: Mechanisms and therapeutic implications. Eur. Heart J. 1999, 20, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, R.; Ali, Y.; Hashizume, R.; Suzuki, N.; Ito, M. BNP as a Major Player in the Heart-Kidney Connection. Int. J. Mol. Sci. 2019, 20, 3581. [Google Scholar] [CrossRef]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 191, 341–366. [Google Scholar]
- Maisel, A.S.; Duran, J.M.; Wettersten, N. Natriuretic Peptides in Heart Failure: Atrial and B-type Natriuretic Peptides. Heart Fail. Clin. 2018, 14, 13–25. [Google Scholar] [CrossRef]
- Farnsworth, C.W.; Bailey, A.L.; Jaffe, A.S.; Scott, M.G. Diagnostic concordance between NT-proBNP and BNP for suspected heart failure. Clin. Biochem. 2018, 59, 50–55. [Google Scholar] [CrossRef]
- Sutanto, H.; Lyon, A.; Lumens, J.; Schotten, U.; Dobrev, D.; Heijman, J. Cardiomyocyte calcium handling in health and disease: Insights from in vitro and in silico studies. Prog. Biophys. Mol. Biol. 2020, 157, 54–75. [Google Scholar] [CrossRef]
- Katrukha, I.A. Human cardiac troponin complex. Structure and functions. Biochemistry 2013, 78, 1447–1465. [Google Scholar] [CrossRef]
- Chaulin, A.M. Cardiac Troponins Metabolism: From Biochemical Mechanisms to Clinical Practice (Literature Review). Int. J. Mol. Sci. 2021, 22, 928. [Google Scholar] [CrossRef]
- Shomanova, Z.; Ohnewein, B.; Schernthaner, C.; Höfer, K.; Pogoda, C.A.; Frommeyer, G.; Wernly, B.; Brandt, M.C.; Dieplinger, A.M.; Reinecke, H.; et al. Classic and Novel Biomarkers as Potential Predictors of Ventricular Arrhythmias and Sudden Cardiac Death. J. Clin. Med. 2020, 9, 578. [Google Scholar] [CrossRef]
- Burke, M.A.; Cotts, W.G. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail. Rev. 2007, 12, 23–36. [Google Scholar] [CrossRef]
- Ammann, P.; Fehr, T.; Minder, E.I.; Günter, C.; Bertel, O. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001, 27, 965–969. [Google Scholar] [CrossRef]
- Mehta, S.; Granton, J.; Gordon, A.C.; Cook, D.J.; Lapinsky, S.; Newton, G.; Bandayrel, K.; Little, A.; Siau, C.; Ayers, D.; et al. Cardiac ischemia in patients with septic shock randomized to vasopressin or norepinephrine. Crit. Care 2013, 17, R117. [Google Scholar] [CrossRef]
- ver Elst, K.M.; Spapen, H.D.; Nguyen, D.N.; Garbar, C.; Huyghens, L.P.; Gorus, F.K. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin. Chem. 2000, 46, 650–657. [Google Scholar] [CrossRef]
- Schupp, T.; Weidner, K.; Rusnak, J.; Jawhar, S.; Forner, J.; Dulatahu, F.; Brück, L.M.; Hoffmann, U.; Bertsch, T.; Weiß, C.; et al. Diagnostic and prognostic value of the AST/ALT ratio in patients with sepsis and septic shock. Scand. J. Gastroenterol. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar]
