Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Protocol of a Randomized Double-Blind Placebo-Controlled Trial
Abstract
1. Introduction
2. Experimental Design
2.1. Aim and Hypothesis
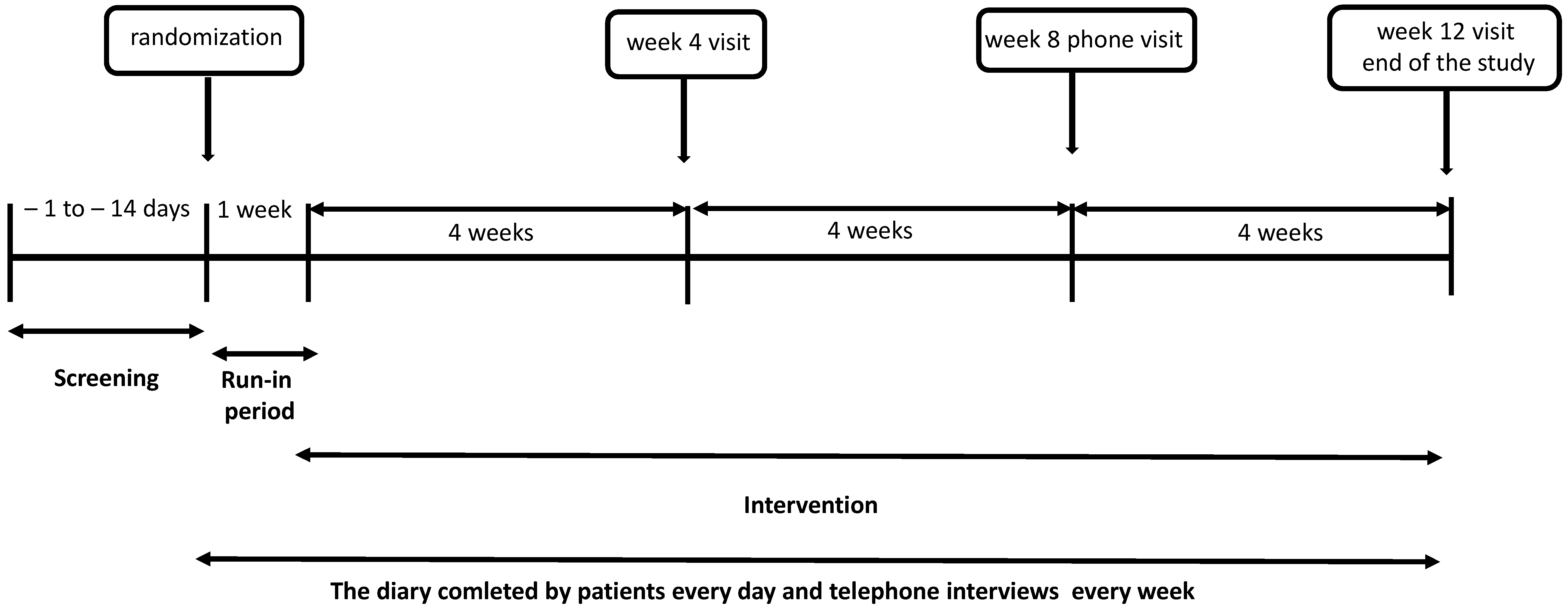
2.2. Study Design
2.3. Randomization
2.4. Intervention
3. Patients
3.1. Inclusion Criteria
- IBS-D—more than 25% of BSF type 6 and 7 stools, and less than 25% of type 1 and 2 stools;
- IBS-C—more than 25% of BSF type 1 and 2 stools, with less than 25% of type 6 and 7 stools;
- IBS-M—more than 25% of BSF type 6 and 7 stools and also more than 25% of type 1 and 2 stools.
- males and females aged from 18 to 70 years, inclusive;
- a good physical and mental condition assessed based on the patient’s history and physical examination;
- laboratory test results (complete blood count, blood chemistry panel) within normal limits or considered not to be clinically significant by the investigator;
- a voluntarily provided written informed consent;
- IBS with at least moderate symptom severity, defined as an IBS-Severity Scoring System (IBS-SSS) score of >175 points [32];
- the ability to strictly adhere to the investigators’ instructions regarding study procedures and protocol requirements.