- Apple, F.S.; Collinson, P.O. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin. Chem. 2012, 58, 54–61. [Google Scholar] [CrossRef]
- Behnes, M.; Espeter, F.; Hoffmann, U.; Lang, S.; Brueckmann, M.; Akin, I.; Borggrefe, M.; Bertsch, T.; Weiss, C.; Neumaier, M.; et al. Diagnostic and Long-Term Prognostic Value of Sensitive Troponin I in Symptomatic Patients Suspected of Acute Heart Failure. Clin. Lab. 2015, 61, 1737–1747. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic Cardio-myopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Barre, M.; Behnes, M.; Hamed, S.; Pauly, D.; Lepiorz, D.; Lang, S.; Akin, I.; Borggrefe, M.; Bertsch, T.; Hoffmann, U. Revisiting the prognostic value of monocyte chemotactic protein 1 and interleukin-6 in the sepsis-3 era. J. Crit. Care 2018, 43, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Babuin, L.; Jaffe, A.S. Troponin: The biomarker of choice for the detection of cardiac injury. CMAJ 2005, 173, 1191–1202. [Google Scholar] [CrossRef]
- Altmann, D.R.; Korte, W.; Maeder, M.T.; Fehr, T.; Haager, P.; Rickli, H.; Kleger, G.R.; Rodriguez, R.; Ammann, P. Elevated cardiac troponin I in sepsis and septic shock: No evidence for thrombus associated myocardial necrosis. PLoS ONE 2010, 5, e9017. [Google Scholar] [CrossRef]
- Ammann, P.; Maggiorini, M.; Bertel, O.; Haenseler, E.; Joller-Jemelka, H.I.; Oechslin, E.; Minder, E.I.; Rickli, H.; Fehr, T. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, 2004–2009. [Google Scholar] [CrossRef]
- Agewall, S.; Giannitsis, E.; Jernberg, T.; Katus, H. Troponin elevation in coronary vs. non-coronary disease. Eur. Heart J. 2011, 32, 404–411. [Google Scholar] [CrossRef]
- Vasile, V.C.; Chai, H.S.; Abdeldayem, D.; Afessa, B.; Jaffe, A.S. Elevated cardiac troponin T levels in critically ill patients with sepsis. Am. J. Med. 2013, 126, 1114–1121. [Google Scholar] [CrossRef]
- Kalla, C.; Raveh, D.; Algur, N.; Rudensky, B.; Yinnon, A.M.; Balkin, J. Incidence and significance of a positive troponin test in bacteremic patients without acute coronary syndrome. Am. J. Med. 2008, 121, 909–915. [Google Scholar] [CrossRef]
- Wen, K.; Du, H.; Tang, B.; Xiong, B.; Zhang, A.; Wang, P. Complete Blood Count and Myocardial Markers Combination with Sequential Organ Failure Assessment Score Can Effectively Predict the Mortality in Sepsis: A Derivation and Validation Study. Int. J. Gen. Med. 2022, 15, 3265–3280. [Google Scholar] [CrossRef]