3.2. Exclusion Criteria
- unclassified IBS;
- gastrointestinal conditions other than IBS, including clinical or endoscopic diagnosis of gastroenteritis, celiac disease, or inflammatory bowel disease;
- other diseases, such as respiratory disorders (asthma, chronic obstructive pulmonary disease); cardiovascular disorders, including uncontrolled hypertension (blood pressure > 170/100 mmHg); endocrine disorders, including diabetes mellitus (fasting blood glucose > 11 mmol/L) or thyroid diseases; severe neurological conditions, including psychosis; malignancy; and hepatic or renal impairment;
- unexplained blood biochemistry abnormalities: serum creatinine levels over twice the upper limit of normal, AST or ALT levels over twice the upper limit of normal;
- pregnancy or breastfeeding;
- hypersensitivity to soy or other food allergens;
- lactose intolerance;
- a surgical procedure scheduled during the course of the clinical study;
- the use of gastrointestinal motility stimulants or dietary fiber supplements during the two weeks preceding the clinical study;
- the use of antithrombotic drugs;
- current use of gut microbiota-targeted dietary supplements or drugs, such as probiotics, prebiotics, synbiotics, SCFAs, and a refusal to undergo a 1-month washout period;
- antibiotic therapy during the one month preceding the study;
- antibiotic use during the study;
- any drugs, except contraceptive pills or intramuscular contraceptives, hormone replacement therapy (estrogen/progesterone), L-thyroxine, antidepressants at low doses (up to 25 mg of amitriptyline, nortriptyline, or a selective serotonin reuptake inhibitor per day), antihypertensive drugs at low doses (diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists) and provided they have been used at a stable dose and for at least one month prior to the study;
- being included in another clinical study during the previous three months;
- a history of alcohol or substance abuse;
- COVID-19 infection, contact with any COVID-19-positive individuals during the previous two weeks.
3.3. Withdrawal Criteria
- informed consent withdrawal,
- a less than 80% adherence to the study protocol-required product/placebo supplementation,
- non-attendance at the study visits,
- the lack of contact with the telephone interviewer,
- any exclusion criteria found after enrollment,
- any serious adverse event during the intervention period.
4. Detailed Procedures
- A physical examination—at each visit;
- Anthropometric measurements, including weight, height, body mass index (BMI), waist-to-hip ratio (WHR), and arm or calf circumference—at visit 0 and at weeks 4 and 12 of the study intervention;
- Nutritional status assessment and body composition analysis, including skin fold measurements and bioelectrical impedance analysis (BIA) with the use of a Bodystat machine—at visit 0 and at weeks 4 and 12 of the study intervention;
- IBS symptom severity assessment, with the use of IBS-SSS [32]—at visit 0 and at weeks 4, 8, and 12 of the study intervention, and with patient-rated (on a Likert scale) symptom severity from patient diaries—weekly;
- Improvement or worsening of IBS symptoms, with the use of the IBS-SSS, IBS-Global Improvement Scale (IBS-GIS), and IBS—Adequate Relief (IBS-AR) [33]—at weeks 4, 8, and 12 of the study intervention;
- Adverse events based on the data from telephone interviewers and obtained by the investigators during the whole trial;
- Quality of life (QOF) assessment, with the use of IBS-QOL questionnaire [34]—at visit 0 and at weeks 4 and 12,
- Laboratory tests (including complete blood count; liver function tests (ALT, AST); bilirubin, amylase, creatinine, C-reactive protein, glucose, and electrolyte levels at the screening visit; and cytokine (interleukin 6 (IL-6) and macrophage inflammatory protein 1β (MIP-1ß)) levels at visit 0 and at weeks 4 and 12 of the study intervention.
4.1. IBS-SSS
- the severity of abdominal pain (IBS-SSS1),
- the frequency of abdominal pain over the last 10 days (IBS-SSS2),
- the severity of abdominal bloating (IBS-SSS3),
- dissatisfaction with bowel habits (IBS-SSS4),
- interference with quality of life over the past 10 days (IBS-SSS5) [32].
4.2. IBS-GIS
- 1 point—“I feel that the symptoms have worsened significantly”;
- 2 points—“I feel that the symptoms have moderately worsened”;
- 3 points—“I feel that the symptoms have slightly worsened”;
- 4 points—“I feel no change”;
- 5 points—“I feel a slight improvement”;
- 6 points—“I feel moderate improvement”;
- 7 points—“I feel significant improvement”.
4.3. IBS-AR
4.4. BSF Scale
4.5. IBS-QOL
4.6. Patient’s Diary
4.7. Telephone Interview
4.8. Study Endpoint Definition
- the number and type of stools assessed with the BSF scale,
- pain, bloating/abdominal distension, stool urgency, feeling of incomplete evacuation after a bowel movement assessed with a patient-rated Likert scale,
- adverse events,
- anthropometric measurements and BMI,
- body composition,
- cytokine (IL-6, MIP-1ß) levels.
4.9. Adverse Events
4.10. Statistical Analyses
4.10.1. Sample Size Calculation
4.10.2. Statistics
5. Expected Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Imren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2020, 160, 99–114. [Google Scholar] [CrossRef]
- Vivinus-Nébot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the microbiota-gut-brain axis in visceral pain: Relevance to irritable bowel syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Gillilland, M.; Wu, X.; Leelasinjaroen, P.; Zhang, G.; Zhou, H.; Ye, B.; Lu, Y.; Owyang, C. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin. Investig. 2018, 128, 267–280. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Muniz Pedrogo, D.A.; Kashyap, P.C. Irritable bowel syndrome: A gut microbiota-related disorder? Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G52–G62. [Google Scholar] [CrossRef]
- Mari, A.; Baker, F.A.; Mahamid, M.; Sbeit, W.; Khoury, T. The evolving role of gut microbiota in the management of irritable bowel syndrome: An overview of the current knowledge. J. Clin. Med. 2020, 9, 685. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Carroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2012, 24, 521–530. [Google Scholar] [CrossRef]
- Kerckhoffs, A.P.; Samsom, M.; van der Rest, M.E.; de Vogel, J.; Knol, J.; Ben-Amor, K.; Akkermans, L.M.A. Lower Bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J. Gastroenterol. 2009, 15, 2887–2892. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.C.; Chen, Y.J.; Shen, X.Z.; Liu, T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Campos-Perez, W.; Martinez-Lopez, E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158900. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 11, 277. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Z.; Liu, J.; Pei, L.; Wang, Y.; Li, A.; Kulyar, M.F.; Shahzad, M.; Mehmood, K.; Li, J.; et al. Effects of Short-Chain Fatty Acid Modulation on Potentially Diarrhea-Causing Pathogens in Yaks Through Metagenomic Sequencing. Front. Cell. Infect. Microbiol. 2022, 12, 805481. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 18, 6025–6041. [Google Scholar] [CrossRef]
- Banasiewicz, T.; Krokowicz, Ł.; Stojcev, Z.; Kaczmarek, B.F.; Kaczmarek, E.; Maik, J.; Marciniak, R.; Krokowicz, P.; Walkowiak, J.; Drews, M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Color. Dis. 2013, 15, 204–209. [Google Scholar] [CrossRef]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Li, B.; Liang, L.; Deng, H.; Guo, J.; Shu, H.; Zhang, L. Efficacy and safety of probiotics in irritable bowel syndrome: A systematic review and meta-Analysis. Front. Pharmacol. 2020, 11, 332. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Skrzydło-Radomańska, B.; Prozorow-Król, B.; Cichoż-Lach, H.; Majsiak, E.; Bierła, J.B.; Kosikowski, W.; Szczerbiński, M.; Gantzel, J.; Cukrowska, B. The effectiveness of synbiotic preparation containing Lactobacillus and Bifidobacterium probiotic strains and short chain fructooligosaccharides in patients with diarrhea predominant irritable bowel syndrome-a randomized double-blind, placebo-controlled study. Nutrients 2020, 12, 1999. [Google Scholar] [CrossRef]