- Brueckmann, M.; Huhle, G.; Lang, S.; Haase, K.K.; Bertsch, T.; Weiss, C.; Kaden, J.J.; Putensen, C.; Borggrefe, M.; Hoffmann, U. Prognostic value of plasma N-terminal pro-brain natriuretic peptide in patients with severe sepsis. Circulation 2005, 112, 527–534. [Google Scholar] [CrossRef]
- Khoury, J.; Arow, M.; Elias, A.; Makhoul, B.F.; Berger, G.; Kaplan, M.; Mashiach, T.; Ismael-Badarneh, R.; Aronson, D.; Azzam, Z.S. The prognostic value of brain natriuretic peptide (BNP) in non-cardiac patients with sepsis, ultra-long follow-up. J. Crit. Care 2017, 42, 117–122. [Google Scholar] [CrossRef]
- Yang, Y.; Leng, J.; Tian, X.; Wang, H.; Hao, C. Brain natriuretic peptide and cardiac troponin I for prediction of the prognosis in cancer patients with sepsis. BMC Anesthesiol. 2021, 21, 159. [Google Scholar] [CrossRef]
- Qian, A.; Zhang, M.; Zhao, G. Dynamic detection of N-terminal pro-B-type natriuretic peptide helps to predict the outcome of patients with major trauma. Eur. J. Trauma Emerg. Surg. 2015, 41, 57–64. [Google Scholar] [CrossRef]
- Andersson, P.; Frigyesi, A. High-sensitivity troponin T is an important independent predictor in addition to the Simplified Acute Physiology Score for short-term ICU mortality, particularly in patients with sepsis. J. Crit. Care 2019, 53, 218–222. [Google Scholar] [CrossRef]
- de Groot, B.; Verdoorn, R.C.; Lameijer, J.; van der Velden, J. High-sensitivity cardiac troponin T is an independent predictor of inhospital mortality in emergency department patients with suspected infection: A prospective observational derivation study. Emerg. Med. J. 2014, 31, 882–888. [Google Scholar] [CrossRef]
- Mehta, N.J.; Khan, I.A.; Gupta, V.; Jani, K.; Gowda, R.M.; Smith, P.R. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int. J. Cardiol. 2004, 95, 13–17. [Google Scholar] [CrossRef]
- Hai, P.D.; Binh, N.T.; Tot, N.H.; Hung, H.M.; Hoa, L.T.V.; Hien, N.V.Q.; Son, P.N. Diagnostic Value of High-Sensitivity Troponin T for Subclinical Left Ventricular Systolic Dysfunction in Patients with Sepsis. Cardiol. Res. Pract. 2021, 2021, 8897738. [Google Scholar] [CrossRef]
- Yu, J.; Zheng, R.; Yang, P.; Wang, D. Construction of a predictive model and prognosis of left ventricular systolic dysfunction in patients with sepsis based on the diagnosis using left ventricular global longitudinal strain. J. Intensive Care 2022, 10, 29. [Google Scholar] [CrossRef]

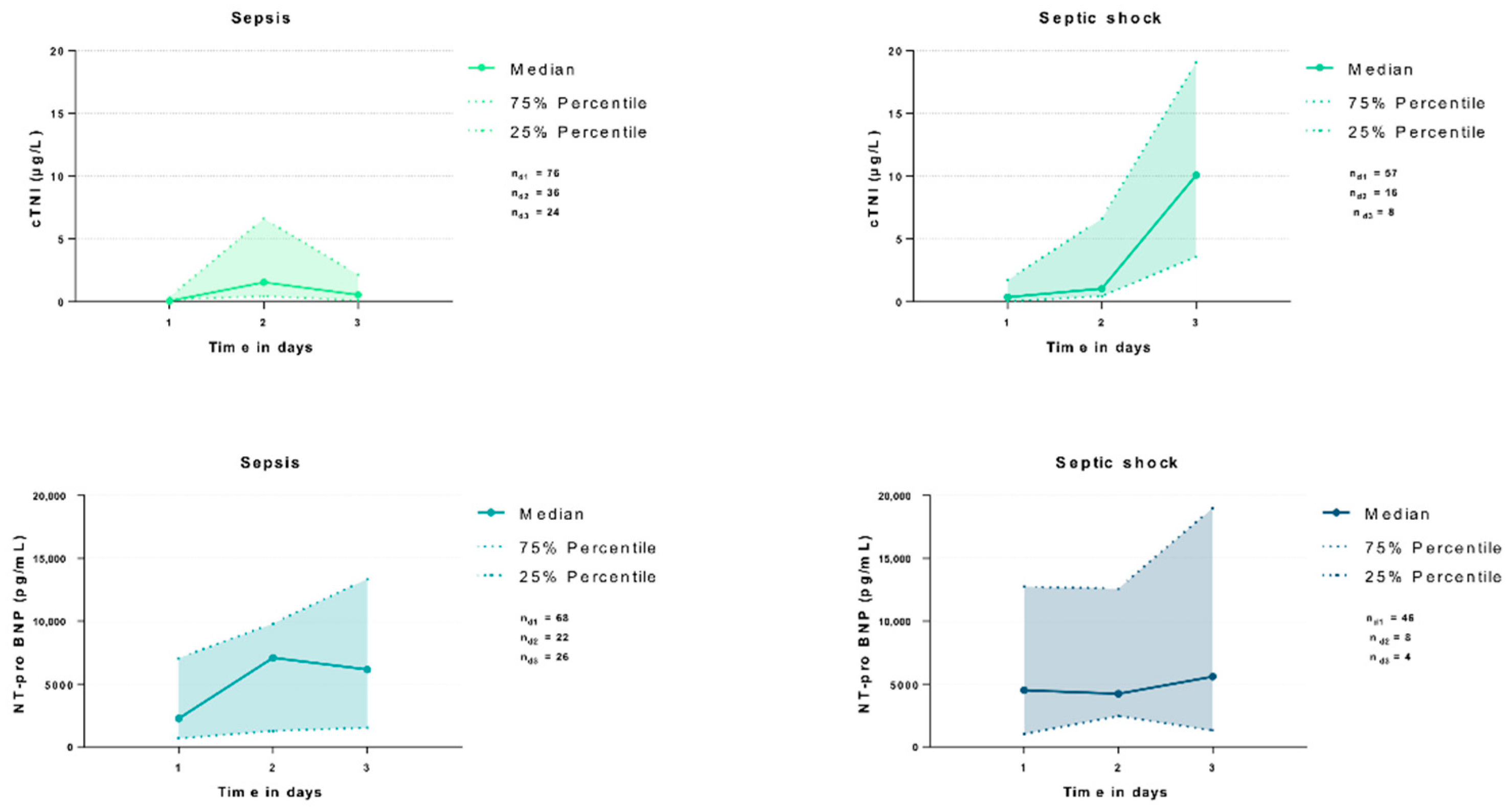


| All Patients (n = 162) | Sepsis (n = 93) | Septic Shock (n = 69) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Age, median; (IQR) | 70 | (61–78) | 70 | (60–78) | 70 | (60–80) | 0.966 |
| Male sex, n (%) | 106 | (65.4) | 61 | (65.6) | 45 | (65.2) | 0.961 |
| Body mass index (kg/m2), median; (IQR) | 26.67 | (24.22–30.86) | 26.58 | (23.77–29.89) | 26.73 | (24.69–32.65) | 0.410 |