- Suresh, K.P. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]
- Cole, E.T.; Scott, R.A.; Connor, A.L.; Wilding, I.R.; Petereit, H.U.; Schminke, C.; Beckert, T.; Cadé, D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002, 231, 83–95. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Riegler, G.; Esposito, I. Bristol scale stool form: A still valid help in medical practice and clinical research. Tech. Coloproctol. 2001, 5, 163–164. [Google Scholar] [CrossRef]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef]
- Gordon, S.; Ameen, V.; Bagby, B.; Shahan, B.; Jhingran, P.; Carter, E. Validation of irritable bowel syndrome Global Improvement Scale: An integrated symptom end point for assessing treatment efficacy. Dig. Dis. Sci. 2003, 48, 1317–1323. [Google Scholar] [CrossRef]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; DiCesare, J.; Puder, K.L. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig. Dis. Sci. 1998, 43, 400–411. [Google Scholar] [CrossRef]
- Niu, H.L.; Xiao, J.Y. The efficacy and safety of probiotics in patients with irritable bowel syndrome: Evidence based on 35 randomized controlled trials. Int. J. Surg. 2020, 75, 116–127. [Google Scholar] [CrossRef]
- Liang, D.; Longgui, N.; Guoqiang, X. Efficacy of different probiotic protocols in irritable bowel syndrome: A network meta-analysis. Medicine 2019, 98, e16068. [Google Scholar] [CrossRef]
- El-Salhy, M.; Valeur, J.; Hausken, T.; Gunnar Hatlebakk, J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2021, 33, e13983. [Google Scholar] [CrossRef]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Siciliano, R.A.; Reale, A.; Mazzeo, M.F.; Morandi, S.; Silvetti, T.; Brasca, M. Paraprobiotics: A New Perspective for Functional Foods and Nutraceuticals. Nutrients 2021, 13, 1225. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
| Active | Dose per Capsule * |
|---|---|
| Microencapsulated sodium butyrate | 300 mg (equal to 150 mg sodium butyrate) |
| Bifidobacterium lactis DSMZ 32269 | 5.2 × 108 CFU |
| Bifidobacterium longum DSMZ 32946 | 1.0 × 108 CFU |
| Bifidobacterium bifidum DSMZ 32403 | 1.0 × 108 CFU |
| Lactobacillus acidophilus DSMZ 32418 | 1.4 × 108 CFU |
| Lactobacillus rhamnosus FloraActive19070-2 | 1.4 × 108 CFU |
| scFOS | 64 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gąsiorowska, A.; Romanowski, M.; Walecka-Kapica, E.; Kaczka, A.; Chojnacki, C.; Padysz, M.; Siedlecka, M.; Bierła, J.B.; Steinert, R.E.; Cukrowska, B. Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Protocol of a Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Med. 2022, 11, 6587. https://doi.org/10.3390/jcm11216587
Gąsiorowska A, Romanowski M, Walecka-Kapica E, Kaczka A, Chojnacki C, Padysz M, Siedlecka M, Bierła JB, Steinert RE, Cukrowska B. Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Protocol of a Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine. 2022; 11(21):6587. https://doi.org/10.3390/jcm11216587
Chicago/Turabian StyleGąsiorowska, Anita, Marek Romanowski, Ewa Walecka-Kapica, Aleksandra Kaczka, Cezary Chojnacki, Milena Padysz, Marta Siedlecka, Joanna B. Bierła, Robert E. Steinert, and Bożena Cukrowska. 2022. "Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Protocol of a Randomized Double-Blind Placebo-Controlled Trial" Journal of Clinical Medicine 11, no. 21: 6587. https://doi.org/10.3390/jcm11216587
APA StyleGąsiorowska, A., Romanowski, M., Walecka-Kapica, E., Kaczka, A., Chojnacki, C., Padysz, M., Siedlecka, M., Bierła, J. B., Steinert, R. E., & Cukrowska, B. (2022). Effects of Microencapsulated Sodium Butyrate, Probiotics and Short Chain Fructooligosaccharides in Patients with Irritable Bowel Syndrome: A Study Protocol of a Randomized Double-Blind Placebo-Controlled Trial. Journal of Clinical Medicine, 11(21), 6587. https://doi.org/10.3390/jcm11216587