| Entry criteria, median; (IQR) | |||||||
| Body temperature (°C) | 36.8 | (36–37.6) | 36.9 | (36.1–37.4) | 36.6 | (35.6–37.9) | 0.381 |
| Heart rate (bpm) | 102 | (87–115) | 96 | (85–111) | 108 | (90–123) | 0.027 |
| Systolic blood pressure (mmHg) | 111 | (96–129) | 114 | (99–133) | 108 | (88–125) | 0.010 |
| Respiratory rate (breaths/minute) | 22 | (18–26) | 22 | (18–26) | 21 | (18–26) | 0.563 |
| Cardiovascular risk factors, n (%) | |||||||
| Arterial hypertension | 113 | (69.8) | 65 | (69.9) | 48 | (69.6) | 0.964 |
| Diabetes mellitus | 60 | (37.0) | 35 | (37.6) | 25 | (36.2) | 0.855 |
| Hyperlipidemia | 51 | (31.5) | 24 | (25.8) | 27 | (39.1) | 0.071 |
| Smoking | 44 | (27.3) | 27 | (29.3) | 17 | (24.6) | 0.507 |
| Prior medical history, n (%) | |||||||
| Coronary artery disease | 67 | (41.4) | 36 | (38.7) | 31 | (44.9) | 0.427 |
| Congestive heart failure | 39 | (24.1) | 18 | (19.4) | 21 | (30.4) | 0.103 |
| Atrial fibrillation | 50 | (30.9) | 25 | (26.9) | 25 | (36.2) | 0.203 |
| Chronic kidney disease | 32 | (19.8) | 22 | (23.7) | 10 | (14.5) | 0.147 |
| COPD | 32 | (19.8) | 18 | (19.4) | 14 | (20.3) | 0.882 |
| Liver cirrhosis | 7 | (4.3) | 4 | (4.3) | 3 | (4.3) | 0.988 |
| Malignancy | 48 | (29.6) | 26 | (28.0) | 22 | (31.9) | 0.588 |
| Immunosuppression | 19 | (12.1) | 13 | (14.8) | 6 | (8.7) | 0.247 |
| LVEF at admission, n (%) | |||||||
| ≥55% | 46 | (28.4) | 26 | (28.0) | 20 | (29.0) | 0.886 |
| 54–45 | 44 | (27.2) | 35 | (37.6) | 9 | (13.0) | 0.001 |
| 44–35% | 35 | (21.6) | 15 | (16.1) | 20 | (29.0) | 0.049 |
| <35% | 37 | (22.8) | 17 | (18.3) | 20 | (29.0) | 0.108 |
| Cardiopulmonary resuscitation, n (%) | 26 | (16.0) | 5 | (5.4) | 21 | (30.4) | 0.001 |
| In-hospital | 7 | (4.3) | 2 | (2.2) | 5 | (7.2) | 0.001 |
| Out-of-hospital | 19 | (11.7) | 3 | (3.2) | 16 | (23.2) | |
| All Patients (n = 162) | Sepsis (n = 93) | Septic Shock (n = 69) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Sepsis scores, median; (IQR) | |||||||
| DIC | 1 | (1–2) | 1 | (0–2) | 2 | (1–3) | 0.001 |
| Acute physiology score | 17 | (12–23) | 13 | (8–19) | 22 | (15–25) | 0.001 |
| APACHE II | 24 | (18–30) | 20 | (14–27) | 27 | (21–33) | 0.001 |
| SOFA | 10 | (8–13) | 9 | (6–12) | 13 | (10–15) | 0.001 |
| ISARIC-4C-Mortality score | 15 | (12–16) | 14 | (12–16) | 15 | (12–16) | 0.615 |
| Infection focus, n (%) | |||||||
| Pulmonary | 93 | (57.4) | 53 | (57.0) | 40 | (58.0) | 0.216 |
| Urogenital | 19 | (11.7) | 15 | (16.1) | 4 | (5.8) | |
| Intra-abdominal | 12 | (7.4) | 6 | (6.5) | 6 | (8.7) | |
| Wound | 1 | (0.6) | 0 | (0.0) | 1 | (1.4) | |
| Unknown | 37 | (22.8) | 19 | (20.4) | 18 | (26.1) | |
| SARS-CoV-2 infection, n (%) | 24 | (14.8) | 19 | (20.4) | 5 | (7.2) | 0.020 |
| Multiple organ support during ICU | |||||||
| Vasopressor support norepinephrine, n (%) | 141 | (87.0) | 73 | (78.5) | 68 | (98.6) | 0.001 |
| Dose of norepinephrine (µg; median (IQR)) | 51.7 | (5.8–158.3) | 25.0 | (1.8–104.3) | 105.4 | (24.3–281.5) | 0.001 |
| Dialysis during hospitalization, n (%) | 75 | (46.3) | 31 | (33.2) | 44 | (63.8) | 0.001 |
| Extracorporeal membrane oxygenation, n (%) | 14 | (8.6) | 9 | (9.7) | 5 | (7.2) | 0.586 |
| Respiratory status | |||||||
| Mechanical ventilation, n (%) | 96 | (59.3) | 49 | (52.3) | 47 | (68.1) | 0.048 |
| Invasive mechanical ventilation, n (%) | 73 | (45.1) | 29 | (31.2) | 44 | (63.8) | 0.001 |
| Duration of mechanical ventilation (days; mean, (range)) | 5 | (1–15) | 5 | (1–16) | 3 | (1–15) | 0.715 |
| PaO2/FiO2 ratio (median; (IQR)) | 191 | (129–285) | 192 | (129–297) | 191 | (127–278) | 0.866 |
| PaO2 (median; (IQR)) | 91 | (72–123) | 87 | (69–117) | 97 | (80–126) | 0.081 |
| Liver function | |||||||
| Acute liver failure, n (%) | 15 | (9.3) | 6 | (6.5) | 9 | (13.0) | 0.152 |
| Renal function, median; (IQR) | |||||||
| Serum creatinine (mg/dL) | 1.9 | (1.28–3.03) | 1.69 | (1.09–2.85) | 2.16 | (1.56–3.49) | 0.010 |
| GFR (mL/min) | 31.49 | (19.2–51.88) | 34.93 | (21.7–62.01) | 26.87 | (16.2–40.48) | 0.008 |
| Urine output (mL) | 790 | (179–1493) | 900 | (415–1650) | 510 | (40–1270) | 0.022 |
| Dialysis (days) | 0 | (0–4) | 0 | (0–4) | 2 | (0–6) | 0.001 |
| Baseline laboratory values, median; (IQR) | |||||||
| pH | 7.37 | (7.28–7.42) | 7.39 | (7.31–7.44) | 7.33 | (7.22–7.40) | 0.001 |
| Lactate (mmol/L) | 2.1 | (1.2–3.9) | 1.4 | (1.0–2.2) | 3.5 | (2.3–8.6) | 0.001 |
| Serum sodium (mmol/L) | 139 | (135–143) | 138 | (135–142) | 140 | (135–145) | 0.181 |
| Serum potassium (mmol/L) | 4.2 | (3.7–4.7) | 4.1 | (3.6–4.6) | 4.2 | (3.8–4.8) | 0.289 |
| Hemoglobin (g/dL) | 10.8 | (9.0–12.5) | 10.7 | (9.0–12.9) | 10.8 | (9.0–12.3) | 0.691 |
| WBC (106/mL) | 13.13 | (8.45–17.76) | 13.06 | (8.21–17.58) | 13.97 | (8.92–19.62) | 0.657 |
| Platelets (106/mL) | 201 | (132–281) | 212 | (136–280) | 197 | (119–297) | 0.649 |
| INR | 1.18 | (1.08–1.32) | 1.13 | (1.06–1.23) | 1.28 | (1.12–1.64) | 0.001 |
| Fibrinogen (g/L) | 4.40 | (2.80–5.86) | 4.93 | (3.40–6.34) | 3.67 | (2.53–5.61) | 0.074 |
| D-dimer (µg/L) | 4.13 | (1.50–15.25) | 2.68 | (1.28–10.34) | 11.59 | (4.04–30.54) | 0.001 |
| AST (U/L) | 61 | (35–147) | 50 | (29–83) | 85 | (46–247) | 0.003 |
| ALT (U/L) | 33 | (18–97) | 30 | (18–87) | 39 | (17–125) | 0.352 |
| Bilirubin (mg/dL) | 0.85 | (0.50–1.36) | 0.74 | (0.49–1.31) | 0.97 | (0.53–1.54) | 0.224 |
| Troponin I (µg/L) | 0.14 | (0.03–0.92) | 0.08 | (0.02–0.37) | 0.37 | (0.05–1.73) | 0.002 |
| NT-pro BNP (pg/mL) | 2794 | (913–7978) | 2256 | (668–7053) | 4500 | (1033–12,742) | 0.085 |
| Procalcitonin (ng/mL) | 2.44 | (0.57–17.85) | 1.65 | (0.50–9.78) | 5.66 | (0.74–26.68) | 0.042 |
| CRP (mg/L) | 144 | (76–225) | 147 | (87–226) | 137 | (47–221) | 0.204 |
| Primary endpoint | |||||||
| All-cause mortality at 30 days, n (%) | 80 | (49.4) | 39 | (41.9) | 41 | (59.4) | 0.028 |
| Follow up data, n (%) | |||||||
| ICU time (days; median; (IQR)) | 7 | (3–17) | 8 | (3–18) | 5 | (3–16) | 0.098 |
| Death ICU, n (%) | 75 | (46.3) | 33 | (35.5) | 42 | (60.9) | 0.001 |
| cTNI | NT-Pro BNP | |||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| Age | 0.092 | 0.293 | 0.222 | 0.018 |
| BMI | 0.000 | 0.997 | −0.137 | 0.155 |
| Hb (g/dL) | −0.045 | 0.607 | −0.080 | 0.399 |
| WBC (106/mL) | −0.050 | 0.571 | −0.159 | 0.091 |
| Platelets (106/mL) | −0.160 | 0.067 | −0.225 | 0.016 |
| Albumin (g/L) | −0.064 | 0.520 | −0.170 | 0.112 |
| Bilirubin (mg/dL) | 0.072 | 0.493 | 0.185 | 0.088 |
| cTNI (µg/L) | - | - | 0.528 | 0.001 |
| NT-pro BNP (pg/mL) | 0.528 | 0.001 | - | - |
| LVEF | 0.307 | 0.001 | 0.439 | 0.001 |
| CRP (mg/L) | −0.141 | 0.110 | 0.081 | 0.403 |
| PCT (ng/mL) | 0.209 | 0.029 | 0.247 | 0.012 |
| PaO2/FiO2 ratio | 0.115 | 0.201 | 0.202 | 0.037 |
| Mechanical ventilation days | −0.053 | 0.544 | −0.342 | 0.001 |
| Creatinine (mg/dL) | 0.154 | 0.078 | 0.291 | 0.002 |
| Renal replacement days | 0.053 | 0.545 | −0.021 | 0.824 |
| SOFA score | 0.201 | 0.020 | 0.226 | 0.015 |
| Acute Physiology score | 0.239 | 0.006 | 0.192 | 0.040 |
| APACHE II score | 0.220 | 0.011 | 0.281 | 0.002 |
| MAP (mmHg) | 0.013 | 0.884 | −0.199 | 0.034 |
| Catecholamine use | 0.133 | 0.127 | −0.074 | 0.432 |
| Intensive-care days | −0.080 | 0.358 | −0.304 | 0.001 |
| cTNI | NT-Pro BNP | p-Value for AUC Difference | |
|---|---|---|---|
| Day 1 | 0.658 (0.564–0.753); p = 0.002 | 0.595 (0.488–0.702); p = 0.085 | 0.389 |
| Day 1: Controls n = 93 patients with sepsis | |||
| Day 2 | 0.547 (0.382–0.712); p = 0.592 | 0.517 (0.284–0.750); p = 0.888 | 0.842 |
| Day 2: Controls n = 103 patients with sepsis | |||
| Day 3 | 0.885 (0.770–1.000); p = 0.001 | 0.481 (0.162–0.800); p = 0.903 | 0.022 |
| Day 3: Controls n = 100 patients with sepsis | |||
| cTNI | NT-Pro BNP | p-Value for AUC Difference | |
|---|---|---|---|
| Day 1 | 0.635 (0.541–0.729); p = 0.007 | 0.582 (0.477–0.687); p = 0.132 | 0.462 |
| Day 2 | 0.687 (0.540–0.834); p = 0.021 | 0.537 (0.317–0.757); p = 0.735 | 0.255 |
| Day 3 | 0.633 (0.436–0.830); p = 0.200 | 0.525 (0.315–0.735); p = 0.813 | 0.455 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age | 1.013 | 0.995–1.030 | 0.151 | 0.994 | 0.966–1.023 | 0.688 |
| Sodium (mmol/L) | 1.027 | 0.998–1.057 | 0.066 | 1.023 | 0.950–1.102 | 0.548 |
| Potassium (mmol/L) | 0.989 | 0.736–1.329 | 0.944 | 0.669 | 0.354–1.266 | 0.217 |
| pH | 0.101 | 0.015–0.686 | 0.019 | 0.067 | 0.002–2.793 | 0.155 |
| WBC (106/mL) | 0.978 | 0.954–1.002 | 0.069 | 0.982 | 0.940–1.026 | 0.424 |
| Platelets (106/mL) | 0.998 | 0.996–1.000 | 0.042 | 0.998 | 0.995–1.001 | 0.179 |
| Malignancy | 1.255 | 0.786–2.005 | 0.341 | 3.439 | 1.389–8.511 | 0.008 |
| Immunosuppression | 0.559 | 0.243–1.287 | 0.172 | 0.520 | 0.106–2.540 | 0.419 |
| Respiratory rate > 22/min | 0.793 | 0.511–1.231 | 0.301 | 0.696 | 0.306–1.582 | 0.387 |
| Heart rate > 100 bpm | 0.923 | 0.595–1.431 | 0.721 | 0.549 | 0.236–1.276 | 0.163 |
| Systolic BP < 100 mmHg | 0.851 | 0.520–1.392 | 0.520 | 1.457 | 0.619–3.429 | 0.389 |
| Creatinine (mg/dL) | 1.004 | 0.896–1.126 | 0.940 | 1.035 | 0.847–1.266 | 0.735 |
| LVEF < 35% | 1.239 | 0.747–2.055 | 0.407 | 4.084 | 1.475–11.304 | 0.007 |
| cTNI > 0.136 µg/L | 1.703 | 1.030–2.814 | 0.038 | 2.251 | 1.017–4.981 | 0.045 |
| NT-pro BNP > 2793.5 pg/mL | 1.769 | 1.048–2.986 | 0.033 | 1.364 | 0.528–3.521 | 0.522 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forner, J.; Schupp, T.; Weidner, K.; Rusnak, J.; Jawhar, S.; Dulatahu, F.; Brück, L.M.; Behnes, M.; Hoffmann, U.; Bertsch, T.; et al. Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock. J. Clin. Med. 2022, 11, 6592. https://doi.org/10.3390/jcm11216592
Forner J, Schupp T, Weidner K, Rusnak J, Jawhar S, Dulatahu F, Brück LM, Behnes M, Hoffmann U, Bertsch T, et al. Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock. Journal of Clinical Medicine. 2022; 11(21):6592. https://doi.org/10.3390/jcm11216592
Chicago/Turabian StyleForner, Jan, Tobias Schupp, Kathrin Weidner, Jonas Rusnak, Schanas Jawhar, Floriana Dulatahu, Lea Marie Brück, Michael Behnes, Ursula Hoffmann, Thomas Bertsch, and et al. 2022. "Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock" Journal of Clinical Medicine 11, no. 21: 6592. https://doi.org/10.3390/jcm11216592
APA StyleForner, J., Schupp, T., Weidner, K., Rusnak, J., Jawhar, S., Dulatahu, F., Brück, L. M., Behnes, M., Hoffmann, U., Bertsch, T., Kittel, M., & Akin, I. (2022). Cardiac Troponin I Reveals Diagnostic and Prognostic Superiority to Aminoterminal Pro-B-Type Natriuretic Peptide in Sepsis and Septic Shock. Journal of Clinical Medicine, 11(21), 6592. https://doi.org/10.3390/jcm11216592







